Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 13
Neuroprotective Effects of Leptin on the APP/PS1 Alzheimer’s Disease Mouse Model: Role of Microglial and Neuroinflammation
Authors Ma J, Hou YH, Liao ZY, Ma Z, Zhang XX , Wang JL, Zhu YB, Shan HL, Wang PY, Li CB, Lv YL, Wei YL, Dou JZ
Received 21 July 2023
Accepted for publication 5 October 2023
Published 25 October 2023 Volume 2023:13 Pages 69—79
DOI https://doi.org/10.2147/DNND.S427781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Thomas Müller
Jing Ma,1 Yi-Hui Hou,2 Zhe-Yan Liao,2 Zheng Ma,1 Xiao-Xuan Zhang,1 Jian-Li Wang,3 Yun-Bo Zhu,1 Hai-Lei Shan,1 Ping-Yue Wang,1 Cheng-Bo Li,1 Ying-Lei Lv,1 Yi-Lan Wei,1 Jie-Zhi Dou1
1Department of Neurology, Chengde Medical University Affiliated Hospital, Chengde Medical University, Chengde, People’s Republic of China; 2Department of Neurology, Chengde Medical University Affiliated Hospital, School of Medicine, Chengde Medical University, Chengde, People’s Republic of China; 3Department of Hepatobiliary Surgery, Chengde Medical University Affiliated Hospital, Chengde Medical University, Chengde, People’s Republic of China
Correspondence: Jie-Zhi Dou, Department of Neurology, Chengde Medical University Affiliated Hospital, Chengde Medical University, No. 36 Nanyingzi Road, Chengde, People’s Republic of China, Email [email protected]
Background: Microglia are closely linked to Alzheimer’s disease (AD) many years ago; however, the pathological mechanisms of AD remain unclear. The purpose of this study was to determine whether leptin affected microglia in the hippocampus of young and aged male APP/PS1 mice.
Objective: In a transgenic model of AD, we investigated the association between intraperitoneal injection of leptin and microglia.
Methods: We intraperitoneal injection of leptin (1mg/kg) every day for one week and analyzed inflammatory markers in microglia in the hippocampus of adult (6 months) and aged (12 months) APP/PS1 mice.
Results: In all leptin treatment group, the brain Aβ levels were decrease. We found increased levels of IL-1β, IL-6 and microglial activation in the hippocampus of adult mice. Using aged mice as an experimental model for chronic neuroinflammation and leptin resistance, the number of Iba-1+ microglia and the levels of IL-1β/IL-6 in the hippocampus were greatly increased as compared to the adult. But between the leptin treatment and un-treatment, there were no difference.
Conclusion: Leptin signaling would regulate the activation of microglia and the release of inflammatory factors, but it is not the only underlying mechanism in the neuroprotective effects of AD pathogenesis.
Keywords: Alzheimer’s disease, leptin, microglial, neuroinflammation, aged
Introduction
Alzheimer’s disease (AD) is a complex neurological disorder of the central nervous system (CNS) with increasing cognitive dysfunction and behavioral impairment, resulting in death between 3 and 9 years after diagnosis.1 The neuropathological changes of AD brain include abnormal generation and deposition of A-amyloid (Aβ) peptides, either in Aβ40 or Aβ42 fragments, and hyperphosphorylated Tau protein clumps (neurofibrillary tangles) that are accompanied by astrogliosis and microglial cell activation.2 As a multifactorial disease, the prevalence and progression of AD are underpinned by a variety of contributing factors and complicated mechanisms. Besides genetic factors, aging, unhealthy lifestyles, obesity, etc. can directly or indirectly facilitate the occurrence of AD.3 So, it is a challenge to address its patho-physiology and thus therapeutic strategies because of the complexity of various processes and their interlinking between them.
