Back to Journals » Psychology Research and Behavior Management » Volume 16
Neurogenetics and Epigenetics of Loneliness
Authors Bowirrat A , Elman I , Dennen CA, Gondré-Lewis MC, Cadet JL, Khalsa J, Baron D, Soni D, Gold MS, McLaughlin TJ , Bagchi D , Braverman ER, Ceccanti M , Thanos PK, Modestino EJ , Sunder K, Jafari N, Zeine F , Badgaiyan RD , Barh D, Makale M, Murphy KT, Blum K
Received 30 July 2023
Accepted for publication 14 November 2023
Published 29 November 2023 Volume 2023:16 Pages 4839—4857
DOI https://doi.org/10.2147/PRBM.S423802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Mei-Chun Cheung
Abdalla Bowirrat,1 Igor Elman,2 Catherine A Dennen,3 Marjorie C Gondré-Lewis,4 Jean Lud Cadet,5 Jag Khalsa,6 David Baron,7 Diwanshu Soni,8 Mark S Gold,9 Thomas J McLaughlin,10 Debasis Bagchi,11 Eric R Braverman,12 Mauro Ceccanti,13 Panayotis K Thanos,14,15 Edward Justin Modestino,16 Keerthy Sunder,17,18 Nicole Jafari,19,20 Foojan Zeine,21,22 Rajendra D Badgaiyan,23 Debmalya Barh,24,25 Milan Makale,26 Kevin T Murphy,27 Kenneth Blum1,7,10,12,18,20,24,28,29
1Department of Molecular Biology, Adelson School of Medicine, Ariel University, Ariel, 40700, Israel; 2Cambridge Health Alliance, Harvard Medical School, Cambridge, MA, 02139, USA; 3Department of Family Medicine, Jefferson Health Northeast, Philadelphia, PA, USA; 4Neuropsychopharmacology Laboratory, Department of Anatomy, Howard University College of Medicine, Washington, DC, 20059, USA; 5Molecular Neuropsychiatry Research Branch, NIH National Institute on Drug Abuse, Bethesda, MD, 20892, USA; 6Department of Microbiology, Immunology and Tropical Medicine, George Washington University, School of Medicine, Washington, DC, USA; 7Division of Addiction Research & Education, Center for Sports, Exercise, and Mental Health, Western University of Health Sciences, Pomona, CA, 91766, USA; 8Western University Health Sciences School of Medicine, Pomona, CA, USA; 9Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, 63110, USA; 10Division of Reward Deficiency Clinics, TranspliceGen Therapeutics, Inc, Austin, TX, USA; 11Department of Pharmaceutical Sciences, Texas Southern University College of Pharmacy, Houston, TX, USA; 12Division of Clinical Neurology, The Kenneth Blum Institute of Neurogenetics & Behavior, LLC, Austin, TX, USA; 13Alcohol Addiction Program, Latium Region Referral Center, Sapienza University of Rome, Roma, 00185, Italy; 14Behavioral Neuropharmacology and Neuroimaging Laboratory on Addictions, Clinical Research Institute on Addictions, Department of Pharmacology and Toxicology, Jacobs School of Medicine and Biosciences, State University of New York at Buffalo, Buffalo, NY, 14203, USA; 15Department of Psychology, State University of New York at Buffalo, Buffalo, NY, 14203, USA; 16Department of Psychology, Curry College, Milton, MA, USA; 17Karma Doctors & Karma TMS, and Suder Foundation, Palm Springs, CA, USA; 18Department of Medicine, University of California, Riverside School of Medicine, Riverside, CA, USA; 19Department of Human Development, California State University at Long Beach, Long Beach, CA, USA; 20Division of Personalized Medicine, Cross-Cultural Research and Educational Institute, San Clemente, CA, USA; 21Awareness Integration Institute, San Clemente, CA, USA; 22Department of Health Science, California State University at Long Beach, Long Beach, CA, USA; 23Department of Psychiatry, Mt. Sinai School of Medicine, New York, NY, USA; 24Centre for Genomics and Applied Gene Technology, Institute of Integrative Omics and Applied Biotechnology (IIOAB), Purba Medinipur, WB, 721172, India; 25Departamento de Genética, Ecologia e Evolução, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil; 26Department of Radiation Medicine and Applied Sciences, UC San Diego, La Jolla, CA, 92093-0819, USA; 27Department of Radiation Oncology, University of California San Diego, La Jolla, CA, USA; 28Department of Psychiatry, University of Vermont School of Medicine, Burlington, VA, USA; 29Institute of Psychology, ELTE Eötvös Loránd University, Budapest, Hungary
Correspondence: Igor Elman; Kenneth Blum, Email [email protected]; [email protected]
Abstract: Loneliness, an established risk factor for both, mental and physical morbidity, is a mounting public health concern. However, the neurobiological mechanisms underlying loneliness-related morbidity are not yet well defined. Here we examined the role of genes and associated DNA risk polymorphic variants that are implicated in loneliness via genetic and epigenetic mechanisms and may thus point to specific therapeutic targets. Searches were conducted on PubMed, Medline, and EMBASE databases using specific Medical Subject Headings terms such as loneliness and genes, neuro- and epigenetics, addiction, affective disorders, alcohol, anti-reward, anxiety, depression, dopamine, cancer, cardiovascular, cognitive, hypodopaminergia, medical, motivation, (neuro)psychopathology, social isolation, and reward deficiency. The narrative literature review yielded recursive collections of scientific and clinical evidence, which were subsequently condensed and summarized in the following key areas: (1) Genetic Antecedents: Exploration of multiple genes mediating reward, stress, immunity and other important vital functions; (2) Genes and Mental Health: Examination of genes linked to personality traits and mental illnesses providing insights into the intricate network of interaction converging on the experience of loneliness; (3) Epigenetic Effects: Inquiry into instances of loneliness and social isolation that are driven by epigenetic methylations associated with negative childhood experiences; and (4) Neural Correlates: Analysis of loneliness-related affective states and cognitions with a focus on hypodopaminergic reward deficiency arising in the context of early life stress, eg, maternal separation, underscoring the importance of parental support early in life. Identification of the individual contributions by various (epi)genetic factors presents opportunities for the creation of innovative preventive, diagnostic, and therapeutic approaches for individuals who cope with persistent feelings of loneliness. The clinical facets and therapeutic prospects associated with the current understanding of loneliness, are discussed emphasizing the relevance of genes and DNA risk polymorphic variants in the context of loneliness-related morbidity.
Keywords: addiction, affective, alcohol, anti-reward, anxiety, depression, dopamine, cancer, genes, cardiovascular, cognitive, medical, motivation, social isolation, reward deficiency
Oh, loneliness, how harsh your temper is
Your shining compasses of iron
Is what you link your freezing circle with
Not heeding useless promises or vows.
Bella Akhmadulina
As it was, we all acted alone, we were caught alone, and every one of us will have to die alone. But that doesn’t mean that we are alone.
Hans Fallada
Introduction
Emotional and physical consequences of present or past collective traumatic events, like the COVID-19 pandemic, extend beyond those directly impacted by the virus to millions of individuals who experience prolonged loneliness1 because of self- and/or government-imposed social isolation.2 By and large, people undergoing such periods resort to constructive coping strategies, eg, structured daily routine, regular contacts with family and friends, physical exercise, and productive vocational engagements.3 However, a sizable population segment succumbs to anxious and depressive affective states,4 cognitive distortions,5 memory problems,6 an enhanced propensity for psychosis7 and suicide.8 Dependence on social attachments may be juxtaposed to addictive disorders.9,10 So, longing for meaningful social interactions may be akin to craving and hunger11 and, if not satisfied, could lead to the worsening of behavioral and chemical addictions, overeating, obesity, and promiscuity.12–14 Hence, it appears that loneliness encompasses a comprehensive set of systemic changes that coincide with alterations in immunometabolism patterns15 and contribute to the development of cardiovascular, glucoregulatory and oncological morbidity.16–19
Given such a profound impact on emotional and physical wellbeing,20 it is reasonable to assume a neurobiological basis for loneliness.21 In fact, findings from neuroscience research substantiate the notion that loneliness should be considered as part of a broader spectrum involving reward deficiency and anti-reward.22,23 This spectrum encompasses personality traits and mental disorders characterized by a state of reduced dopamine activity in the reward circuitry, resulting in diminished motivation, inability to experience pleasure23–26 in conjunction with heightened stress that is not effectively alleviated by rewards.27 The dopaminergic neurons in the dorsal raphe nucleus are implicated in the negative affects arising in the context of loneliness,28,29 along with the exhilaration experienced with the termination of solitude28 and reestablishment of the normative reward and motivational function.30 Thus, dopaminergic activity in the limbic-raphe-corticostriatal circuits is associated with motivational longings, ie, drugs, food31 and loved ones.32–34
However, loneliness is not a unitary entity defined by straightforward behavioral and physiological mechanisms. Quite the reverse, it is a complex biopsychosocial phenomenon comprising genetic factors that play an important role in the vulnerability, course, and outcomes of various loneliness-associated conditions. The present review will elucidate loneliness’ neuro- and epigenetic underpinnings. To that end, an English-language literature search on loneliness-related genes, and related topics was undertaken using PubMed, Medline, and EMBASE from launch until March 2023. The selected Medical Subject Headings encompassed addiction, affective, alcohol, anti-reward, anxiety, depression, dopamine, cancer, genes, cardiovascular, cognitive, medical, motivation, social isolation, and reward deficiency. Data on the neuroscience of addiction and the neurobiology/neurochemistry of social attachments were also drawn from comprehensive reviews of these topics.9,11,21,27,35–40 To expand the search, we also conducted manual searches within the reference lists of the selected papers and utilized PubMed’s Similar Articles function to identify related articles. The resulting paper consists of ten sections, including this Introduction. The second section offers clinical and epidemiological perspectives on loneliness. The subsequent six sections delve into the neuropharmacology of reward, as well as the neurogenetics and epigenetics associated with loneliness. The final ninth and tenth sections present limitations, therapeutic implications with summary and conclusions.
Loneliness: Epidemiological and Clinical Context
Loneliness is an intensifying public health problem in the US41 and throughout the world.42,43 While most of surveyed people across all ages consistently report alienation, the risk of loneliness seems to increase with age.41 According to systematic reviews,44 27.6% (95% CI: 22.6–33.0%) and 31.3% (95% CI: 21.0–42.7%) of 65–75- and over 75-year-old people surveyed, respectively, reported loneliness.44 A study of adolescents,45 uncovered persistent loneliness and paucity of meaningful friendships in 18.1% (95% CI: 16.4–20.0%) with girls, in comparison to boys, displaying greater propensity for feeling lonely (14.6% vs 9.2%), while boys were more concerned about not having close friends (8.7% vs 7.2%). These data may partially inform the Global School-based Student Health Surveys46 implicating loneliness as a significant risk factor for suicidal behavior among adolescents; similar trends have been observed in adults.8 The ongoing recognition of loneliness’ societal costs has naturally spurred the development of therapeutic interventions targeting social skills, social support, social contacts, and social cognition.20 However, given the ongoing rise in loneliness prevalence,47 there is a pressing need for fresh perspectives that can contribute to enhanced prevention, identification, and management strategies. Inquiry into genetic mechanisms may have heuristic value in terms of mapping pathophysiologic pathways driven by vulnerability genes underlying given traits as well as the environmental impacts reflected in the epigenetic alterations.
