Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Neurological Manifestations of Long COVID: A Single-Center One-Year Experience
Authors Taruffi L, Muccioli L, Mitolo M, Ferri L, Descovich C, Mazzoni S, Michelucci R, Lodi R, Liguori R , Cortelli P, Tonon C, Bisulli F
Received 1 September 2022
Accepted for publication 6 December 2022
Published 3 February 2023 Volume 2023:19 Pages 311—319
DOI https://doi.org/10.2147/NDT.S387501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Lisa Taruffi,1,* Lorenzo Muccioli,1,* Micaela Mitolo,2,3 Lorenzo Ferri,1 Carlo Descovich,4 Stefania Mazzoni,2 Roberto Michelucci,2 Raffaele Lodi,1,2 Rocco Liguori,1,2 Pietro Cortelli,1,2 Caterina Tonon,1,2 Francesca Bisulli1,2
1Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy; 2IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy; 3Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Bologna, Italy; 4Clinical Governance, Research, Education and Quality Improvement Unit, AUSL Bologna, Bologna, Italy
*These authors contributed equally to this work
Correspondence: Francesca Bisulli, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy, Tel +39 0514966937, Fax +39 05149669993, Email [email protected]
Purpose: We report our single-center experience on the neurological manifestations of long COVID.
Patients and Methods: This is a retrospective observational study. All consecutive patients referred to the neurological long COVID outpatient clinic of our institute from January 21 2021 to December 9 2021 underwent a general neurological objective examination. Treatments and investigations (brain MRI, neuropsychological evaluation, or others) were prescribed on an individual basis as per standard clinical practice. A follow-up visit was performed when appropriate. Descriptive statistics were presented as absolute and relative frequencies for categorical variables and as means, median, and ranges for continuous variables.
Results: One hundred and three patients were visited (mean age 50.5 ± 36 years, 62 females). The average time from acute COVID-19 infection to the first visit to our outpatient clinic was 243 days. Most patients presented with a mild form of acute COVID-19, with only 24 cases requiring hospitalization. The neurological symptoms mostly (n=70/103, 68%) started during the acute phase (before a negative swab for SARS-CoV-2). The most frequent acute manifestations reported, which lately became persistent, were fatigue (n=58/103, 56%), olfactory/taste dysfunction (n=58/103, 56%), headache (n=47/103, 46%), cognitive disorders (n=46/103, 45%), sleep disorders (n=30/103, 29%), sensitivity alterations (n=29/103, 28%), and dizziness (n=7/103, 7%). Tremor was also reported (n=8/103, 7%). Neuropsychological evaluation was performed in 30 patients and revealed alterations in executive functions (n=6/30, 20%), memory (n=11/30, 37%), with pathological depressive (n=9/30, 30%) and anxiety (n=8/30, 27%) scores. Brain MRIs have been performed in 41 cases, revealing nonspecific abnormal findings only in 4 cases. Thirty-six patients underwent a follow-up, where a general improvement was observed but rarely (n=2/36) a complete recovery.
Conclusion: The majority of patients presenting persistent neurological symptoms (most frequently fatigue, cognitive disorders, and olfactory dysfunctions) developed a previous mild form of COVID-19. Further studies are required to develop therapeutic strategies.
Keywords: COVID 19, neuroCOVID, follow-up, cognitive disorders, neuropsychology, hypo/anosmia
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Li has been published for this article.
Introduction
A significant number of COVID-19 patients develop a post-acute syndrome known as long COVID, which may last for several months and be highly debilitating. Long COVID was initially defined as signs and symptoms that develop during or following COVID-19, which continue for more than 4 weeks and are not explained by an alternative diagnosis.1 Recent studies have identified further subdivisions that are (1) subacute or ongoing symptomatic COVID-19, which includes symptoms and abnormalities persisting for 4–12 weeks beyond acute COVID-19, and (2) chronic or post-COVID-19 syndrome, which includes symptoms and abnormalities persisting beyond 12 weeks after acute COVID-19.2,3
While first studies hypothesized an association between long COVID development and the severity of acute infection or pre-existing comorbidities,2 the most recent ones have shown that long COVID is frequent also in non-hospitalized patients.4 Indeed, the frequency of persistent symptoms in patients after mild COVID-19 ranges between 10% and 35%.4 The most common neurological symptoms in long COVID patients described so far are chronic malaise, diffuse myalgia, depressive symptoms, non-restorative sleep, headache, loss of taste and smell, and cognitive impairment.2 In particular, brain fog is the term used to describe typical cognitive disorders associated with COVID-19, which affects especially executive function, attention, memory, and language.5
The underlying pathophysiology is still unclear, although evidence primarily implicates immune dysfunction, including nonspecific neuroinflammation secondary to cytokine storm and antineuronal autoimmunity.5 Indeed, first hypothesis expected SARS-CoV-2 to enter the central nervous system via migration through the nasal cavity and the olfactory pathway or crossing the blood–brain barrier, but no relevant results were obtained by trying to detect viral RNA in the cerebrospinal fluid (CSF) of patients with neuropsychiatric manifestations. Conversely, a dysregulated immune activation was shown both in the CSF and in peripheral tissues.6
Consistently, high blood levels of inflammatory markers were detected 3 months after the infection, with a correlation with cognitive disorders.7
Considering the high prevalence of long COVID, a systematic study of this condition is needed to develop evidence-based multidisciplinary teams that can support these patients, allowing the development of multi-specialty COVID-19 clinics.2,8
Herein, we report the one-year experience of a referral center for neurological manifestations of long COVID.