Microglia are resident immune cells in the CNS that are able to detect (micro) environmental variations and tissue damage.4–6 Besides well-characterized roles in neuroinflammation, removing cellular debris and repairing injuries, microglia also provide trophic neuronal support, synaptic pruning, and homeostasis for living neurons, which is essential for the remodeling of neural circuits and plasticity of synapses.7 When brain homeostasis is disturbed, microglia become “activated”, a state marked by morphological alterations, such as process retraction and thickness, and produced inflammatory cytokines such as IL-1β and IL-6.8 Numerous searches revealed that phenotypic changes keep occurring in AD and that microglia communicate extensively with astrocytes, oligodendrocytes, neurons, and peripheral innate immune cells through certain signaling pathways and cytokines.9–13 The most important functions of microglia are synaptic remodeling throughout life and complement-dependent synaptic pruning throughout CNS maturation.14,15 Brain-derived neurotrophic factor (BDNF), for example, promotes the creation of synapses that are dependent on learning and protects neurons from damage to the brain.16,17 Furthermore, the activation of microglia in AD pathogenesis is a double-edged sword since it may promote the removal of Aβ and tau while also having the ability to cause neuroinflammation and the neuronal impairments linked to AD.18–20
As a polypeptide hormone, leptin is primarily secreted by adipocytes and performs its biological activity mostly in the brain, with potential metabolic effects on neurological systems.21,22 It was found to be expressed in various brain regions associated with higher cognitive functions, including the cortex and hippocampus, 2 main brain areas affected by AD.23,24 Numerous studies have shown that the aging process significantly reduces the sensitivity of neurons to leptin.25 Additionally, clinical research has found a link between circulating leptin levels and the chance of developing specific neurological diseases including Alzheimer’s disease (AD).26 According to growing research, leptin appears to have cognitively enhancing and neuroprotective effects in multiple AD models. Numerous studies have identified that leptin has been linked to improved hippocampal neuron survival, inhibition of neurodegeneration, promotion of NMDA-receptor dependent synaptic plasticity and glutamate receptor trafficking, facilitation of long-term potentiation, reduction of Aβ levels, and inhibition of tau phosphorylation.25,27–31 It has been reported that astrocyte and microglia express leptin receptors using RT-PCR analysis, suggesting that inflammation and immune responses are directly modulated by leptin.32 Microglial pro-inflammatory responses can be enhanced by leptin, including IL-6 production, IL-1β release and lipopolysaccharide (LPS)-induced pro-inflammatory responses.33,34 Following avulsion of the preganglionic cervical root, leptin is positive correlated with the expansion of microglia and necessary for their differentiation into an active state.35 Moreover, inhibition of leptin activity in cerebral ischemia-reperfusion (IR) lesions model preserved the viability of ipsilateral hippocampal CA1 neurons, probably through reducing apoptosis and local inflammation.36 On the other hand, previous research observed that treatment with leptin reversed the upregulation of pro-inflammatory cytokines expression (IL-1β, IL-6, and TNF-α) in AD mice.37 Furthermore, in comparison to untreated animals, leptin treatment reduced Iba1+ cell number and increased microglial span ratio in the hippocampus of 12-months-old 2xTgAD animals.38 Fernández-Martos et al also found that leptin induced microglial neuroprotective phenotypes and reduced inflammatory responses in a spinal cord injury model.39 Myeloid cell-specific LepR deficient mice showed less ramified microglia and reduced phagocytosis, suggesting that leptin directly regulates homeostatic microglial phenotypes.40 Microglia carry out a variety of physiological functions and their abnormal activation can lead to harm (eg, neuroinflammation). It is possible that leptin signaling in microglia could influence the central nervous system’s homeostasis.
Leptin can enter the brain via the blood–brain barrier, this study aims to determine whether injection of leptin into the abdomen has neuroprotective effect on the AD mouse model, and to better understand leptin signaling in microglia and CNS homeostasis.
Methods and Materials
Animals
Male APP/PS1 mice (n = 40) used in experiments were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). A 12-hour light/dark cycle was maintained in a humidity and temperature-controlled environment with free access to food and water for all the animals. Animals were used in experiments in a minimal number and their suffering was minimized. Research on animals was conducted strictly in accordance with the Laboratory Animal Protection Handbook, as well as receiving approval from the Chengde Medical University Affiliated Hospital Ethics Committee (CYFYLL2019007) for animal experiments.
Leptin Treatment
Mice were divided randomly into four groups: adult model group, leptin + adult model group, aged model group and leptin + age model group (n = 10 in each group). Recombinant mouse leptin (Elabscience. Catalog No. PKSM041419) was dissolved in sterile saline solution (0.9% NaCl). Seven days of leptin (1mg/kg) or saline injections were given intraperitoneally (i.p.) every day after 8 hours of fasting to both adult and aged models.38 The control and experimental groups were concealed from the investigators during experiments and analysis.