Numerous adoption and twin studies involving both children and adults have indicated48–51 that the perception of social isolation has a genetic basis while clinical research has uncovered a significant correlation between social isolation and loneliness,52 suggesting that these concepts share common elements (around 15% of variance) and are both linked to depression. When entered into a regression analysis together, loneliness had a stronger association with depression than did social isolation. Nevertheless, comparable degrees of genetic impact were noted for both social isolation (40%) and loneliness (38%), though depressive symptoms displayed a lesser genetic influence (29%). This indicates that the unexplained variability can be attributed to factors in the non-shared environment. The investigation unveiled noteworthy genetic correlations, with an r-value of 0.65 between isolation and loneliness, and an r-value of 0.63 between loneliness and depression implying a substantial genetic influence on the simultaneous occurrence of these characteristics. Thus, individuals who experience loneliness may be prone to depression due to shared genetic factors influencing both conditions.
Loneliness Construct
In ancient times, individuals deprived of social connections often faced a grim fate in terms of demise from violence and starvation. Such harsh reality laid the evolutionary foundation for the preponderance of extended families, tribes, states, nations, and religions across various cultures and locations. And so, the innate drive to seek social bonding and the subsequent rewards and stresses associated with its attainment and loss are deeply ingrained. It is worth noting that addictive drugs target the same neural mechanisms that are naturally designated to facilitate social attachments,9 leading to the proposition that social bonding can be seen as the “primary form of addiction”.9,53,54 In fact, the initial euphoria experienced upon forming a romantic attachment shares similarities with the euphoria induced by drug use. Similarly, phenomena such as tolerance, withdrawal, and craving, as well as giving up important activities, are observed in the context of broken romantic ties,54,55 along with psychological distress.56 For example, in the War and Peace novel, the protagonist describes feeling “an electric shock” and experiencing “some fearful pain” that seemed to pierce her heart upon learning about the loss of her brother. The heroine of yet another masterpiece by the same author, Ana Karenina, resorts to the use of morphine to cope with her reactive depression following the rejection by a beloved person.
Even though several studies identified genetic associations between loneliness and genetic polymorphisms, these findings may not be specific enough since various psychological traits may have common neurobiological and neurochemical correlates and vice versa. Hence, a recent investigation57 explored the overlap of genome-wide association studies (GWAS) within the major Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) disorders (such as anorexia nervosa, anxiety disorders, attention deficit hyperactivity disorder, autism, bipolar disorder, major depressive disorder, and schizophrenia) across various brain morphometric indices, cognitive traits (educational attainment and general cognitive function), and personality features (agreeableness, aggressive behavior, conscientiousness, depressive symptoms, extraversion, loneliness, neuroticism, openness to experience, and subjective well-being). The researchers identified 48 genes containing distinct markers associated with multiple traits (gene-level pleiotropy), along with nine genes possessing different markers that independently correlated with specific traits (allelic heterogeneity). This warrants the question of whether the construct of loneliness per se can be considered a realistic endophenotype or is just a symptom of the reward deficiency anti-reward syndrome impacted by the environment (epigenetics).
Indeed, when it comes to elucidating the genetic mechanisms underlying loneliness, the initial challenge lies in determining a precise definition and operationalization for this widely discussed concept, which has been defined in numerous ways throughout the literature. Loneliness can be described as a distressing state that arises when the need for human connection is not sufficiently fulfilled or when an individual’s social network does not align with the preferences, whether in terms of quantity or quality.15 Various perspectives, including philosophical, emotional, poetic, and social,58–60 contribute to the definition of loneliness. Here we propose a psychopathological entity entailing an allostatic deficit in the reward and motivation systems26 resulting from perceived or actual alienation or social isolation61 and manifested in emotional numbing and pain, stress (ie, anti-reward), longing for social interactions, compensatory consumption of substances, or engagement in activities with high hedonic impact (eg, palatable food, gambling, or sexual promiscuity.11,24 While there are multiple valid approaches to operationalizing “loneliness”, the method described here offers several notable benefits. Firstly, it provides a clear definition based on pathophysiological criteria involving a combination of reward deficiency and anti-reward syndromes.11,27,39 Secondly, it is built upon a solid foundation of clinical research.21,22 Lastly, its connection to loss of social ties and reward-seeking behaviors that yield negative outcomes has been extensively supported by evidence.62–64
Neuropharmacological Underpinnings of Reward
The fundamental Brain Reward Cascade (Figure 1) comprises a sequential circuit connecting the ventral tegmental area, nucleus accumbens, and ventral pallidum via the medial forebrain bundle.65 A shared characteristic observed in addictive drugs is their propensity to be actively self-administered by laboratory animals. Addictive drugs also enhance the activity of the brain’s dopaminergic reward circuitry, resulting in the sought-after “high” experienced by drug users.35
 |
Figure 1 Displays the interplay of multiple neurotransmitter pathways involved in the Brain Reward Cascade (BRC). The process commences with an environmental stimulus triggering the release of serotonin in the hypothalamus, which, in turn, activates the release of opioid peptides from opioid peptide neurons, mediated by 5HT-2a receptors (indicated by a green equal sign). Subsequently, the opioid peptides have two distinct effects through different opioid receptors. One effect involves inhibition (indicated by a red hash sign) via the mu-opioid receptor, which projects to GABAA neurons in the Substantia Nigra. The other effect involves stimulation (indicated by a green equal sign) of cannabinoid neurons, facilitated by delta receptors linked to Beta-Endorphin. The activated cannabinoid neurons inhibit GABAA neurons in the Substantia Nigra. Additionally, cannabinoids, particularly 2-archidonylglycerol, can indirectly disinhibit (indicated by a red hash sign) GABAA neurons by activating G1/0 coupled to CB1 receptors in the Substantia Nigra. In the Dorsal Raphe Nuclei (DRN), glutamate neurons indirectly disinhibit GABAA neurons in the Substantia Nigra through the activation of GLU M3 receptors (indicated by a red hash sign). When GABAA neurons are activated, they strongly inhibit (indicated by red hash signs) the glutaminergic drive from the Ventral Tegmental Area (VTA) through GABAB3 neurons. Stimulation of acetylcholine (ACH) neurons in the Nucleus Accumbens activates both muscarinic (indicated by a red hash sign) and nicotinic (indicated by a green hash sign) receptors. Finally, glutamate neurons in the VTA project to dopamine neurons via NMDA receptors (indicated by a green equal sign), leading to the predominant release of dopamine at the Nucleus Accumbens, depicted as a bullseye, representing a euphoric or motivational (“wanting”) response. The result is that low dopamine release leads to feelings of unhappiness, while maintaining a balanced dopamine homeostatic tonic set point is crucial for overall well-being and happiness. Notes: Reprinted from Blum K, McLaughlin T, Bowirrat A, Modestino EJ, Baron D, Gomez LL, Ceccanti M, Braverman ER, Thanos PK, Cadet JL, Elman I, Badgaiyan RD, Jalali R, Green R, Simpatico TA, Gupta A, Gold MS. Reward Deficiency Syndrome (RDS) Surprisingly Is Evolutionary and Found Everywhere: Is It “Blowin’ in the Wind”? J Pers Med. 2022 Feb 21;12(2):321. Creative Commons.25 |
Although originally assumed to only determine the hedonic set point as a key component of hedonostasis,27 these circuits are now postulated to be functionally far more complex, also encoding attention, expectancy of outcomes, discrepancy between the actual and expected outcome (prediction error), contextual processing, incentive motivation, and even the specific construct of loneliness.11,24,27,66,67 It is now generally accepted that allostatic dysregulation within these circuits leads to combined reward deficiency and anti-reward syndromes (see below), including substance use disorders and other types of addiction.11,27,39 Drug self-administration is regulated by the nucleus accumbens’ dopamine concentration, which is normatively kept within a physiologically defined hedonostatic range.27 Chronic use of certain addictive drugs, such as opioids, leads to the development of tolerance to their euphoric effects. As a result, post-use dysphoria develops due to allostatic processes in the reward circuit’s hedonic tone. Consequently, addicted individuals use drugs not to experience “high”, but merely to feel normal or “get straight”, depending on the extent of impairment.11,27,68 There are important genetic variations in vulnerability to drug addiction, yet environmental factors such as stress and social defeat also alter brain reward mechanisms to impart epigenetic vulnerability to addiction11,24,25,27,39,69–85 in the context of its biopsychosocial nature.86
Addiction, exemplified by combined reward deficiency and anti-reward syndrome, is marked by a dysfunctional brain reward circuitry. This dysfunction involves reduced dopaminergic activity and abnormalities in noradrenergic, serotonergic, opioidergic, endocannabinoid, GABAergic, and glutamatergic neurotransmission.39 The anti-reward component is manifested by outpouring of stress-related hormones38 within the extended amygdala structures involved in stress, anxiety, and fear87 and through the habenula, which further inhibits dopamine activity thus aggravating reward deficiency.88–91 From a neuroanatomical perspective, the progression from occasional recreational substance use to impulsive and eventually uncontrollable consumption is implicated in a neuroanatomical shift from the ventral striatum (nucleus accumbens) to the dorsal striatum, which underlies compulsive drug-seeking behavior and craving.92,93
Craving is commonly elicited by exposure to addictive drugs, stress, and environmental cues associated with drug-taking behavior,35 activating the shared circuitry involving frontostriatal glutamatergic pathways,36 the nucleus accumbens and related dopaminergic circuitry,94–96 as well as the basolateral nucleus of the amygdala, the hippocampus, the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and the noradrenergic nuclei in the brain stem, which are associated with norepinephrine and corticotropin-releasing factor (CRF) release during stress-induced activation of anti-reward pathways.97–100 Loneliness may be relevant to all these triggers. When repeatedly paired with drugs, withdrawal-related stress, and environmental cues, loneliness can become a conditioned stimulus that independently triggers various forms of craving. Therefore, loneliness is included in the HALT acronym, which represents the major relapse risks: being hungry, angry, lonely, and tired.101
While the HALT acronym encompasses common stressors, loneliness is also strongly connected to traumatic events and subsequent post-traumatic stress disorder (PTSD).102 To explore the developmental links between victimization and loneliness, data were extracted from the Environmental Risk (E-Risk) Longitudinal Twin Study, which involves a birth cohort of 2232 children born in England and Wales.103 The findings revealed a unique association between childhood bullying victimization and loneliness, independent of concurrent psychopathology, social isolation, and genetic risk. Moreover, childhood bullying victimization predicted loneliness in young adulthood, even after the bullying had ceased. Analyses within twin pairs suggested that genetic factors played a role in explaining this longitudinal association. During adolescence, various forms of victimization were correlated with loneliness, maltreatment, social neglect, and cyber victimization remaining significant even after controlling for genetic influences.103 These results indicate that vulnerability to loneliness among victimized young individuals may be influenced by genetic factors.