Materials and Methods
This was a single-center retrospective observational study of patients attending a “long NeuroCOVID” weekly outpatient clinic established at our Institute from 21 January 2021 to 9 December 2021. Patients were referred by their general practitioner when they detected a persistent (≥1 month from the onset) neurological disturbance that arose during acute COVID-19 or after its resolution following indications of the local health trust of Bologna, Italy (Figure 1). The diagnosis of COVID-19 was confirmed by PCR test in all patients. In each medical examination, the following data were collected: demographics, comorbidities, and acute COVID-19 clinical data (duration of symptoms, diagnostic modality details, hospitalization, oxygen therapy, and/or ventilation). We considered mild infection a form that has been managed in the outpatient setting.4
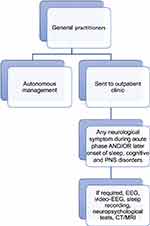 |
Figure 1 Flowchart of patients’ selection by their general practitioners, according to the provisions of the local health authorities. Abbreviation: PNS, peripheral nervous system. |
In addition, during the clinical interview, the following data on long-term neurological disturbances were collected: onset time (during or after acute COVID-19); new-onset symptoms, or aggravation of pre-existing ones. Following a qualitative description, the reported symptoms were classified into seven categories: sleep disorders, headache, cognitive disorders, sensory alterations, chronic fatigue, dizziness, and olfactory/taste dysfunctions. The neurological symptoms were divided into acute and persistent (considering as persistent symptoms that last more than 4 weeks and that therefore determine the long COVID syndrome). Myalgia was identified when patients referred muscle pain, while fatigue when a decrease in the ability of the muscle to produce force or power was reported.9,10 Previous treatments taken specifically for these symptoms were noted. A complete neurological physical examination was performed on each patient, and specific therapies or exams were prescribed according to the clinical picture, including brain MRI, brain CT, EEG, EMG, neuropsychological examination, Sniffin’ Sticks test,11 and other specialist visits as per standard clinical practice. If needed, follow-up visits were scheduled at three or six months to evaluate the outcome, based on individual needs with no predetermined criteria.
Descriptive statistics were presented as absolute (N) and relative frequencies (%) for categorical variables and as means, median, and ranges for continuous variables. Due to the descriptive nature of the study, no statistical comparisons were performed.
Neuropsychological Evaluation
At the discretion of the neurologist, a complete neuropsychological evaluation was performed, on the basis of patients’ availability to undergo neuropsychological testing. The standardized battery12 included a general screening test (ie, Montreal Cognitive Assessment MoCA) and investigated language skills, verbal and visuospatial memory, visuoconstructional abilities, and attention. In addition, we also administered two scales: the Depression, Anxiety, and Stress Scale-21 Items (DASS-21)13 and the Modified Fatigue Impact Scale (MFIS).14 All raw scores were corrected by age and education, according to published norms, and performances were considered impaired if below the cut-off values.
Ethical Approval and Informed Consent
Ethical approval was obtained by the Institutional Review Board “Area Vasta Emilia Centro” (AVEC). The local IRB waives the requirement to obtain informed consent for observational retrospective studies performed within IRCCS (Scientific Institute for Research, Hospitalization and Healthcare).