Tissue Collection
Isoflurane anesthesia was used to deeply anesthetize fasting mice. Brain tissue was collected from half of the subjects, flash frozen on liquid nitrogen, and stored at −80°C for later analysis of protein expression, while the second half of the subjects’ brains were postfixed for 24 h at 4°C in 4% paraformaldehyde.
Tissue Homogenates and ELISA Assays
Using a glass homogenizer on ice, frozen hippocampal samples of mice (n = 5 per group, 10mg) were homogenized in PBS (tissue weight (g): PBS volume (mL) =1:9). We sonicate the suspension with an ultrasonic cell disrupter to further break down the cells. The homogenates are then centrifuged for 5–10 min at 5000×g at 2–8°C to get the supernatant and centrifuge it for 5–10 minutes at 5000RPM at 2–8°C in order to obtain the supernatants.
Levels of Aβ1-40 (Amyloid Beta 1–40), Aβ1-42 (Amyloid Beta 1–42), IL-1β and IL-6 in brain tissues were measured by ELISA kits according to the instructions of the manufacturers. The concentration of total protein, which was determined using a BCA Protein Assay Kit, was used to adjust the results of ELISA measurements in hippocampal homogenates. The sensitivities and detection range of the assays were 4.69 (7.81–500) pg/mL for Aβ1-40 (Amyloid Beta 1–40), 1.88 (3.13–200) pg/mL for Aβ1-42, 4.69 (7.81–500) pg/mL for IL-1β and 18.75 (31.25–2000) pg/mL for IL-6. All samples were assayed in duplicate and the coefficient of variation was less than 5% for both assays.
Western Blotting (WB)
Proteins were extracted from frozen hippocampi samples using ice-cold RIPA lysis buffer supplemented with protease and phosphatase inhibitors. The Bradford assay was used to determine the protein concentration of the supernatant. The expression level of synaptophysin and leptin receptor at various epitopes in the transgenic mice was determined by WB analysis. A 10% SDS-PAGE gel was loaded into an electrophoresis running buffer (Tris/Glycine/SDS) and electrophoresed for 1 hour at 120 volts, followed by transfer to a polyvinylidene difluoride membrane. 5% Tris-buffered saline with 0.1% Tween (TBS-T) buffer was used to block the membranes, and then mouse anti-synaptophysin (Proteintech 17,785-1-AP), anti-leptin receptor (Affbiotech DF7139), and anti-GAPDH antibodies (Elabscience E-AB-40337) were used to incubate them. Overnight at 4°C, the membranes were incubated with primary antibodies on a shaker. We rinsed the membranes with TBS-T and incubated them for one hour with HRP-linked secondary antibodies (Elabscience E-AB-1003), followed by a second rinse with TBS-T. Finally, we scanned the membranes with a GS-800 Densitometer using ECL to determine density of bands. We used GAPDH to normalized the synaptophysin and leptin receptor densities. ImageJ was used to quantify signal density.
Immunofluorescent Staining and Measurement of the Percentage Area of Immuno-Positive Staining
Paraformaldehyde-fixed mouse brains were processed for paraffin blocks after 24 hours to perform immunohistochemistry assays. According to stereotaxic atlas, hippocampus staining was carried out on sections between coordinates 1.4 and 2.4 mm of lateral plane from medial line, then sliced into 4 μm coronal sections using a rotary microtome (Leica, Germany). Sections were air-dried after mounting them on gelatin-coated slides. After deparaffinization in xylene for five minutes, rehydration in ethanol at decreasing concentrations, they were hydrated with distilled water for five minutes. For IBA1 staining, sections were incubated in EDTA Antigen Retrieval Solution or citrate buffer (0.1 M) for 30 minutes for antigen retrieval, followed by washing with PBS. In order to decrease endogenous tissue peroxidase activity, 3% H2O2 was used to treatment for 10 min and PBS containing 0.3% Triton X-100 and 5% BSA was performed to block for 30-min. Sections were subsequently incubated with rabbit anti-IBA1 (Proteintech, 10,904-1-AP) overnight at 4°C. Polyperoxidase-anti-Rabbit/Mouse IgG (Elabscience E-IR-R221) was applied to incubate the sections after washing, then 3.30-diaminobenzidine was added to create color. To evaluate non-specific staining, sections were treated without the primary antibody and served as the negative control. In the end, sections were dehydrated in increasing grades of ethanol while being faintly counterstained with haematoxylin. Following air drying, xylene clearing, and mounting with DPX (Sigma-Aldrich, 06522), the sections were prepared. The Olympus BX 51 was used for the microscopic examination. The hippocampus of CA1, CA3 and DG were chosen and evaluated using ImageJ software (National Institutes of Health) to determine the cell number for Iba1. Per hippocampus subregion, three ROIs were consistently positioned in the same location across all pictures. All analyses were done blinded.