Neurogenetics of Loneliness
Multiple research studies indicate that there is an overlap between genes associated with slight variations in loneliness and genes involved in deviant behaviors, including those related to substance use.104 Understanding why some lonely individuals become addicted following exposure to addictive drugs and/or behaviors while others do not remains a major challenge in devising science-driven interventions in addiction medicine and mental health.90 Imaging studies generally align with preclinical discoveries, suggesting that brain circuits modulated by dopamine and implicated in reward, memory, executive function, and motivation may explain certain aspects of the individual differences observed in the shared tendencies for addiction and loneliness.105–107 Genetic polymorphism in the dopamine and opioid systems seems to be an important intermediator.108 Furthermore, in both drug addiction and obesity, the enhanced salience of the respective reinforcer (drugs or food) is modulated by genetic factors90,109 or personality features,110 underscoring the importance of multidimensional approaches in understanding and treating addictive disorders in the context of loneliness.111,112
GWAS studies and candidate gene analysis have supported loneliness’ heritable nature, pointing to genes associated with dopamine, serotonin, and oxytocin systems.113–117 For example, the dopamine D2 receptor candidate gene (DRD2) has been confirmed through GWAS as being linked to schizophrenia and has been found to be associated with major depressive disorder.118–120 Our group has uncovered significant links between schizoid-avoidant behavior and the DRD2 Taq A1 allele, as well as with the dopamine transporter gene (DAT1).121 Furthermore, shared genetic risk factors and specific polymorphisms within the ANKKI/DRD2 gene have been associated with both schizoid and avoidant personality disorders.121,122 Notably, schizoid personality disorder, which involves social avoidance and ambivalence toward social contact, has been subdivided into avoidant personality disorder and schizoid-avoidant behavior, corresponding to the concept of loneliness as described in DSM-5 TR.123 Individuals with schizoid-avoidant personalities or traits have a heightened susceptibility to addictions, with the heritability of perceived isolation estimated at around 50%.124 Additionally, a study has linked polymorphism of the oxytocin receptor gene (OXTR) to the development of loneliness.125
GWAS split-half validation analyses have shown that individuals with autism spectrum disorders, bipolar disorder, major depression, and schizophrenia tend to have lower sociability scores.118 The sociability score was found to be significantly heritable, composed of 18 independent loci and 56 gene-wide significant genes, including ARNTL, DRD2, and ELAVL2. Importantly, the sociability score demonstrated negative genetic correlations with autism spectrum disorders, depression, schizophrenia, loneliness, and social anxiety.118 The strongest association in the single-nucleotide polymorphism (SNP)-based GWAS was observed on chromosome 11p15, encompassing the ARNTL gene, which is a circadian clock gene.126 Another GWAS reported 19 independent genetic associations in 16 loci, while our research group identified 58 genome-wide significant genes associated with loneliness across subjects from five Western countries.127 The genetic association signals were enriched in genes expressed in specific brain regions, particularly cortical and cerebellar regions, and the genetic risk for loneliness was associated with various health-related traits. Some of the top candidate regions and genes included TCF4, PHF2, AC091969.1, ERBB4, RP11-6M13.1, BPTF, EPB41l2, STAUI, TAOK3, RP11–259G18.1, CELF1, and RERE.109
According to the five annual waves longitudinal study utilizing the Latent Growth Curve Modeling, the 5-HTTLPR genotype is associated with the development of loneliness.117 Specifically, individuals carrying the short allele exhibited steady levels of loneliness over time, whereas those with the long-long allele genotype reported diminishing loneliness. Interactions were observed between maternal support and the 5-HTTLPR genotype, indicating that adolescents perceiving low levels of maternal support and carrying a short allele were at a heightened risk of developing loneliness. Additionally, recent studies have demonstrated a significant association between a rs1044396 variation in the CHRNA4 gene (encoding the neuronal nicotinic acetylcholine receptor alpha-4 subunit) with loneliness and depression.128 Notably, the same CHRNA4 gene variation affects acetylcholine regulation of dopamine release in the nucleus accumbens (Figure 1). Further investigations have identified two key regions, 1p22.2-BARHL2 and 3p21.31-CAMKV, associated not only with social isolation but also with pleiotropy across various complex traits. For instance, there is an association between the polygenic score for loneliness in the oxytocin signaling pathway (154 genes) and apolipoprotein A1, a major protein component of HDL.61,129 Another study130 revealed that the GG genotype for the OXTR gene is linked to the development of loneliness in adolescence, and this association is moderated by both sex and genotype for a dopamine-related gene (DRD2 A1 allele). Moreover, adults who experience loneliness also excessively express pro-inflammatory genes that are responsive to mindfulness-based stress reduction programs.130
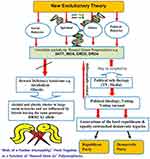
The relationship between the brain-derived neurotrophic factor (BDNF) gene polymorphism and loneliness vary in accordance with gender. The presence of the Met allele in girls and the Val Val allele in boys is linked to distinct alterations in dopamine release from the nucleus accumbens, thereby influencing loneliness experience.113 Latent Growth Curve Modeling has demonstrated interactions between parental support and DRD2 genotype, indicating that adolescents with the A2A2 allele (normal) who perceive limited support from their parents exhibit the highest baseline levels of loneliness. Moreover, adolescent carriers of the DRD2 A1 allele corresponding to reduced DRD2 receptors’ expression, are insensitive to the rewarding effect of parental support, potentially due to hypofunctional reward circuitry. The Methylenetetrahydrofolate reductase (MTHFR) C677T is another relevant polymorphism, which is linked to loneliness and reward deficiency, independent of age, education, cognitive function, and mood.131,132 Stress plays an important role in addiction98,133,134 and is also a part of loneliness experience. In older adults, the impact of stress inherent in rare contact with children and low levels of social support on loneliness is influenced by two SNPs, namely rs1876831 and rs242938, located within the corticotropin-releasing hormone receptor 1 (CRHR1) gene.135 Those homozygous for the C allele of rs1876831, in comparison to the carries CT/TT allele are lonelier in the context of infrequent encounters with the children and their consequently diminished support. See Figure 2 for summary.
 |
Figure 2 A schematic showing a proposed DNA antecedent map to the loneliness construct. |
Human Social Networks and Genetics
In humans, heritable personality traits have been associated with dominance,136 and superior status interacts with various neurotransmitter systems, including dopamine D2/D3 receptor binding. Higher binding of these receptors is linked to higher social status,137–139 suggesting the presence of biological mechanisms that process information related to social rank and hierarchies.140 Brain responses to perceiving superiority or inferiority appear to be distinct, both when encountering an individual of a specific status and when faced with outcomes that can impact one’s position in the hierarchy.140 The perception of a superior individual triggers the engagement of perceptual-attentional, saliency, and cognitive systems, particularly the dorsolateral prefrontal cortex. Additionally, the hierarchical social consequences of performance are neurally separate and hold comparable significance to monetary rewards, providing a neural foundation for the strong motivational value of status. This research highlights the significance of hierarchical status in social networks, linking status to the reward circuitry, a crucial site for emotions and well-being.11,27
To some extent, an individual’s happiness is influenced by the people they are connected to within their social network.141 Clusters of happy and unhappy individuals can be observed within the network, and the influence of happiness can extend up to three degrees of separation, reaching the friends of one’s friends’ friends. Individuals who are surrounded by happy people and those who hold central positions in the network are likely to be happy themselves. Having a nearby happy friend, a happy spouse, a happy sibling, or just a happy next-door neighbor increases the proband’s happiness probability by 25% (95% CI: 1% to 57%), 8% (CI: 0.2% to 16%), 14% (CI: 1% to 28%), and 34% (CI: 7% to 70%), respectively. The National Longitudinal Study of Adolescent Health and the Framingham Heart Study revealed the presence of the DRD2 A1 allele’s positive correlation (homophily) and CYP2A6 (SNP rs1801272) allele’s negative correlation (heterophily) in the context of friendship networks.142 These unique findings indicate that genetic homophily and heterophily occur at the allelic level, suggesting that association tests should consider the genes of friends and evolutionary theories should account for the fact that humans may be metagenomic in relation to those around them. This supports the notion that like-minded individuals tend to associate with each other143 and even indicates a potential impact on political affiliation144 (Figure 3). Relevant to the topic of happiness genes and social networks, our original study associated the DRD2 A1 allele with severe alcoholism.145 Furthermore, the DRD2 A1 allele has been linked to various behaviors related to reward deficiency syndrome.146 There are genetic data beyond SNPs, such as copy number variation studies and genome sequencing studies focused on rare variants (SNVs), which are also relevant.147
Clusters of individuals who engage in drinking or abstaining behavior were found within the network, and these clusters extended up to three degrees of separation.148 These clusters were not solely a result of selective social ties among drinkers but also indicated interpersonal influence. Changes in alcohol consumption of a person’s social network (eg, relatives and friends), not immediate neighbors and coworkers, had a statistically significant impact on that person’s subsequent intake of alcohol. Similarly, clusters of obese individuals were present in the network and also extended up to three degrees of separation.141 These clusters did not solely arise from selective social ties among obese individuals. The likelihood of an individual becoming obese increased by 57% (95% CI, 6 to 123), 40% (95% CI, 21 to 60) or 37% (95% CI, 7 to 73) if a friend, sibling or spouse gained a substantial amount within a certain time period. The same effects were not observed among neighbors in the immediate geographic location. Interestingly, in comparison to the opposite sex, same sex individuals had a relatively stronger influence on each other. These findings align with our own data in a five-generational genotyping for the DRD2 A1 allele, using reward deficiency syndrome as a generalized phenotype, showed that every member of the family carrying the DRD2 A1 allele married a person who also carried the same gene.100,149
Epigenetics and Loneliness
Much has been learned on how physical, chemical, and social environments affect human health, predisposing certain subpopulations to adverse health outcomes, especially the socio-environmentally disadvantaged. Translational data on gene and adverse environment, termed aberrant epigenomic modulation, translates into impaired gene expression via messenger ribonucleic acid dysregulation, reflecting abnormal protein synthesis and hence dysfunctional cellular differentiation and maturation. The epigenetic influence on gene expression observed in most literature includes the physical, chemical, physicochemical, and, recently, social environment.150
Loneliness is a multifaceted concept influenced by various genetic and environmental factors.151 Studies focusing on candidate genes and gene expression have identified genes associated with neurotransmitters115 and the immune system,152,153 which are likely to be related to loneliness. These findings align with the evolutionary theory of loneliness (ETL),104 which suggests that loneliness is an inherited adaptation that signals a threat to social connections and motivates individuals to reconnect with others. The exploration of the genetic basis of loneliness has been greatly influenced by the fundamental principles of the ETL, prompting researchers to delve deeper into the genetic roots of this emotion. The ensuing research on gene-environment interactions have discovered that social-environmental factors, like low social support, can significantly affect feelings of loneliness, especially in individuals who have sensitive variants of certain candidate genes.104
Loneliness or social isolation elevate the risk of stress-related major depressive disorder and PTSD that are linked via shared neuroinflammatory etiology.152,153 In a mice model of PTSD/suicide-like behavior four weeks of social isolation cause a reduction in peroxisome proliferator-activated receptor (PPAR) ligand-activated nuclear receptor and transcription factor that in addition to enhancing neurosteroid biosynthesis and exerting anti-inflammatory effect improves anxiety and depressive symptomatology.154 Such reduction is accompanied by enhanced methylation of cytosines in CpG-rich regions of the PPAR-α gene, evident in impaired neurosteroid biosynthesis and in histone deacetylases (HDAC)1 and methyl-cytosine binding protein (MeCP)2 increases along with decreased expression of ten-eleven translocator (TET)2, favoring a state of hypermethylation. These changes correlated with elevated activation of toll-like receptor 4 (TLR-4) and pro-inflammatory markers, such as TNF-α, in the hippocampus, mediated by NF-κB signaling (known to be implicated in loneliness). The induction of social isolation stress targeted epigenetic modifications associated with PPAR-α downregulation, suggesting a potential therapeutic approach to counteract the detrimental effects of loneliness.154
A mice study explored how social isolation affects the epigenome. The researchers observed global DNA methylation changes with an increase in DNA methyltransferase activity. They also found di- and trimethylation of global histone H3 lysine 4 (H3K4), alongside heightened activities of histone methyltransferases, histone acetyltransferases, and histone deacetylases resulting in an overall increase of histone H3 lysine 9 (H3K9) acetylation.155 Furthermore, this study also revealed gene-specific impacts caused by social isolation. Genes such as Hdac1, Hdac3, and the serotonin transporter Slc6a4 displayed abnormal DNA methylation patterns, with decreased methylation in the CpG islands. This led to the downregulation of the Slc6a4 gene and a general increase in DNA methylation.155 These findings provide valuable insights into how social isolation can influence the epigenetic mechanisms underlying specific behavioral effects in mice. A recent human study likewise revealed significant association between loneliness and methylation in stress related BDNF and the glucocorticoid receptor gene.156
Early life stress (ELS) triggers complex neurochemical processes that influence interconnected neurophysiopathology, including behaviors associated with addiction and a sense of loneliness.157,158 Rats exposed to maternal separation stress (MS), a model of ELS, displayed a threefold increase in ethanol consumption over a three-week period, along with a significant reduction in dopaminergic ventral tegmental area neurons positive for tyrosine hydroxylase.158 These rats exhibited depressive-like symptoms and anhedonia, which are clinically associated with the construct of loneliness.159 Furthermore, MS rats displayed twofold higher immobility time in the forced swim test and reduced sucrose drinking compared to control rats. The motivation to seek social contact can be influenced by both positive and negative emotional states, as social interaction can be rewarding while social isolation can be aversive.28
Therapeutic Considerations
Genes inherited at birth from parents determine a hardwired portion of the perception and responsivity to loneliness as well as the potential to develop loneliness-induced neuropsychiatric and/or medical morbidity. Studies in monozygotic twins albeit showed high correlation yet only about 35% of the variance in loneliness may be attributable to the genetic substrate160,161 c.f. 90% heritability for height,162 50% heritability for body mass index163 and 50% heritability for happiness.164 Hence symptoms and sequelae of loneliness may be targeted in accordance with individualized formulations, based on genetic risk assessment battery akin to the one designed by us for the assessment of the propensity for addiction165 in conjunction with clinical assessments, functional neuroimaging, psychotherapy, and psychopharmacology.