Results
A total of 103 patients were visited (62 females, mean age 50.5 ± 36.5 years). The average time from acute COVID-19 infection to the first referral was 243 days (22 to 618 days). The majority of patients (n=79/103, 76.7%) had a mild form of COVID-19, whereas only 24 patients were hospitalized and three of them required invasive mechanical ventilation. The average time before a negative respiratory sample was 28 days (6 to 92 days). A total of 12 patients had previous comorbidities. Demographic details are fully reported in Table 1, whereas infection details are reported in Table 2.
 |
Table 1 Demographic and Comorbidity Details |
 |
Table 2 Acute Infection Details |
The most common neurological symptoms in the acute phase were fatigue (n=58/103, 56%), olfactory/taste dysfunction (n=58/103, 56%), headache (n=47/103, 46%), cognitive disorders (n=46/103, 45%), sleep disorders, including both insomnia and hypersomnia (n=30/103, 29%), sensitivity alterations, including paraesthesia, neuropathic pain, and dysesthesia (n=29/103, 28%) and dizziness (n=7/103, 7%). Tremor was also reported (n=8/103, 7%).
Conversely, the most common persistent neurological symptoms in order of frequency were fatigue (n=49/58, 84.5% of the acute cases went persistent), cognitive disorders (n=42/46, 91.3%), olfactory/taste dysfunction (n=40/58, 69%) sleep disorders (n=25/30, 83.3%), sensitivity alterations (n=27/29, 93.1%), headache (n=24/47, 51.1%), and dizziness (n=3/7, 42.9%). A comparison between acute and persistent neurological symptoms is reported in Figure 2.
 |
Figure 2 Prevalence of neurological symptoms in acute and persistent phase. |
The average onset time of neurological symptoms since the COVID-19 infection was 17 days, with most patients (n=70/103, 68%) developing them while still positive. Acute neurological symptoms were mainly new onset (n=89/103, 86.4%); when already present before infection (n=14/103, 13.6%), symptoms (mainly headache and cognitive disorders) were aggravated in all cases. Further neurological details related to the infection are reported in Table 3.
 |
Table 3 Neuro-COVID Details |
Chronic fatigue was described as a lack of strength that could have arisen during the acute phase or even weeks later, not associated with muscular ache or shortness of breath but able to force them to reduce the amount of daily activities due to their sense of weakness.
Cognitive impairment, often reported as an increased forgetfulness or as an inability to recall whatever the patient was about to do or say, even if it was planned a few seconds before, was persistent, with only 9% of patients affected during the acute infection considered returning to their previous cognitive functioning.
Sleep disorders referred to our attention were extremely different, including difficulties in falling or staying asleep or, conversely, the tendency to sleep more hours than usual.
Only one patient developed acute COVID-19–related encephalopathy during hospitalization, characterized by delirium and frontal lobe dysfunction with akinetic mutism without significant EEG and MRI alterations, and was successfully treated with pulse steroid therapy. Debilitating persistent cognitive disorders were observed during follow-up.
Of the 103 patients visited, 67 were prescribed a supplement (palmitoylethanolamide-based supplements for patients with fatigue, olfactory, and cognitive related symptoms, sometimes associated with homotaurine or multivitamin-based for the latter, and melatonin-based ones for sleep disorders), and 35 a specific medication, including mainly antidepressant drugs. Thirty-six patients required a follow-up of one or more visits, often showing a general improvement but rarely (2/36) a return to usual health. Patients who did not present any improvement had several previous comorbidities in most cases. Thirty-one of the 36 patients who have been followed up (mean 96.5 days, range: 27–175 days) underwent at least one instrumental investigation or specialistic visit.
Forty-one MRIs were performed, mostly in patients without previous neurological comorbidities, revealing normal findings in most cases (n=37/41, 90.2%). Abnormal findings were identified in four cases: 1. Mild brain cortical atrophy (>temporal), small vessel disease; 2. Diffuse mild brain cortical atrophy, small vessel disease; 3–4: small vessel disease of moderate entity. Abnormal findings were also found in EEG (two out of the seven performed: 1. slight frontal slow abnormalities, rare, nonspecific; 2. symmetrical anterior sharp slow abnormalities during hyperpnea) and EMG (1. ulnar neuropathy; 2. chronic root disease).
Twenty-three patients underwent olfactory investigation through Sniffin’ Sticks, resulting in one patient with functional anosmia, 21 patients with hyposmia (16.25–30.5), and one patient with normosmia (scoring 30.75, the cut-off value), but concomitant dysosmia and gustatory dysfunction. Nine patients were also presenting persistent dysosmia (n=9/23, 40%), which includes parosmia, cacosmia, and phantosmia.
Neuropsychological assessment was administered to a subsample of 30 patients, and all different cognitive domains were explored. A total of n = 6/30 (20%) showed attentive and executive function deficits; memory was impaired in a total of n = 11/30 (37%); instead, language and visuospatial abilities were the cognitive domains mainly preserved (n = 1/30, 3%). Moreover, mood was also assessed with specific scales, showing the presence of depressive mood in 9/30 (30%), and a high level of anxiety in 8/30 (27%) patients.