Statistical Analysis
GraphPad Prism (version 5.01 for Windows; Graph Pad Software, USA) was used for statistical analysis and graphs preparation. Although the D’Agostino & Pearson omnibus normality test and the ROUT test for outliers were used to determine the data’s normality, no data points were left out of the analysis. Our work needs to be viewed as exploratory given the small sample size. Two-way Analysis of Variance (ANOVA) was used to analyze data comparing four groups with two variables (treatment and age), followed by the multiple comparisons Tukey posthoc test. Differences between measures in 2 groups were analyzed with unpaired t-test. The data are expressed as mean ± standard error of the mean (SEM). We considered the results statistically significant if P < 0.05.
Results
Leptin Reduces Aβ1-40 and Aβ1-42 Level in the Hippocampus of Adult and Aged Mice
APP/PS1 mice, which can generate senile plaques, is a typical Aβ-related AD mice model. We used adult (6 months) and aged (12 months) transgenic mice, treated and untreated with leptin, to describe the ability of leptin to reduce brain Aβ levels. The CA1, CA3 and dentate gyrus (DG) of aged transgenic mice contains higher Aβ1-40 and Aβ1-42 level as compared with adult group, both leptin treatment group and un-treatment. In addition, both adult and aged mice group treated with leptin showed a lower level of Aβ1-40 and Aβ1-42 in the CA1, CA3 and DG of hippocampus (Figure 1A and B) and also demonstrated whenever used leptin has a beneficial effect.
 |
Figure 1 Influence of leptin on the levels of Aβ1-40 (Left) and Aβ1-42 (Right) in the adult + leptin group, adult + saline group, aged + leptin group and aged + saline group. |
Leptin Alleviates the Synaptic Dysfunction in the Hippocampus of Adult and Aged Mice
In AD patients and various animal models of AD, synaptic integrity loss in the hippocampal brain is a crucial characteristic. It has also been shown that APP/PS1 double transgenic mice display a reduction in synapse number, which can be reversed to the previous state (3 months) by leptin treatment.41 We therefore assessed the expression of synaptophysin in the leptin treatment group compared with their age-matched saline treatment. Both adult and aged mice with leptin treatment displayed significantly higher in synaptophysin protein expression levels than age-matched controls (Figures 2 and 3). Additionally, aged mice had significantly lower expression levels of synaptophysin in the hippocampus than the adult mice. According to these findings, AD damages hippocampal synaptic integrity, and leptin treatment may partially alleviate impairment, especially in adult mice.
 |
Figure 2 Western blotting analysis of synaptophysin expression in the adult + leptin group, adult + saline group, aged + leptin group and aged + saline group. |
 |
Figure 3 Semi-quantitative analysis of synaptophysin expression in the adult + leptin group, adult + saline group, aged + leptin group and aged + saline group. |
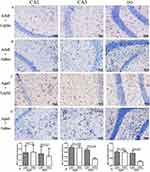
Leptin Treatment Show an Increase in Microglia Immunoreactivity in the Adult Hippocampus
In order to evaluate the effects of leptin on microglia in the hippocampus, Iba-1 staining was performed to access microglia numbers (Figure 4A–D). After seven days of leptin treatment, the number of IBA1-positive cells was increased in CA1 (p = 0.025) (Figure 4E), CA3 (p = 0.006) (Figure 4F) and DG (p = 0.037) (Figure 4G) hippocampal subregions of the adult mice when compared to controls. In old age, the CNS becomes resistant to leptin signaling and becomes more prone to inflammation. Since transgenic animals at this age already have significant Aβ plaque formation in the hippocampus, increased levels of microgliosis and microglial activation, as well as modifications in the cytokine production of microglia,42 we also decided to use 12-month-old mice for this experiment. We administered the same treatment to the aged mice with leptin, however there was no difference in the number of IBA1-positive cells between the two groups (Figure 4E–G). As expected, aged mice showed significantly higher in the number of microglial in the hippocampus than the adult mice – CA1 (p = 0.025), CA3 (p = 0.004) and DG (p = 0.005). According to our findings, leptin increases the number of microglia in the hippocampus subregions with an age-dependent manner.