It is reasonable to expect that variability in the magnitude of (epi)genetic, psychological, social, and environmental contributors to overall symptomatology could define the nature and type of appropriate intervention alleviating symptoms of loneliness and improving general processing of information by the brain. To that end, reward abnormalities can be addressed by the Positive Psychology methods166 including the Positive Affect Treatment167 and the Positive Affect Stimulation and Sustainment techniques.168
Diminished dopaminergic neurotransmission in the mesolimbic pathway underlying reward deficiency,24,165 presents opportunities for therapeutic interventions including antidepressants and cognitive enhancers like bupropion,169 solriamfetol,170 or modafinil.171 Additional methods for restoring dopaminergic hedonstasis27,39 include anticonvulsants, atypical antipsychotics,11,89,172 nootropics,173 and N-methyl-D-aspartate glutamate receptors’ antagonists.174 A dietary supplement, acetyl-L-carnitine175 with neurotrophic, neuroprotective, and antidepressant properties likewise presents heuristic value in managing reward deficiency.176
Adrenergic agents as α2 adrenoceptor agonists or α1 adrenoceptor antagonists (eg, clonidine, guanfacine, or prazosin) is a rational therapeutic approach to anti-reward symptomatology. Such agents are already used successfully for other conditions that are frequently associated with feelings of loneliness102,177 eg, substance use disorders178,179 and PTSD.180 Despite the complexities surrounding reward deficiency and anti-reward mechanisms, exploring these therapeutic options provides hope for better understanding and potentially alleviating loneliness and its related effects.
Limitations
Limitations that should be considered in interpreting our data refer to causality, scope, generalizability, the nature of the review and ongoing research. Establishing causality is challenging given the predominance of observational studies, and the cross-sectional design limiting the ability to firmly conclude whether loneliness directly causes neurobiological alterations or the other way around. Further research with prospective cohorts and comprehensive measures of loneliness is warranted to better understand the complex interplay between gene, loneliness, and neurobiological mechanisms. Also, the cited studies were conducted in specific populations and may not capture the full range of genetic and epigenetic factors influencing loneliness in different cultural, social, or age groups pointing to limited external validity.
Being a narrative review, the emphasis was placed on integrating key concepts and insights derived from existing knowledge and expertise rather than rigorous adherence to stringent selection criteria and quality assessment for the sources typical of systematic reviews. Systemic reviews may be vulnerable to publication bias as studies with significant findings are more likely to be published, while those with non-significant or negative results might be omitted, potentially skewing the overall picture. Given that discussed topics are subjects of ongoing research including the heritability of loneliness and the findings in this paper are not exhaustive, combining both narrative and systematic reviews that are representing the depth of experts’ insight with the breadth of extant evidence is an important prerequisite for fostering new discoveries and evolving insights that may reveal additional dimensions or nuances that are not covered here.
Concluding Remarks
Key findings of this exploration of loneliness, a well-documented risk factor for both mental and physical morbidity, can be abridged within the domains of genetic antecedents, genes and mental health, epigenetic influences, and neural correlates. Regarding genetic antecedents, multiple genes appear to be involved in the interrelated processes of reward, stress, immunity, and loneliness as they are modulated by environmental factors. From the mental health perspective, genetic associations with personality traits and underlying neuropsychopathology provide insights into the intricate network of interactions converging on the experience of loneliness, enriching our understanding of its multifaceted nature. Epigenetically, loneliness and social isolation are significantly impacted by DNA methylations arising in the context of negative childhood experiences, underscoring the pervasive effects of early life adversity on loneliness-related outcomes. On the neural level being critical for the survival, loneliness system is embedded within the overlapping reward and stress circuitry responsible for continued existence of individuals and species via pursuit of food, water, sex, and social affiliations9,11,89 while loneliness-related affective states and cognitions may be driven by hypodopaminergic reward deficiency,23–25 particularly in the context of early life stress, such as maternal separation.
About 55 years after its release, the “All You Need Is Love” hit by the Beatles remains an anthem for peace-loving people throughout the world. This is part due to its epitomizing the universal human longing for connectedness and acceptance or in the words of a Poet (Robert Burns, 1794) “I could range the world around, For the sake o’ Somebody”. Even though various neuro(epi)genetic mechanisms have been put forward to explain yearning for social attachments, they do not seem to fully explain its complexity. Once it became evident that genes associated with reward deficiency and anti-reward neuroadaptations are involved in loneliness, a subsequent question arises: Are some individuals, even in the absence of actual loneliness, predisposed to serious health problems due to a neuropathological vulnerability inherent in their genetic makeup, specifically in terms of social anhedonia?181 This concept is supported by neuroimaging studies that implicate a reward deficiency state characterized by depressed dopaminergic activity in the striatum, which corresponds to feeling “several drinks behind” the rest of the world.182 This state is a risk factor for the development of substance use disorders,183 with consequently reduced social engagement leading to loneliness.184
And so, people afflicted with social anhedonia are vulnerable to experiencing loneliness due hereditary and/or acquired neuropsychopathological alterations inherent in their genome. These alterations may be worsened by addictive substances consumption accompanied by excessive stress exposure. No consensus has yet been established on optimal therapeutic stratagems for social anhedonia and other conditions within the combined reward deficiency and anti-reward spectrum perhaps because further research is warranted on the specific pathways that lead to social anhedonia or loneliness sequelae, or whether a target psychotherapeutic or psychopharmacologic approach might be suitable to address the underlying cause. In this review we identified a potential system involved in reward and stress, which may be aimed at in clinical trials and in mechanistic research.
If the findings of clinical studies confirm our insights, they could have significant implications for the prophylactic measures against loneliness. Identifying neurogenetic vulnerability factors for loneliness may enable primary prevention in terms of screening and genetic counseling for individuals at risk that can help making informed decisions about emotional well-being and reproductive choices. High loneliness vulnerability due to genetically determined impairments in reward and stress function could prompt guidance to avoid loneliness, manage stress, and steer clear of addictive behaviors. To that end, healthcare providers may offer targeted interventions such as social support programs, cognitive-behavioral therapies at an early age as well as customized interventions in the form of behavioral and pharmacological treatments addressing the specific genetic and epigenetic factors contributing to feelings of loneliness. Detection of the epigenetic factors related to negative childhood experiences and their impact on loneliness can guide the development of therapeutic strategies aimed at addressing early life trauma and its long-term consequences on social and emotional well-being. Furthermore, patients could be targeted for early interventions, even in the presence of mild loneliness-related issues, as a secondary preventive approach. Governments and healthcare organizations can use this knowledge on a broader scale to design and implement public health initiatives to combat loneliness and its associated health risks, particularly in vulnerable populations. Such innovative preventive, diagnostic, and therapeutic approaches hold promise in addressing the growing public health concern of loneliness and its associated mental and physical morbidity.
In view of the high prevalence of loneliness among psychiatric and medical patients, we anticipate that understanding the role of genes and associated DNA risk polymorphic variants impacted by the environment through epigenetic mechanisms (eg, methylation/acetylation on histones) will provide substantial relief to the at risk- and the afflicted population. Early genetic assessment of the loneliness construct, when available coupled with structured psychometric assessments following required research, could offer valuable insights for generations to come and help provide for better psychological and medical management tailored to unique needs and biopsychosocial characteristics of each individual. This journey is far from complete, and the current stage is but a steppingstone in a broader quest to fully grasp the complex nature of the loneliness construct and its profound implications for health and disease.
Disclosure
Dr Milan Makale reports personal fees from PeakLogic Inc, outside the submitted work; In addition, Dr Milan Makale has a patent “Miniaturized rTMS system” pending. Dr Kenneth Blum reports royalties from and discloses many pending USA and foreign patents on GARS and KB220 variants licensed to Synaptamine. The authors report no other conflicts of interest in this work.