The simultaneous presence of mood deflection and memory impairment was reported as particularly frustrating, as was involving both the personal and the working area.
Discussion
In our experience, we have observed that the majority of patients referred for long-COVID neurological symptoms had had a mild form of acute COVID-19 infection. Accordingly, persistent neurological symptoms were observed in approximately 10% of patients affected by mild forms of infection after a mean follow-up of 13 weeks in a previous study.4 The mean time of active infection before negative respiratory samples in our cohort was 28 days, with rare cases of long positivity, up to 92 days, which are numbers slightly above average, that is 17.0 days, with a maximum shedding duration of 83 days in upper respiratory tract samples.15 In our cohort, the main general acute symptoms were fever, asthenia, respiratory and GI symptoms, headache, myalgia, hyposmia, and hypogeusia, which are the most common findings reported in the literature.16 While myalgia, identified as general or localized muscular pain, was a quite uncommon finding, asthenia was present in more than half of the patients, persisting in the majority of them despite negative respiratory samples, determining a condition of chronic fatigue, one of the most frequent manifestations of long COVID, even more than dyspnoea.4 Olfactory and taste dysfunction were both present in the acute disease (56%) and in the persistent (39%) form and represented the most frequent referral motivation. Accordingly, the prevalence of this symptom range from 11% up to 68% in the literature.17 We observed both new-onset headache and the worsening of a pre-existing headache; it was reported from 45.6% of patients in the acute phase, resolving in about half of them (49%) after COVID-19 resolution. Similarly, in a previous study, 77% of patients complained of headache in the first 6 months and only 2% in the following 6 months.18 Cognitive disorders represented a further frequent referral motivation. Patients suffered from impaired concentration, memory, and difficulty finding words or focusing on the required task, which is particularly problematic in the workplace.19 In our cohort of patients who underwent neuropsychological tests, we found that memory was the most impaired cognitive ability, with a prevalence of 37% of patients. In addition, one-third of patients also presented depressive mood and high anxiety levels. Sleep disturbances were less common than the other symptoms so far specified but in line with the literature.2 Sensitivity alterations were less common than other symptoms but more frequent than in other studies.20 Finally, as expected, dizziness was a rare symptom both in the acute and chronic phases.18
The most common persistent neurological symptoms observed in this cohort (fatigue, cognitive impairment, and olfactory dysfunction) are consistent with those previously reported,2,21 but with a slightly higher prevalence of cognitive disorders compared to other studies.7,8 However, the study of Rass et al (2022),7 which has divided self-reported cognitive impairment into concentration difficulties and forgetfulness, shows that both symptoms tend to persist at 3-month and 1-year follow-ups, similarly to our findings.
Furthermore, delirium, which we have observed in a single patient who developed encephalopathy, has been correlated with poorer cognitive outcomes.22 All other patients who reported cognitive impairment did not experience delirium, which is more typical of hospitalized patients, notably in the ICU, compared to those managed as outpatients.
In our cohort, brain MRI did not show pathological findings in the vast majority of patients, similar to previously reported findings.23
Regarding pathophysiology, neurological symptoms do not seem attributable to virus-specific pathophysiologic changes, as the evidence of the presence of SARS-CoV-2 in both cerebrospinal fluid and neurological tissues is scarce.6 So far, the most likely explanations are residual immune activation or persistent autoimmune disturbance, ongoing endothelial activation, vascular dysfunction, or residual injury accrued during acute disease.6
Due to the unclearness of the pathophysiology, there are no specific medications at present days that can be used for long COVID neurological symptoms. We have used different supplements, especially palmitoylethanolamide (PEA) based ones, for fatigue, olfactory, and cognitive related symptoms, as the anti-inflammatory, pain-relieving, antimicrobial, immunomodulatory, and neuroprotective effects of PEA have recently been observed.24
The present study has several limitations including selection bias, as not the totality of patients that reported these symptoms to their general practitioner was sent to our outpatient clinic, so the actual prevalence of disorders may variate. Additionally, we cannot exclude with certainty that all symptoms complained of by the patients and all the pathological findings we had at diagnostic investigations were directly related to COVID-19. Furthermore, a control cohort of patients with previous COVID-19 that did not develop long COVID would have enriched the findings. Finally, self-reported symptoms were not necessarily correlated to specific examinations and confirmed, ie, neuropsychological tests were not prescribed to all patients who complained of cognitive disorders but only to those who considered this symptom particularly debilitating in their everyday life.
Future studies are needed to shed light on the pathophysiology of long COVID and to provide an effective treatment acting on the mechanism that determines the persistent symptoms.