Leptin Increases IL-1β and IL-6 Levels in the Hippocampus of Adult Mice
Leptin increases the amounts of pro-inflammatory cytokines in the hippocampus, which is evidence that it affects microglia dynamics.33,34 Here, we aimed to assess and confirm the effect of leptin on IL-1β and IL-6 levels in the hippocampus in adult and aged mice. For this purpose, the levels of IL-1β and IL-6 expression in hippocampus tissue from adult (6 months) and aged (12 months) mice were measured. Leptin significantly increased the level of IL-1β and IL-6 in adult mice compared to the adult saline group. In aged animals, we did not detect an increase in the level of IL-1β and IL-6 between in leptin treatment group and the saline treatment group, but it contains higher level of IL-1β in the control group as compared with the adult (p = 0.007), but no IL-6 level (p = 0.095) (Figure 5). In addition, we also conducted a correlation between toxic Aβ conformer levels and pro-inflammatory cytokine, but there was no significant correlation in all groups.
 |
Figure 5 Influence of leptin on the levels of IL-1β (Left), IL-6 (Right) in the adult + leptin group, adult + saline group, aged + leptin group and aged + saline group. |
Leptin Resistance in the Aged Mice Group
Leptin Resistance is the condition where circulating levels of leptin increased and leptin sensitivity diminished, which affects in body fat or food intake.32 It is not only found in obesity, but also in aging and neurodegenerative diseases. Leptin levels were increased in the hippocampus and CSF, but leptin receptor mRNA was downregulated in the hippocampus and this could suggest a novel neuronal leptin resistance in AD.43 On the other hand, age-related variations in LepR expression levels are also shown in an animal model of AD.30 In our study, there was an age-dependent reduction of LepR expression in the hippocampus at 12 months of age in APP/PS1 mice (Figures 6 and Figure 7). Injection of leptin can increase the level of LepR expression in the adult group; however, this difference was not detectable in the aged group. Based on the results described above, brain leptin resistance may play a role in the pathophysiology of AD.
 |
Figure 6 Western blotting analysis of leptin receptor expression in the adult + leptin group, adult + saline group, aged + leptin group and aged + saline group. |
 |
Figure 7 Semi-quantitative analysis of leptin receptor expression in the adult + leptin group, adult + saline group, aged + leptin group and aged + saline group. |
Discussion
According to our research, adult and aged transgenic mice showed different inflammatory reactions to leptin. This theory is confirmed by the fact that adult mice, but not aged mice, exhibited an increase in Iba-positive microglia in the CA1, CA3 and DG of hippocampus subfields in response to leptin. In addition, the study of the inflammatory markers linked to microglia revealed concurrently higher IL-1 and IL-6 levels in the hippocampus of adult mice. Here, it is suggested that leptin affects spatial memory processing and inflammatory mediator expression via activating microglia, especially in the hippocampus of adult mice. After leptin treatment, we discovered that pattern of microglia activation of adult mice was accompanied by elevated levels of IL-1 and IL-6, supporting the link between microglia phenotypes and memory performance.8 Although researchers have already discussed the beneficial benefits of both IL-1 and IL-6 on memory,44–47 our findings largely link this signature to leptin influencing higher brain functions, mostly through microglia. Moreover, this is the first time demonstrated that the IL-1β/IL-6 level in the hippocampus of 6-month-old mice was increased but in the hippocampus of aged mice was not changed. Aged mice did not respond to leptin challenges by activating microglia and increasing the levels of IL-1/IL-6 in the hippocampus region as was seen in adult mice. Additionally, we also found that there was an age-dependent reduction of LepR expression in the hippocampus at 12 months of age in APP/PS1 mice, suggesting a different mechanism underlying brain leptin resistance during aging. It is important to note that both our study and the referred study did not provide evidence to exclude possible abnormalities in leptin resistance caused by aging. Age-related declines in peripheral and central leptin signaling seem to impair microglial responses.48,49 Additionally, despite disagreements, it has also been established that a little quantity of leptin is created in the brain, however its precise function and potential impact on extracellular levels in aging brains are still unknown.50