References
1. Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46(3 Suppl):S39–S52. PMID: 14563073. doi:10.1353/pbm.2003.0049
2. Bzdok D, Dunbar RIM. Social isolation and the brain in the pandemic era. Nat Hum Behav. 2022;6(10):1333–1343. PMID: 36258130. doi:10.1038/s41562-022-01453-0
3. Coping with isolation: 25 strategies for optimizing mental health [Internet]. Sbu.edu; 2020 [cited January 26, 2023]. Available from: https://online.sbu.edu/news/coping-with-isolation.
4. Okruszek Ł, Aniszewska-Stańczuk A, Piejka A, Wiśniewska M, Żurek K. Safe but lonely? Loneliness, Anxiety, and depression symptoms and COVID-19. Front Psychol. 2020;11:579181. PMID: 33343454; PMCID: PMC7747668. doi:10.3389/fpsyg.2020.579181
5. Zhou Z, Mao F, Zhang W, Towne SD, Wang P, Fang Y. The association between loneliness and cognitive impairment among older men and women in China: a nationwide longitudinal study. Int J Environ Res Public Health. 2019;16(16):2877. PMID: 31408955; PMCID: PMC6721226. doi:10.3390/ijerph16162877
6. Solé-Padullés C, Macià D, Andersson M, et al. No association between loneliness, episodic memory and hippocampal volume change in young and healthy older adults: a Longitudinal European Multicenter Study. Front Aging Neurosci. 2022;14:795764. PMID: 35283753; PMCID: PMC8905540. doi:10.3389/fnagi.2022.795764
7. Lim MH, Gleeson JFM, Alvarez-Jimenez M, Penn DL. Loneliness in psychosis: a systematic review. Soc Psychiatry Psychiatr Epidemiol. 2018;53(3):221–238. PMID: 29327166. doi:10.1007/s00127-018-1482-5
8. Goldman-Mellor SJ, Caspi A, Harrington H, et al. Suicide attempt in young people: a signal for long-term health care and social needs. JAMA Psychiatry. 2014;71(2):119–127. PMID: 24306041; PMCID: PMC3946312. doi:10.1001/jamapsychiatry.2013.2803
9. Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79(3):351–357. PMID: 12954430. doi:10.1016/s0031-9384(03)00148-3
10. Christie NC. The role of social isolation in opioid addiction. Soc Cogn Affect Neurosci. 2021;16(7):645–656. PMID: 33681992; PMCID: PMC8259283. doi:10.1093/scan/nsab029
11. Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27. PMID: 23827972; PMCID: PMC4827340. doi:10.1016/j.pneurobio.2013.06.003
12. Herchenroeder L, Post SM, Stock ML, Yeung EW. Loneliness and alcohol-related problems among college students who report binge drinking behavior: the moderating role of food and alcohol disturbance. Int J Environ Res Public Health. 2022;19(21):13954. PMID: 36360841; PMCID: PMC9658452. doi:10.3390/ijerph192113954
13. Buechler J. The loneliness epidemic persists: a post-pandemic look at the state of loneliness among U.S. adults [Internet]. Cigna Newsroom; [cited January 26, 2023]. Available from: https://newsroom.cigna.com/loneliness-epidemic-persists-post-pandemic-look.
14. Lion EG, Jambor HM, Corrigan HG, Bradway KP. An experiment in the psychiatric treatment of promiscuous girls [Internet]; 1945:68. Available from: https://psycnet.apa.org/fulltext/1947-01913-000.pdf.
15. Pourriyahi H, Yazdanpanah N, Saghazadeh A, Rezaei N. Loneliness: an immunometabolic syndrome. Int J Environ Res Public Health. 2021;18(22):12162. PMID: 34831917; PMCID: PMC8618012. doi:10.3390/ijerph182212162
16. Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160(8):805–811. PMID: 16894079. doi:10.1001/archpedi.160.8.805
17. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218–227. PMID: 20652462; PMCID: PMC3874845. doi:10.1007/s12160-010-9210-8
18. Rødevand L, Bahrami S, Frei O, et al. Polygenic overlap and shared genetic loci between loneliness, severe mental disorders, and cardiovascular disease risk factors suggest shared molecular mechanisms. Transl Psychiatry. 2021;11(1):3. PMID: 33414458; PMCID: PMC7790035. doi:10.1038/s41398-020-01142-4
19. Kraav SL, Lehto SM, Kauhanen J, Hantunen S, Tolmunen T. Loneliness and social isolation increase cancer incidence in a cohort of Finnish middle-aged men. A longitudinal study. Psychiatry Res. 2021;299:113868. PMID: 33774371. doi:10.1016/j.psychres.2021.113868
20. Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011;15(3):219–266. PMID: 20716644; PMCID: PMC3865701. doi:10.1177/1088868310377394
21. Lam JA, Murray ER, Yu KE, et al. Neurobiology of loneliness: a systematic review. Neuropsychopharmacology. 2021;46(11):1873–1887. PMID: 34230607; PMCID: PMC8258736. doi:10.1038/s41386-021-01058-7
22. Vitale EM, Smith AS. Neurobiology of loneliness, isolation, and loss: integrating human and animal perspectives. Front Behav Neurosci. 2022;16:846315. PMID: 35464141; PMCID: PMC9029604. doi:10.3389/fnbeh.2022.846315
23. Gold MS, Blum K, Febo M, et al. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front Biosci. 2018;10(2):309–325. PMID: 29293435. doi:10.2741/s518
24. Blum K, Bowirrat A, Braverman ER, et al. Reward Deficiency Syndrome (RDS): a cytoarchitectural common neurobiological trait of all addictions. Int J Environ Res Public Health. 2021;18(21):11529. PMID: 34770047; PMCID: PMC8582845. doi:10.3390/ijerph182111529
25. Blum K, McLaughlin T, Bowirrat A, et al. Reward Deficiency Syndrome (RDS) surprisingly is evolutionary and found everywhere: is it “Blowin’ in the Wind”? J Pers Med. 2022;12(2):321. PMID: 35207809; PMCID: PMC8875142. doi:10.3390/jpm12020321
26. Wilkialis L, Rodrigues N, Majeed A, et al. Loneliness-based impaired reward system pathway: theoretical and clinical analysis and application. Psychiatry Res. 2021;298:113800. PMID: 33618235. doi:10.1016/j.psychres.2021.113800
27. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11–36. PMID: 26748087. doi:10.1016/j.neuron.2015.11.027
28. Matthews GA, Nieh EH, Vander Weele CM, et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell. 2016;164(4):617–631. PMID: 26871628; PMCID: PMC4752823. doi:10.1016/j.cell.2015.12.040
29. Pomrenze MB, Cardozo Pinto DF, Neumann PA, et al. Modulation of 5-HT release by dynorphin mediates social deficits during opioid withdrawal. Neuron. 2022;110(24):4125–4143.e6. PMID: 36202097; PMCID: PMC9789200. doi:10.1016/j.neuron.2022.09.024
30. Krach S, Paulus FM, Bodden M, Kircher T. The rewarding nature of social interactions. Front Behav Neurosci. 2010;4:22. PMID: 20577590; PMCID: PMC2889690. doi:10.3389/fnbeh.2010.00022
31. Grigson PS. Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Behav. 2002;76(3):389–395. PMID: 12117575. doi:10.1016/s0031-9384(02)00758-8
32. O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain’s reward center. Neuroimage. 2008;42(2):969–972. PMID: 18559294; PMCID: PMC2553561. doi:10.1016/j.neuroimage.2008.04.256
33. Inagaki TK, Muscatell KA, Moieni M, et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc Cogn Affect Neurosci. 2016;11(7):1096–1101. PMID: 26084531; PMCID: PMC4927031. doi:10.1093/scan/nsv076
34. Dicker-Oren SD, Gelkopf M, Greene T. The dynamic network associations of food craving, restrained eating, hunger and negative emotions. Appetite. 2022;175:106019. PMID: 35500722. doi:10.1016/j.appet.2022.106019
35. Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. PMID: 21508625; PMCID: PMC4549070. doi:10.1159/000324065
36. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. PMID: 16055761. doi:10.1176/appi.ajp.162.8.1403
37. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. PMID: 27475769; PMCID: PMC6135092. doi:10.1016/S2215-0366(16)00104-8
38. Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. PMID: 31919349. doi:10.1038/s41572-019-0137-5
39. Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–297. PMID: 27246519. doi:10.1016/j.neubiorev.2016.05.033
40. Elman I, Borsook D. Threat Response System: parallel Brain Processes in Pain vis-à-vis Fear and Anxiety. Front Psychiatry. 2018;9:29. PMID: 29515465; PMCID: PMC5826179. doi:10.3389/fpsyt.2018.00029
41. Rameer VM. US loneliness statistics 2023: are Americans lonely? [Internet]. Science of People; 2022 [cited January 26, 2023]. Available from: https://www.scienceofpeople.com/loneliness-statistics.
42. Surkalim DL, Luo M, Eres R, et al. The prevalence of loneliness across 113 countries: systematic review and meta-analysis. BMJ. 2022;376:e067068. PMID: 35140066; PMCID: PMC8826180. doi:10.1136/bmj-2021-067068
43. Loneliness among adults worldwide by country 2021 [Internet]. Statista; [cited January 26, 2023]. Available from: https://www.statista.com/statistics/1222815/loneliness-among-adults-by-country/.
44. Chawla K, Kunonga TP, Stow D, Barker R, Craig D, Hanratty B. Prevalence of loneliness amongst older people in high-income countries: a systematic review and meta-analysis. PLoS One. 2021;16(7):e0255088. PMID: 34310643; PMCID: PMC8312979. doi:10.1371/journal.pone.0255088
45. Sauter SR, Kim LP, Jacobsen KH. Loneliness and friendlessness among adolescents in 25 countries in Latin America and the Caribbean. Child Adolesc Ment Health. 2020;25(1):21–27. PMID: 32285635. doi:10.1111/camh.12358
46. Abio A, Owusu PN, Posti JP, et al. Cross-national examination of adolescent suicidal behavior: a pooled and multilevel analysis of 193,484 students from 53 LMIC countries. Soc Psychiatry Psychiatr Epidemiol. 2022;57(8):1603–1613. PMID: 35445842; PMCID: PMC9288956. doi:10.1007/s00127-022-02287-x
47. Buecker S, Mund M, Chwastek S, Sostmann M, Luhmann M. Is loneliness in emerging adults increasing over time? A preregistered cross-temporal meta-analysis and systematic review. Psychol Bull. 2021;147(8):787–805. PMID: 34898234. doi:10.1037/bul0000332
48. Bartels M, Cacioppo JT, Hudziak JJ, Boomsma DI. Genetic and environmental contributions to stability in loneliness throughout childhood. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):385–391. PMID: 17918194. doi:10.1002/ajmg.b.30608
49. McGuire S, Clifford J. Genetic and environmental contributions to loneliness in children. Psychol Sci. 2000;11(6):487–491. PMID: 11202494. doi:10.1111/1467-9280.00293
50. Boomsma DI, Willemsen G, Dolan CV, Hawkley LC, Cacioppo JT. Genetic and environmental contributions to loneliness in adults: the Netherlands twin register study. Behav Genet. 2005;35(6):745–752. PMID: 16273322. doi:10.1007/s10519-005-6040-8
51. Boomsma DI, Cacioppo JT, Slagboom PE, Posthuma D. Genetic linkage and association analysis for loneliness in Dutch twin and sibling pairs points to a region on chromosome 12q23-24. Behav Genet. 2006;36(1):137–146. PMID: 16378171. doi:10.1007/s10519-005-9005-z
52. Matthews T, Danese A, Wertz J, et al. Social isolation, loneliness and depression in young adulthood: a behavioural genetic analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(3):339–348. PMID: 26843197; PMCID: PMC4819590. doi:10.1007/s00127-016-1178-7
53. Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology. 2012;224(1):1–26. PMID: 22885871; PMCID: PMC3469771. doi:10.1007/s00213-012-2794-x
54. Zellner MR, Watt DF, Solms M, Panksepp J. Affective neuroscientific and neuropsychoanalytic approaches to two intractable psychiatric problems: why depression feels so bad and what addicts really want. Neurosci Biobehav Rev. 2011;35(9):2000–2008. PMID: 21241736. doi:10.1016/j.neubiorev.2011.01.003
55. Katriel. Emotional withdrawal after a breakup [Internet]. Positive Progress, Rick Merillat, LCSW; 2019 [cited January 26, 2023]. Available from: https://capecoralcounselor.com/emotional-withdrawal-after-a-breakup/.