Conclusion
Most patients presenting with neurological manifestations of long COVID referred to our outpatient clinic have been affected by mild acute COVID-19 at the onset. The most common persistent symptoms at the time of evaluation were chronic fatigue, cognitive disorders, and olfactory and taste dysfunction. The lack of clear-cut pathological findings at brain neuroimaging studies and of knowledge of the pathophysiological processes determining this heterogeneous clinical picture limits the development of target therapeutic strategies.
Acknowledgments
We thank all our colleagues and residents working at the IRCSS Institute of Neurological Sciences of Bologna (ISNB) who worked in our neurological long COVID outpatient clinic.
Disclosure
Prof. Dr. Rocco Liguori reports personal fees from SUMMEET s.r.l., ALEXION PHARMA ITALY s.r.l., UCB PHARMA S.p.A., ARGENX BV, AMICUS THERAPEUTICS s.r.l., EDITREE s.r.l., outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sivan M, Taylor S. NICE guideline on long covid. BMJ. 2020;m4938. doi:10.1136/bmj.m4938
2. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi:10.1038/s41591-021-01283-z
3. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;n136. doi:10.1136/bmj.n136
4. van Kessel SAM, Olde Hartman TC, Lucassen PLBJ, van Jaarsveld CHM. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2022;39(1):159–167. doi:10.1093/fampra/cmab076
5. Pensato U, Muccioli L, Cani I, et al. Brain dysfunction in COVID‐19 and CAR‐T therapy: cytokine storm‐associated encephalopathy. Ann Clin Transl Neurol. 2021;8(4):968–979. doi:10.1002/acn3.51348
6. Spudich S, Nath A. Nervous system consequences of COVID-19. Science. 2022;375(6578):267–269. doi:10.1126/science.abm2052
7. Rass V, Beer R, Schiefecker AJ, et al. Neurological outcomes 1 year after COVID-19 diagnosis: a prospective longitudinal cohort study. Eur J Neurol. 2022;29(6):1685–1696. doi:10.1111/ene.15307
8. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi:10.15585/mmwr.mm6930e1
9. Bockman CS, Eckerson J, McCarson KE. Myalgia. Reference Module Biomed Sci. 2015;B9780128012383052000. doi:10.1016/B978-0-12-801238-3.05161-8
10. Enoka RM. Fatigue. Larry R, ed:Squire, Encyclopedia of Neuroscience, Academic Press: 2009. 201–206.
11. Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34(4):222–226.
12. Barletta-Rodolfi C, Gasparini F, Ghidoni E. Kit del neuropsicologo italiano. Bologna Soc Ital Neuropsicol. 2011;1:498.
13. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335–343. doi:10.1016/0005-7967(94)00075-U
14. Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013;15(1):15–20. doi:10.7224/1537-2073.2012-019
15. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi:10.1016/S2666-5247(20)30172-5
16. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi:10.1038/s41591-020-0968-3
17. Xydakis MS, Albers MW, Holbrook EH, et al. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20(9):753–761. doi:10.1016/S1474-4422(21)00182-4
18. Stefanou MI, Palaiodimou L, Bakola E, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. 2022;13:20406223221076890. doi:10.1177/20406223221076890
19. Callan C, Ladds E, Husain L, Pattinson K, Greenhalgh T. ‘I can’t cope with multiple inputs’: a qualitative study of the lived experience of ‘brain fog’ after COVID-19. BMJ Open. 2022;12(2):e056366. doi:10.1136/bmjopen-2021-056366
20. Pilotto A, Cristillo V, Cotti Piccinelli S, et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol Sci. 2021;42(12):4903–4907. doi:10.1007/s10072-021-05586-4
21. Tabacof L, Tosto-Mancuso J, Wood J, et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehabil. 2022;101(1):48–52. doi:10.1097/PHM.0000000000001910
22. Smith CJ, Renshaw P, Yurgelun-Todd D, Sheth C. Acute and chronic neuropsychiatric symptoms in novel coronavirus disease 2019 (COVID-19) patients: a qualitative review. Front Public Health. 2022;10:772335. doi:10.3389/fpubh.2022.772335
23. Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: a systematic review in 116 patients. J Neuroradiol J Neuroradiol. 2021;48(1):43–50. doi:10.1016/j.neurad.2020.06.007
24. D’Ascanio L, Vitelli F, Cingolani C, Maranzano M, Brenner MJ, Di Stadio A. Randomized clinical trial “olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: preliminary results. Eur Rev Med Pharmacol Sci. 2021;25(11):4156–4162. doi:10.26355/eurrev_202106_26059
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