It is widely acknowledged that the formation of Aβ oligomers in the AD brain is toxic to neural cells which can trigger AD pathogenesis.2 An analysis of APP/PS1 mouse brains showed that the Aβ plaques were localized in the hippocampus. After 8 weeks of leptin treatment, the levels of brain Aβ were significantly decreased in adult CRND8 transgenic mice (6-months old).51 According to other researchers, leptin therapy prevented Aβ deposits from accumulation in aged 2xTgAD mice (12-months old).38 Our findings found that leptin treatment decreased Aβ1-40 and Aβ1-42 levels in the hippocampus of 6 months-age and 12 months-age mice. Leptin is known to play a role in all aspects of Aβ metabolism, including its synthesis, clearance, and breakdown, which suggests that it lowers Aβ levels.52 Leptin was additionally reported to promote the absorption of Aβ by microglia, which subsequently target Aβ for intracellular breakdown, although the underlying physiological functions were unknown.37
As a polypeptide hormone, leptin is primarily secreted by adipocytes and performs its biological activity mostly in the brain, with potential metabolic effects on neurological systems.21,22 Additionally, it alters immune cells’ immunometabolism.32 The activation of leptin signaling pathways causes intracellular metabolic changes, including an increase in glucose uptake and glycolysis and a decrease in oxidative phosphorylation (OXPHOS), which is linked to immune cells’ pro-inflammatory phenotype.32 Our study showed that leptin increased the microglia number and then led to higher IL-1β/IL-6 production in adult mice. Additionally, when leptin was treated in aged mice there was neither the levels of IL-1β/IL-6 nor the number of microglia difference between the leptin treatment group and controls. However, the microglia are more activated in aged mice than in adult ones at basal conditions, which sustained inflammation hypothesis in the course of aging. There is evidence that the proliferation of microglia may be promoted by disease pathology. Microglial activation has been reported as an important factor in the pathological development of AD.8,53 It has a neuroprotective effect and may lessen AD pathogenesis by lowering Aβ levels and maintaining synaptic integrity in APP-based models.54 Our research also demonstrated that adult mice treated with leptin had relieved synaptic dysfunction and increased Iba-positive microglia in the hippocampus. An earlier study showed that a rapid microglial response is caused by the high accumulation of Aβ.55 In general, moderate rises in these cytokines have been identified as a natural aspect of aging.8,56 However, significant elevations, as seen in AD, result in excessive neurotoxicity, which fuels the neurodegenerative process and, as a result, may be related to the pathology of AD.57 In disorders of the brain, severe neuronal damage can cause chronic activation that results in the persistent produce of pro-inflammatory chemicals and the damaging generation of ROS, both of which have negative effects.57 However, lots of convincing evidence performed that microglia can affect neurons by secreting substances that damage these cells, raising the possibility that leptin signaling in the hippocampus may play a role in the regulation of the microglia activation in AD.58
In conclusion, our data revealed that leptin signaling would regulate the activation of microglia and the release of inflammatory factors, but it is not the only underlying mechanism in the neuroprotective effects of AD pathogenesis. Activation of leptin signaling pathways affects a variety of functions, including food intake and energy expenditure, as well as immunometabolism in immune cells.37 Leptin receptor activation leads to changes in intracellular metabolic activity, including increased glucose uptake, glycolytic activity, and OXPHOS reduction, which are associated with pro-inflammatory phenotype of immune cells.52 Our study showed that leptin resistance of the CNS is indicated by the apparent paucity of leptin receptors seen in older brains. A new pathological mechanism underlying leptin resistance and cognitive decline in the aging brain is the rupture between leptin signaling in the hippocampus and microglia activation. Leptin has been demonstrated as a potentially useful therapeutic tool for AD, but essential features must be met to ensure its translation into the clinic in the future.
Ethics Approval
Chengde Medical University Affiliated Hospital Ethics Committee (CYFYLL2019007).
Consent for Publication
All authors have given final approval of the version and agreed with the publication of this study here.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Natural Science Foundation of Hebei Province (H2019406165).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397:1577–1590. doi:10.1016/S0140-6736(20)32205-4
2. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189–a006189. doi:10.1101/cshperspect.a006189