56. Baryshnikov I, Isometsä E. Psychological pain and suicidal behavior: a review. Front Psychiatry. 2022;13:981353. PMID: 36203837; PMCID: PMC9531162. doi:10.3389/fpsyt.2022.981353
57. Polushina T, Banerjee N, Giddaluru S, et al. Identification of pleiotropy at the gene level between psychiatric disorders and related traits. Transl Psychiatry. 2021;11(1):410. PMID: 34326310; PMCID: PMC8322263. doi:10.1038/s41398-021-01530-4
58. Hawkley LC, Browne MW, Cacioppo JT. How can I connect with thee? Let me count the ways. Psychol Sci. 2005;16(10):798–804. PMID: 16181443. doi:10.1111/j.1467-9280.2005.01617.x
59. Primack BA, Shensa A, Sidani JE, et al. Social media use and perceived social isolation among young adults in the US. Am J Prev Med. 2017;53(1):1–8. PMID: 28279545; PMCID: PMC5722463. doi:10.1016/j.amepre.2017.01.010
60. The 3 types of loneliness and how to combat them. Psychology Today [Internet]; [cited January 26, 2023]. Available from: https://www.psychologytoday.com/us/blog/lifetime-connections/201907/the-3-types-loneliness-and-how-combat-them.
61. Day FR, Ong KK, Perry JRB. Elucidating the genetic basis of social interaction and isolation. Nat Commun. 2018;9(1):2457. PMID: 29970889; PMCID: PMC6030100. doi:10.1038/s41467-018-04930-1
62. Mastrogiovanni NA, Wheeler AK, Clemens KJ. Social isolation enhances cued reinstatement of sucrose and nicotine seeking, but this is reversed by a return to social housing. Sci Rep. 2021;11(1):2422. PMID: 33510269; PMCID: PMC7843648. doi:10.1038/s41598-021-81966-2
63. Panksepp J. The psycho-neurology of cross-species affective/social neuroscience: understanding animal affective states as a guide to development of novel psychiatric treatments. Curr Top Behav Neurosci. 2017;30:109–125. PMID: 27696337. doi:10.1007/7854_2016_458
64. Hofford RS. Isolation drives reward-seeking in rats. Lab Anim. 2021;50(5):125–126. PMID: 33782618. doi:10.1038/s41684-021-00756-5
65. Gold MS, Baron D, Bowirrat A, Blum K. Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): do homo sapiens acquire or have a reward deficiency syndrome? J Neurol Sci. 2020;418:117137. PMID: 32957037; PMCID: PMC7490287. doi:10.1016/j.jns.2020.117137
66. Elman I, Upadhyay J, Lowen S, Karunakaran K, Albanese M, Borsook D. Mechanisms underlying unconscious processing and their alterations in post-traumatic stress disorder: neuroimaging of zero monetary outcomes contextually framed as “No Losses” vs “No Gains”. Front Neurosci. 2020;14:604867. PMID: 33390889; PMCID: PMC7772193. doi:10.3389/fnins.2020.604867
67. Elman I, Ariely D, Tsoy-Podosenin M, et al. Contextual processing and its alterations in patients with addictive disorders. Addict Neurosci. 2023;7:100100. doi:10.1016/j.addicn.2023.100100
68. Badgaiyan RD, Sinha S, Sajjad M, Wack DS. Attenuated tonic and enhanced phasic release of dopamine in attention deficit hyperactivity disorder. PLoS One. 2015;10(9):e0137326. PMID: 26422146; PMCID: PMC4589406. doi:10.1371/journal.pone.0137326
69. Borsook D, Becerra L, Carlezon WA, et al. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007;11(1):7–20. PMID: 16495096. doi:10.1016/j.ejpain.2005.12.005
70. Elman I, Upadhyay J, Langleben DD, Albanese M, Becerra L, Borsook D. Reward and aversion processing in patients with posttraumatic stress disorder: functional neuroimaging with visual and thermal stimuli. Transl Psychiatry. 2018;8(1):240. PMID: 30389908; PMCID: PMC6214971. doi:10.1038/s41398-018-0292-6
71. Hopper JW, Pitman RK, Su Z, et al. Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res. 2008;42(10):802–807. PMID: 18068725; PMCID: PMC2551554. doi:10.1016/j.jpsychires.2007.10.008
72. Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1083–1090. PMID: 19640506. doi:10.1016/j.biopsych.2009.06.006
73. Blum K, Gondré-Lewis M, Steinberg B, et al. Our evolved unique pleasure circuit makes humans different from apes: reconsideration of data derived from animal studies. J Syst Integr Neurosci. 2018;4(1). PMID: 30956812; PMCID: PMC6446569. doi:10.15761/JSIN.1000191
74. Blum K, Baron D, Jalali R, et al. Polygenic and multi locus heritability of alcoholism: novel therapeutic targets to overcome psychological deficits. J Syst Integr Neurosci. 2020;7. PMID: 34707891; PMCID: PMC8547332. doi:10.15761/JSIN.1000240
75. Hamilton PJ, Nestler EJ. Epigenetics and addiction. Curr Opin Neurobiol. 2019;59:128–136. PMID: 31255844; PMCID: PMC6889055. doi:10.1016/j.conb.2019.05.005
76. Cadet JL. Epigenetics of stress, addiction, and resilience: therapeutic implications. Mol Neurobiol. 2016;53(1):545–560. PMID: 25502297; PMCID: PMC4703633. doi:10.1007/s12035-014-9040-y
77. Cadet JL, McCoy MT, Jayanthi S. Epigenetics and addiction. Clin Pharmacol Ther. 2016;99(5):502–511. PMID: 26841306; PMCID: PMC8252834. doi:10.1002/cpt.345
78. Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122(10):3387–3393. PMID: 23023708; PMCID: PMC3534165. doi:10.1172/JCI60390
79. Beayno A, El Hayek S, Noufi P, Tarabay Y, Shamseddeen W. The role of epigenetics in addiction: clinical overview and recent updates. Methods Mol Biol. 2019;2011:609–631. PMID: 31273724. doi:10.1007/978-1-4939-9554-7_35
80. Ajonijebu DC, Abboussi O, Russell VA, Mabandla MV, Daniels WMU. Epigenetics: a link between addiction and social environment. Cell Mol Life Sci. 2017;74(15):2735–2747. PMID: 28255755. doi:10.1007/s00018-017-2493-1
81. Schmidt HD, McGinty JF, West AE, Sadri-Vakili G. Epigenetics and psychostimulant addiction. Cold Spring Harb Perspect Med. 2013;3(3):a012047. PMID: 23359110; PMCID: PMC3579203. doi:10.1101/cshperspect.a012047
82. Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues Clin Neurosci. 2014;16(3):335–344. PMID: 25364284; PMCID: PMC4214176. doi:10.31887/DCNS.2014.16.3/pkenny
83. Pandey SC, Kyzar EJ, Zhang H. Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology. 2017;122:74–84. PMID: 28174112; PMCID: PMC5479721. doi:10.1016/j.neuropharm.2017.02.002
84. Kyzar EJ, Pandey SC. Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett. 2015;601:11–19. PMID: 25623036; PMCID: PMC4506731. doi:10.1016/j.neulet.2015.01.051
85. Blum K, Brodie MS, Pandey SC, et al. Researching mitigation of alcohol binge drinking in polydrug abuse: KCNK13 and RASGRF2 Gene(s) Risk Polymorphisms Coupled with Genetic Addiction Risk Severity (GARS) guiding precision pro-dopamine regulation. J Pers Med. 2022;12(6):1009. PMID: 35743793; PMCID: PMC9224860. doi:10.3390/jpm12061009
86. Roy AK, Bowirrat A, Smith DE, et al. Neurobiology and spirituality in addiction recovery. Acta Sci Neurol. 2021;4(9):64–71. PMID: 35098052; PMCID: PMC8793770.
87. Koob GF, Buck CL, Cohen A, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. PMID: 23747571; PMCID: PMC3830720. doi:10.1016/j.neuropharm.2013.05.024
88. Mathis V, Kenny PJ. From controlled to compulsive drug-taking: the role of the habenula in addiction. Neurosci Biobehav Rev. 2019;106:102–111. PMID: 29936111; PMCID: PMC9871871. doi:10.1016/j.neubiorev.2018.06.018
89. Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry. 2011;68(1):12–20. PMID: 21199962; PMCID: PMC3085192. doi:10.1001/archgenpsychiatry.2010.174
90. Bach P, de Timary P, Gründer G, Cumming P. Molecular imaging studies of alcohol use disorder. Curr Top Behav Neurosci. 2023. PMID: 36639552. doi:10.1007/7854_2022_414.
91. Luijten M, Schellekens AF, Kühn S, Machielse MW, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74(4):387–398. PMID: 28146248. doi:10.1001/jamapsychiatry.2016.3084
92. Clare K, Pan C, Kim G, et al. Cocaine reduces the neuronal population while upregulating dopamine D2-Receptor-expressing neurons in brain reward regions: sex-effects. Front Pharmacol. 2021;12:624127. PMID: 33912043; PMCID: PMC8072657. doi:10.3389/fphar.2021.624127
93. Fredriksson I, Tsai PJ, Shekara A, et al. Orbitofrontal cortex and dorsal striatum functional connectivity predicts incubation of opioid craving after voluntary abstinence. Proc Natl Acad Sci U S A. 2021;118(43):e2106624118. PMID: 34675078; PMCID: PMC8639358. doi:10.1073/pnas.2106624118
94. Lachowicz M, Chmielowiec J, Chmielowiec K, et al. Significant association of DRD2 and ANKK1 genes with rural heroin dependence and relapse in men. Ann Agric Environ Med. 2020;27(2):269–273. PMID: 32588604. doi:10.26444/aaem/119940
95. Feltmann K, Borroto-Escuela DO, Rüegg J, et al. Effects of long-term alcohol drinking on the dopamine D2 receptor: gene expression and heteroreceptor complexes in the striatum in rats. Alcohol Clin Exp Res. 2018;42(2):338–351. PMID: 29205397; PMCID: PMC5817245. doi:10.1111/acer.13568
96. Dahlgren A, Wargelius HL, Berglund KJ, et al. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol Alcohol. 2011;46(5):509–513. PMID: 21613303. doi:10.1093/alcalc/agr045
97. Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. PMID: 18154498. doi:10.1146/annurev.psych.59.103006.093548
98. Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46(9):1167–1180. PMID: 10560023. doi:10.1016/s0006-3223(99)00164-x
99. Sharp BM. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry. 2017;7(8):e1194. PMID: 28786979; PMCID: PMC5611728. doi:10.1038/tp.2017.161
100. Blum K, Febo M, Badgaiyan RD, et al. Neuronutrient amino-acid therapy protects against reward deficiency syndrome: dopaminergic key to homeostasis and neuroplasticity. Curr Pharm Des. 2016;22(38):5837–5854. PMID: 27510492. doi:10.2174/1381612822666160719111346
101. H.A.L.T. Hungry, Angry, Lonely, Tired [Internet]. Adstv.on.ca; [cited January 26, 2023]. Available from: http://adstv.on.ca/wp-content/uploads/2020/04/Coping-with-Cravings-Handout.pdf.
102. Fox R, McHugh Power J, Coogan AN, Beekman ATF, van Tilburg TG, Hyland P. Posttraumatic stress disorder and loneliness are associated over time: a longitudinal study on PTSD symptoms and loneliness, among older adults. Psychiatry Res. 2021;299:113846. PMID: 33706195. doi:10.1016/j.psychres.2021.113846
103. Matthews T, Caspi A, Danese A, Fisher HL, Moffitt TE, Arseneault L. A longitudinal twin study of victimization and loneliness from childhood to young adulthood. Dev Psychopathol. 2022;34(1):367–377. PMID: 33046153; PMCID: PMC8041922. doi:10.1017/S0954579420001005
104. Spithoven AWM, Cacioppo S, Goossens L, Cacioppo JT. Genetic contributions to loneliness and their relevance to the evolutionary theory of loneliness. Perspect Psychol Sci. 2019;14(3):376–396. PMID: 30844327. doi:10.1177/1745691618812684
105. Wootton RE, Greenstone HSR, Abdellaoui A, et al. Bidirectional effects between loneliness, smoking and alcohol use: evidence from a Mendelian randomization study. Addiction. 2021;116(2):400–406. PMID: 32542815. doi:10.1111/add.15142
106. Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. PMID: 21961707; PMCID: PMC3477468. doi:10.1146/annurev-pharmtox-010611-134625
107. Freilich CD, Mann FD, South SC, Krueger RF. Comparing phenotypic, genetic, and environmental associations between personality and loneliness. J Res Pers. 2022;101:104314. PMID: 36568631; PMCID: PMC9784097. doi:10.1016/j.jrp.2022.104314
108. Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35(2):495–519. PMID: 22640768; PMCID: PMC3506170. doi:10.1016/j.psc.2012.03.010
109. Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. PMID: 18640912; PMCID: PMC2607335. doi:10.1098/rstb.2008.0107
110. Skočibušić S, Zivlak-Radulović N, Hasanović M, et al. Personality dysfunction in opiate addicts on opioid substitution treatment and the risk of HCV infection. Front Public Health. 2022;10:1009413. PMID: 36159261; PMCID: PMC9507475. doi:10.3389/fpubh.2022.1009413
111. Kroll DS, McPherson KL, Manza P, et al. Elevated transferrin saturation in individuals with alcohol use disorder: association with HFE polymorphism and alcohol withdrawal severity. Addict Biol. 2022;27(2):e13144. PMID: 35229939; PMCID: PMC9373047. doi:10.1111/adb.13144
112. Ozawa H, Takashima S. Immunocytochemical development of transferrin and ferritin immunoreactivity in the human pons and cerebellum. J Child Neurol. 1998;13(2):59–63. PMID: 9512304. doi:10.1177/088307389801300203
113. Verhagen M, van Roekel E, Engels RC. Involvement of the BDNF gene in loneliness in adolescence: a report of opposite gene effects in boys and girls. PLoS One. 2014;9(3):e92768. PMID: 24647525; PMCID: PMC3960275. doi:10.1371/journal.pone.0092768
114. van Roekel E, Verhagen M, Engels RC, Goossens L, Scholte RH. Oxytocin receptor gene (OXTR) in relation to loneliness in adolescence: interactions with sex, parental support, and DRD2 and 5-HTTLPR genotypes. Psychiatr Genet. 2013;23(5):204–213. PMID: 23838880. doi:10.1097/YPG.0b013e328363f631
115. van Roekel E, Goossens L, Scholte RH, Engels RC, Verhagen M. The dopamine D2 receptor gene, perceived parental support, and adolescent loneliness: longitudinal evidence for gene-environment interactions. J Child Psychol Psychiatry. 2011;52(10):1044–1051. PMID: 21675993. doi:10.1111/j.1469-7610.2011.02424.x
116. Gao J, Davis LK, Hart AB, et al. Genome-Wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology. 2017;42(4):811–821. PMID: 27629369; PMCID: PMC5312064. doi:10.1038/npp.2016.197
117. van Roekel E, Scholte RH, Verhagen M, Goossens L, Engels RC. Loneliness in adolescence: gene x environment interactions involving the serotonin transporter gene. J Child Psychol Psychiatry. 2010;51(7):747–754. PMID: 20345842. doi:10.1111/j.1469-7610.2010.02225.x
118. Bralten J, Mota NR, Klemann CJHM, et al. Genetic underpinnings of sociability in the general population. Neuropsychopharmacology. 2021;46(9):1627–1634. PMID: 34054130; PMCID: PMC8280100. doi:10.1038/s41386-021-01044-z
119. Berto S, Usui N, Konopka G, Fogel BL. ELAVL2-regulated transcriptional and splicing networks in human neurons link neurodevelopment and autism. Hum Mol Genet. 2016;25(12):2451–2464. PMID: 27260404; PMCID: PMC6086562. doi:10.1093/hmg/ddw110
120. Kõks S, Nikopensius T, Koido K, et al. Analysis of SNP profiles in patients with major depressive disorder. Int J Neuropsychopharmacol. 2006;9(2):167–174. PMID: 15927089. doi:10.1017/S1461145705005468
121. Blum K, Braverman ER, Wu S, et al. Association of polymorphisms of dopamine D2 receptor (DRD2), and dopamine transporter (DAT1) genes with schizoid/avoidant behaviors (SAB). Mol Psychiatry. 1997;2(3):239–246. PMID: 9152988. doi:10.1038/sj.mp.4000261
122. Kendler KS, Aggen SH, Czajkowski N, et al. The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Arch Gen Psychiatry. 2008;65(12):1438–1446. PMID: 19047531; PMCID: PMC2844885. doi:10.1001/archpsyc.65.12.1438
123. Alden LE, Laposa JM, Taylor CT, Ryder AG. Avoidant personality disorder: current status and future directions. J Pers Disord. 2002;16(1):1–29. PMID: 11881158. doi:10.1521/pedi.16.1.1.22558
124. Nerviano VJ. Personality patterns of alcoholics revisited: delineation against the MMPI and clinical implications. Int J Addict. 1981;16(4):723–729. PMID: 7287249. doi:10.3109/10826088109038863
125. Verhagen M, Verweij KJH, Lodder GMA, et al. A SNP, gene, and polygenic risk score approach of oxytocin-vasopressin genes in adolescents’ loneliness. J Res Adolesc. 2020;30(Suppl 2):333–348. PMID: 30697859; PMCID: PMC7277497. doi:10.1111/jora.12480
126. Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. PMID: 15523558; PMCID: PMC524471. doi:10.1371/journal.pbio.0020377
127. Abdellaoui A, Sanchez-Roige S, Sealock J, et al.; 23andme Research Team. Phenome-wide investigation of health outcomes associated with genetic predisposition to loneliness. Hum Mol Genet. 2019;28(22):3853–3865. PMID: 31518406; PMCID: PMC6935385. doi:10.1093/hmg/ddz219
128. Tsai SJ, Yeh HL, Hong CJ, et al. Association of CHRNA4 polymorphism with depression and loneliness in elderly males. Genes Brain Behav. 2012;11(2):230–234. PMID: 22008229. doi:10.1111/j.1601-183X.2011.00741.x
129. Winterton A, Bettella F, Beck D, et al. The oxytocin signaling gene pathway contributes to the association between loneliness and cardiometabolic health. Psychoneuroendocrinology. 2022;144:105875. PMID: 35939863. doi:10.1016/j.psyneuen.2022.105875
130. Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095–1101. PMID: 22820409; PMCID: PMC3635809. doi:10.1016/j.bbi.2012.07.006
131. Almeida OP, Flicker L, Lautenschlager NT, Leedman P, Vasikaran S, van Bockxmeer FM. Contribution of the MTHFR gene to the causal pathway for depression, anxiety and cognitive impairment in later life. Neurobiol Aging. 2005;26(2):251–257. PMID: 15582752. doi:10.1016/j.neurobiolaging.2004.03.007
132. Blum K, Chen AL, Chen TJ, et al. LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Adv Ther. 2008;25(9):894–913. PMID: 18781289. doi:10.1007/s12325-008-0093-z
133. Elman I, Borsook D. The failing cascade: comorbid post traumatic stress- and opioid use disorders. Neurosci Biobehav Rev. 2019;103:374–383. PMID: 31063739. doi:10.1016/j.neubiorev.2019.04.023
134. Elman I, Becerra L, Tschibelu E, Yamamoto R, George E, Borsook D. Yohimbine-induced amygdala activation in pathological gamblers: a pilot study. PLoS One. 2012;7(2):e31118. PMID: 22319607; PMCID: PMC3271103. doi:10.1371/journal.pone.0031118
135. Chou KL, Cacioppo JT, Kumari M, Song YQ. Influence of social environment on loneliness in older adults: moderation by polymorphism in the CRHR1. Am J Geriatr Psychiatry. 2014;22(5):510–518. PMID: 23933425. doi:10.1016/j.jagp.2012.11.002
136. Mehrabian A, Blum JS. Temperament and personality as functions of age. Int J Aging Hum Dev. 1996;42(4):251–269. PMID: 8835610. doi:10.2190/1QCY-VETT-JJAY-3EY1
137. Martinez D, Narendran R. Imaging neurotransmitter release by drugs of abuse. Curr Top Behav Neurosci. 2010;3:219–245. PMID: 21161755. doi:10.1007/7854_2009_34
138. Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. PMID: 15860617. doi:10.1126/science.1106477
139. Moors A, De Houwer J. Automatic processing of dominance and submissiveness. Exp Psychol. 2005;52(4):296–302. PMID: 16302538. doi:10.1027/1618-3169.52.4.296
140. Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58(2):273–283. PMID: 18439411; PMCID: PMC2430590. doi:10.1016/j.neuron.2008.01.025
141. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–379. PMID: 17652652. doi:10.1056/NEJMsa066082
142. Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proc Natl Acad Sci U S A. 2009;106(6):1720–1724. PMID: 19171900; PMCID: PMC2644104. doi:10.1073/pnas.0806746106
143. Blum K, Oscar-Berman M, Bowirrat A, et al. Neuropsychiatric genetics of happiness, friendships, and politics: hypothesizing homophily (“Birds of a Feather Flock Together”) as a function of reward gene polymorphisms. J Genet Syndr Gene Ther. 2012;3(112):1000112. PMID: 23336089; PMCID: PMC3547641. doi:10.4172/2157-7412.1000112
144. Dawes CT, Weinschenk AC. On the genetic basis of political orientation. Curr Opin Behav Sci. 2020;34:173–178.doi:10.1016/j.cobeha.2020.03.012
145. Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263(15):2055–2060. PMID: 1969501. doi:10.1001/jama.1990.03440150063027
146. Chen TJ, Blum K, Chen AL, et al. Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptogen: proposing an addiction candidate gene panel map. J Psychoactive Drugs. 2011;43(2):108–127. PMID: 21858957. doi:10.1080/02791072.2011.587393
147. Benedetti A, Molent C, Barcik W, Papaleo F. Social behavior in 16p11.2 and 22q11.2 copy number variations: insights from mice and humans. Genes Brain Behav. 2022;21(5):e12787. doi:10.1111/gbb.12787
148. Rosenquist JN, Murabito J, Fowler JH, Christakis NA. The spread of alcohol consumption behavior in a large social network. Ann Intern Med. 2010;152(7):426–33, W141. PMID: 20368648; PMCID: PMC3343772. doi:10.7326/0003-4819-152-7-201004060-00007
149. Blum K, Chen AL, Chen TJ, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. PMID: 19014506; PMCID: PMC2615745. doi:10.1186/1742-4682-5-24
150. Holmes L, Chinaka C, Elmi H, et al. Implication of spiritual network support system in epigenomic modulation and health trajectory. Int J Environ Res Public Health. 2019;16(21):4123. PMID: 31717711; PMCID: PMC6862316. doi:10.3390/ijerph16214123
151. Goossens L, van Roekel E, Verhagen M, et al. The genetics of loneliness: linking evolutionary theory to genome-wide genetics, epigenetics, and social science. Perspect Psychol Sci. 2015;10(2):213–226. PMID: 25910391. doi:10.1177/1745691614564878
152. Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. PMID: 17854483; PMCID: PMC2375027. doi:10.1186/gb-2007-8-9-r189
153. Barrientos RM, Sprunger DB, Campeau S, et al. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121(4):847–853. doi:10.1016/S0306-4522(03)00564-5
154. Matrisciano F, Pinna G. PPAR-α hypermethylation in the hippocampus of mice exposed to social isolation stress is associated with enhanced neuroinflammation and aggressive behavior. Int J Mol Sci. 2021;22(19):10678. PMID: 34639019; PMCID: PMC8509148. doi:10.3390/ijms221910678
155. Siuda D, Wu Z, Chen Y, et al. Social isolation-induced epigenetic changes in midbrain of adult mice. J Physiol Pharmacol. 2014;65(2):247–255. PMID: 24781734.