3. Xu W, Tan L, Wang HF, et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol. 2015;86:1299–1306.
4. Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi:10.1016/0306-4522(90)90229-W
5. Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi:10.1007/s11481-009-9164-4
6. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 2007;10:1387–1394. doi:10.1038/nn1997
7. Salter MW, Beggs S. Sublime microglia: expanding roles for The Guardians of the CNS. Cell. 2014;158:15–24. doi:10.1016/j.cell.2014.06.008
8. Wendimu MY, Hooks SB. Microglia phenotypes in aging and neurodegenerative diseases. Cells. 2022;11:2091. doi:10.3390/cells11132091
9. Tcw J, Qian L, Pipalia NH, et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022;185:2213–2233.e2225. doi:10.1016/j.cell.2022.05.017
10. Wang S, Sudan R, Peng V, et al. TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways. Cell. 2022;185:4153–4169.e4119. doi:10.1016/j.cell.2022.09.033
11. Lopes KP, Snijders GJL, Humphrey J, et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat Genet. 2022;54:4–17. doi:10.1038/s41588-021-00976-y
12. Li Y, Li Z, Yang M, et al. Decoding the temporal and regional specification of microglia in the developing human brain. Cell Stem Cell. 2022;29:e626. doi:10.1016/j.stem.2022.02.004
13. Ennerfelt H, Frost EL, Shapiro DA, et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell. 2022;185:e4122. doi:10.1016/j.cell.2022.09.030
14. Schafer DP, Lehrman E, Kautzman A, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi:10.1016/j.neuron.2012.03.026
15. Herz J, Filiano AJ, Wiltbank AT, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46:943–956. doi:10.1016/j.immuni.2017.06.007
16. Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi:10.1016/j.cell.2013.11.030
17. Madinier A, Bertrand N, Mossiat C, et al. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One. 2009;4:e8101. doi:10.1371/journal.pone.0008101
18. Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Molecular Neurobiol. 2010;41:115–128. doi:10.1007/s12035-010-8106-8
19. Leyns CEG, Holtzman DM. Glial contributions to neurodegeneration in tauopathies. Molecular Neurodeg. 2017;12:50. doi:10.1186/s13024-017-0192-x
20. Shippy DC, Ulland TK. Microglial immunometabolism in Alzheimer’s disease. Front Cell Neurosci. 2020;14:563446. doi:10.3389/fncel.2020.563446
21. Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi:10.1038/372425a0
22. Stephens TW, Basinski M, Bristow PK, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi:10.1038/377530a0
23. Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–2638. doi:10.1097/00001756-199611040-00045
24. Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi:10.1016/0092-8674(95)90151-5
25. McGregor G, Clements L, Farah A, Irving AJ, Harvey J. Age-dependent regulation of excitatory synaptic transmission at hippocampal temporoammonic-CA1 synapses by leptin. Neurobiology of Aging. 2018;69:76–93. doi:10.1016/j.neurobiolaging.2018.05.007
26. Lilamand M, Bouaziz-Amar E, Dumurgier J, et al. Plasma leptin is associated with amyloid CSF biomarkers and Alzheimer’s disease diagnosis in cognitively impaired patients. The J Gerontol. 2023;78 645–652.
27. Ha J, Kwak S, Kim KY, et al. Relationship between adipokines, cognition, and brain structures in old age depending on obesity. J Gerontol. 2022.
28. Hamilton K, Harvey J. The neuronal actions of leptin and the implications for treating Alzheimer’s disease. Pharmaceuticals. 2021;14:1.
29. Hamilton K, Harvey J. Leptin regulation of hippocampal synaptic function in health and disease. Vitam Hormon. 2021;115:105–127.
30. King A, Brain A, Hanson K, et al. Disruption of leptin signalling in a mouse model of Alzheimer’s disease. Meta Brain Dis. 2018;33. doi:10.1007/s11011-018-0203-9
31. Fewlass DC, Noboa K, Pi‐Sunyer FX, et al. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J. 2004;18:1870–1878. doi:10.1096/fj.04-2572com
32. Shinjyo N, Kita K. Infection and immunometabolism in the central nervous system: a possible mechanistic link between metabolic imbalance and dementia. Front Cell Neurosci. 2021;15:765217.
33. Tang CH, Lu D-Y, Yang R-S, et al. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappaB, and p300 pathway in microglia. J Immunol. 2007;179:1292–1302. doi:10.4049/jimmunol.179.2.1292
34. Pinteaux E, Inoue W, Schmidt L, et al. Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J Neurochem. 2007;102:826–833. doi:10.1111/j.1471-4159.2007.04559.x
35. Chang KT, Lin Y-L, Lin C-T, et al. Leptin is essential for microglial activation and neuropathic pain after preganglionic cervical root avulsion. Life Sci. 2017;187:31–41. doi:10.1016/j.lfs.2017.08.016
36. Benbenishty A, Schneiderman J, Mongin AA. Intraarterial anti-leptin therapy via ICA protects ipsilateral CA1 neurons subjected to ischemia and reperfusion. PLoS One. 2022;17:e0261644. doi:10.1371/journal.pone.0261644
37. Lu L, Fu Z, Wu B, Zhang D, Wang Y. Leptin ameliorates Abeta1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway. Transl Neurosci. 2023;14. doi:10.1515/tnsci-2022-0270
38. Calió ML, Mosini AC, Marinho DS, et al. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2021;148:105219. doi:10.1016/j.nbd.2020.105219
39. Fernandez-Martos CM, Gonzalez P, Rodriguez FJ, Combs C. Acute leptin treatment enhances functional recovery after spinal cord injury. PLoS One. 2012;7:e35594. doi:10.1371/journal.pone.0035594
40. Davis BM, Salinas-Navarro M, Cordeiro MF, Moons L, De Groef L. Characterizing microglia activation: a spatial statistics approach to maximize information extraction. Sci Rep. 2017;7. doi:10.1038/s41598-017-01747-8
41. Pérez-González R, Alvira-Botero MX, Robayo O, et al. Leptin gene therapy attenuates neuronal damages evoked by amyloid-β and rescues memory deficits in APP/PS1 mice. Gene Therapy. 2014;21:298–308. doi:10.1038/gt.2013.85
42. Babcock AA, Ilkjær L, Clausen BH, et al. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav Immunit. 2015;48:86–101. doi:10.1016/j.bbi.2015.03.006
43. Bonda DJ, Stone JG, Torres SL, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochemist. 2014;128:162–172. doi:10.1111/jnc.12380
44. Takemiya T, Fumizawa K, Yamagata K, Iwakura Y, Kawakami M. Brain interleukin-1 facilitates learning of a water maze spatial memory task in young mice. Front Behav Neurosci. 2017;11. doi:10.3389/fnbeh.2017.00202
45. Del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immu. 2013;33:15–23. doi:10.1016/j.bbi.2013.05.011
46. Balschun D, Wetzel W, Rey A, et al. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi:10.1096/fj.04-1625fje
47. Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learn Mem. 2000;7:400–412. doi:10.1101/lm.32600
48. Ma J, Zhang W, Wang H-F, et al. Peripheral blood adipokines and insulin levels in patients with Alzheimer’s disease: a replication study and meta-analysis. Current Alzheimer Res. 2016;13:223–233. doi:10.2174/156720501303160217111434
49. Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565. doi:10.1001/jama.2009.1836
50. Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway--a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619–632. doi:10.1055/s-2007-979867
51. Greco SJ, Bryan KJ, Sarkar S, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimer’s Dis. 2010;19:1155–1167.
52. Casado ME, Collado-Perez R, Frago LM, Barrios V. Recent advances in the knowledge of the mechanisms of leptin physiology and actions in neurological and metabolic pathologies. Inter J Molec Sci. 2023;24:1.
53. Bivona G, Iemmolo M, Agnello L, et al. Microglial activation and priming in Alzheimer’s disease: state of the art and future perspectives. Inter J Mole Sci. 2023;24:884. doi:10.3390/ijms24010884
54. Michaud JP, Hallé M, Lampron A, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proceed National Acad Sci Unit State Am. 2013;110:1941–1946. doi:10.1073/pnas.1215165110
55. Merighi S, Nigro M, Travagli A, Gessi S. Microglia and Alzheimer’s disease. Inter J Molec Sci. 2022;23:12990. doi:10.3390/ijms232112990
56. McKee CG, Hoffos M, Vecchiarelli HA, Tremblay ME. Microglia: a pharmacological target for the treatment of age-related cognitive decline and Alzheimer’s disease. Front Pharmacol. 2023;14. doi:10.3389/fphar.2023.1125982
57. Bagheri-Mohammadi S. Microglia in Alzheimer’s disease: the role of stem cell-microglia interaction in brain homeostasis. Neurochem Res. 2021;46:141–148. doi:10.1007/s11064-020-03162-4
58. Wu YG, Song LJ, Yin LJ, et al. The effects and potential of microglial polarization and crosstalk with other cells of the central nervous system in the treatment of Alzheimer’s disease. Neural Regen Res. 2023;18:947–954.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.