156. Koopmans Y, Nelemans SA, Bosmans G, Van Den Noortgate W, Van Leeuwen K, Goossens L. Perceived parental support and psychological control, DNA methylation, and loneliness: longitudinal associations across early adolescence. J Youth Adolesc. 2023;52(10):1995–2011. doi:10.1007/s10964-023-01822-6
157. Kotyuk E, Magi A, Eisinger A, et al. Co-occurrences of substance use and other potentially addictive behaviors: epidemiological results from the Psychological and Genetic Factors of the Addictive Behaviors (PGA) Study. J Behav Addict. 2020;9(2):272–288. PMID: 32609628; PMCID: PMC8939407. doi:10.1556/2006.2020.00033
158. Gondré-Lewis MC, Darius PJ, Wang H, Allard JS. Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats. J Chem Neuroanat. 2016;76(Pt B):122–132. PMID: 26939765; PMCID: PMC5010523. doi:10.1016/j.jchemneu.2016.02.004
159. Erzen E, Çikrikci Ö. The effect of loneliness on depression: a meta-analysis. Int J Soc Psychiatry. 2018;64(5):427–435. PMID: 29792097. doi:10.1177/0020764018776349
160. Schermer JA, Colodro-Conde L, Grasby KL, et al. Genetic and environmental causes of individual differences in borderline personality disorder features and loneliness are partially shared. Twin Res Hum Genet. 2020;23(4):214–220. PMID: 32885774. doi:10.1017/thg.2020.62
161. Schermer JA, Martin RA, Vernon PA, et al. Lonely people tend to make fun of themselves: a behavior genetic analysis of humor styles and loneliness. Pers Individ Dif. 2017;117:71–73. doi:10.1016/j.paid.2017.05.042
162. Silventoinen K, Sammalisto S, Perola M, et al. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res. 2003;6(5):399–408. PMID: 14624724. doi:10.1375/136905203770326402
163. Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. PMID: 17566051. doi:10.1093/epirev/mxm004
164. Okbay A, Baselmans BM, De Neve JE, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet. 2016;48(6):624–633. Erratum in: Nat Genet. 2016 Jul 27;48(8):970. Erratum in: Nat Genet. 2016 Nov 29;48(12):1591. PMID: 27089181; PMCID: PMC4884152. doi:10.1038/ng.3552
165. Blum K, Han D, Bowirrat A, et al. Genetic Addiction risk and psychological profiling analyses for “Preaddiction” Severity Index. J Pers Med. 2022;12(11):1772. PMID: 36579510; PMCID: PMC9696872. doi:10.3390/jpm12111772
166. Lim WL, Tierney S. The effectiveness of positive psychology interventions for promoting well-being of adults experiencing depression compared to other active psychological treatments: a systematic review and meta-analysis. J Happiness Stud. 2022;5:1–25. PMID: 36373089; PMCID: PMC9638203. doi:10.1007/s10902-022-00598-z
167. Bryant JS, Gallagher MR, Collins AC, Winer ES. Individuals fearing positivity do not perceive positive affect treatments as strong fits: a novel experimental finding and replication. J Behav Ther Exp Psychiatry. 2022;79:101830. PMID: 36587466. doi:10.1016/j.jbtep.2022.101830
168. McMakin DL, Siegle GJ, Shirk SR. Positive Affect Stimulation and Sustainment (PASS) Module for Depressed Mood: a preliminary investigation of treatment-related effects. Cognit Ther Res. 2011;35(3):217–226. PMID: 22140287; PMCID: PMC3226735. doi:10.1007/s10608-010-9311-5
169. Ikeda Y, Funayama T, Tateno A, Fukayama H, Okubo Y, Suzuki H. Bupropion increases activation in nucleus accumbens during anticipation of monetary reward. Psychopharmacology. 2019;236(12):3655–3665. PMID: 31342097. doi:10.1007/s00213-019-05337-6
170. Krystal AD, Benca RM, Rosenberg R, et al. Solriamfetol treatment of excessive daytime sleepiness in participants with narcolepsy or obstructive sleep apnea with a history of depression. J Psychiatr Res. 2022;155:202–210. doi:10.1016/j.jpsychires.2022.08.018
171. Dias VT, Rosa HZ, D’avila LF, Vey LT, Barcelos RCS, Burger ME. Modafinil reduces amphetamine preference and prevents anxiety-like symptoms during drug withdrawal in young rats: involvement of dopaminergic targets in VTA and striatum. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:199–206. PMID: 30664970. doi:10.1016/j.pnpbp.2019.01.007
172. Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31(10):2091–2120. PMID: 16541087. doi:10.1038/sj.npp.1301051
173. Sagheddu C, Pintori N, Kalaba P, et al. Neurophysiological and neurochemical effects of the putative cognitive enhancer (S)-CE-123 on mesocorticolimbic Dopamine System. Biomolecules. 2020;10(5):779. PMID: 32443397; PMCID: PMC7277835. doi:10.3390/biom10050779
174. Elman I, Pustilnik A, Borsook D. Beating pain with psychedelics: matter over mind? Neurosci Biobehav Rev. 2022;134:104482. PMID: 34922987. doi:10.1016/j.neubiorev.2021.12.005
175. Hoefer ME, Voskanian SJ, Koob GF, Pulvirenti L. Effects of terguride, ropinirole, and acetyl-L-carnitine on methamphetamine withdrawal in the rat. Pharmacol Biochem Behav. 2006;83(3):403–409. PMID: 16647107. doi:10.1016/j.pbb.2006.02.023
176. Traina G. The neurobiology of acetyl-L-carnitine. Front Biosci. 2016;21(7):1314–1329. PMID: 27100509. doi:10.2741/4459
177. Ingram I, Kelly PJ, Deane FP, et al. Loneliness among people with substance use problems: a narrative systematic review. Drug Alcohol Rev. 2020;39(5):447–483. PMID: 32314504. doi:10.1111/dar.13064
178. Solecki WB, Kus N, Gralec K, Klasa A, Pradel K, Przewłocki R. Noradrenergic and corticosteroid receptors regulate somatic and motivational symptoms of morphine withdrawal. Behav Brain Res. 2019;360:146–157. PMID: 30500430. doi:10.1016/j.bbr.2018.11.041
179. Rasmussen DD, Kincaid CL, Froehlich JC. Prazosin + naltrexone decreases alcohol drinking more effectively than does either drug alone in p rats with a protracted history of extensive voluntary alcohol drinking, dependence, and multiple withdrawals. Alcohol Clin Exp Res. 2015;39(9):1832–1841. PMID: 26260061; PMCID: PMC4558320. doi:10.1111/acer.12828
180. Skeie-Larsen M, Stave R, Grønli J, et al. The effects of pharmacological treatment of nightmares: a systematic literature review and meta-analysis of placebo-controlled, randomized clinical trials. Int J Environ Res Public Health. 2022;20(1):777. PMID: 36613097; PMCID: PMC9820008. doi:10.3390/ijerph20010777
181. Blum K, Chen TJ, Meshkin B, et al. Manipulation of catechol-O-methyltransferase (COMT) activity to influence the attenuation of substance seeking behavior, a subtype of Reward Deficiency Syndrome (RDS), is dependent upon gene polymorphisms: a hypothesis. Med Hypotheses. 2007;69(5):1054–1060. PMID: 1746791. doi:10.1016/j.mehy.2006.12.062
182. Blum K, Dennen CA, Elman I, et al. Should Reward Deficiency Syndrome (RDS) be considered an umbrella disorder for mental illness and associated genetic and epigenetic induced dysregulation of brain reward circuitry? J Pers Med. 2022;12(10):1719. PMID: 36294858; PMCID: PMC960460. doi:10.3390/jpm12101719
183. Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39(4):295–307. PMID: 1156704.
184. Tan M, Shallis A, Barkus E. Social anhedonia and social functioning: loneliness as a mediator. Psych J. 2020;9(2):280–289. PMID: 31965741. doi:10.1002/pchj.344
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.