Back to Journals » Cancer Management and Research » Volume 15
Neurofibromatosis Type 1-Associated Optic Pathway Gliomas: Current Challenges and Future Prospects
Authors Tang Y , Gutmann DH
Received 1 March 2023
Accepted for publication 6 June 2023
Published 13 July 2023 Volume 2023:15 Pages 667—681
DOI https://doi.org/10.2147/CMAR.S362678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Yunshuo Tang,1,2 David H Gutmann2
1Department of Ophthalmology, Washington University School of Medicine, St. Louis, MO, USA; 2Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA
Correspondence: David H Gutmann, Department of Neurology, Washington University School of Medicine, Box 8111, 660 S. Euclid Avenue, St. Louis, MO, 63110, USA, Tel +1 314 362 7379, Fax +1 314 362 2388, Email [email protected]
Abstract: Optic pathway glioma (OPG) occurs in as many as one-fifth of individuals with the neurofibromatosis type 1 (NF1) cancer predisposition syndrome. Generally considered low-grade and slow growing, many children with NF1-OPGs remain asymptomatic. However, due to their location within the optic pathway, ~20-30% of those harboring NF1-OPGs will experience symptoms, including progressive vision loss, proptosis, diplopia, and precocious puberty. While treatment with conventional chemotherapy is largely effective at attenuating tumor growth, it is not clear whether there is much long-term recovery of visual function. Additionally, because these tumors predominantly affect young children, there are unique challenges to NF1-OPG diagnosis, monitoring, and longitudinal management. Over the past two decades, the employment of authenticated genetically engineered Nf1-OPG mouse models have provided key insights into the function of the NF1 protein, neurofibromin, as well as the molecular and cellular pathways that contribute to optic gliomagenesis. Findings from these studies have resulted in the identification of new molecular targets whose inhibition blocks murine Nf1-OPG growth in preclinical studies. Some of these promising compounds have now entered into early clinical trials. Future research focused on defining the determinants that underlie optic glioma initiation, expansion, and tumor-induced optic nerve injury will pave the way to personalized risk assessment strategies, improved tumor monitoring, and optimized treatment plans for children with NF1-OPG.
Keywords: neurofibromatosis type 1, optic glioma, vision loss, genetically engineered mouse models
Introduction
Neurofibromatosis type 1 (NF1) is a rare cancer predisposition syndrome, affecting approximately 1 in 3000 live births worldwide.1 As an autosomal dominant condition, NF1 is caused by germline inactivating mutations of the NF1 tumor suppressor gene. However, many of the clinical features of NF1, such as tumor formation, require loss of the remaining functional NF1 allele, a process called copy neutral loss of heterozygosity.2,3 While NF1 is completely penetrant, affected individuals, even those with the same germline NF1 gene mutation, manifest with a wide range of clinical manifestations. In keeping with this variable expressivity, individuals with NF1 usually have café-au-lait macules, skinfold freckling, Lisch nodules, and peripheral nerve sheath tumors (cutaneous neurofibromas), but may also develop brain tumors (gliomas), plexiform neurofibromas, and bony lesions.4 The majority of children with NF1 also exhibit cognitive dysfunction, including impairments in visuospatial function, executive function, language ability, and motor skills, as well as attention-deficit/hyperactivity disorder (ADHD), learning disabilities, autism, sleep disorders, anxiety, and depression.5–10 In addition, adults with NF1 are at increased risk for tumors, including malignant peripheral nerve sheath tumors, malignant gliomas, glomus tumors, pheochromocytoma, and breast cancer.11–13
Optic pathway gliomas (OPG) are classified as World Health Organization grade 1 (pilocytic) astrocytomas.14 Approximately 15–20% of young children with NF1 will develop a pilocytic astrocytoma along the optic pathway, typically detected before 7 years of age.15–20 Although NF1-OPGs are usually considered slow-growing brain tumors, 20-30% of affected children will experience clinical or visual symptoms, such as vision loss or precocious puberty, and require therapeutic intervention.17,19
Due to their location, surgery is rarely performed because of the risk of iatrogenic neurological, ophthalmological, and endocrinological damage.21–25 Radiation therapy is usually contraindicated due to the high risk of secondary malignancy, neurovascular abnormalities (moyamoya), and cognitive impairment in children with a cancer predisposition syndrome.26–28 Currently, the first line therapy for symptomatic OPGs entails the use of chemotherapy agents (eg, vincristine and carboplatin), which are more effective at controlling tumor growth than halting or reversing visual loss.19,29 As such, only 30% of children will experience an improvement in vision after treatment, and as many as 30% will continue to exhibit visual decline despite treatment.19 Vision loss from NF1-OPG can be severe and permanent, which can significantly and negatively impact quality of life.30 Moreover, ~30% children with NF1-OPG will fail first-line therapy and require additional treatment.31 For these reasons, new therapeutic options are urgently needed for this at-risk population, especially those directed at restoring vision loss. In this review, we will describe the recent advances in our understanding of optic glioma initiation and progression, as well as promising therapeutic targets identified through preclinical drug discovery in Nf1-OPG small animal models.
Clinical Presentation
The majority of individuals with NF1 affected by OPG are young children, less than 7 years of age,15,32 with a mean age at OPG diagnosis of 4.5 years.17,33 A small fraction of children with NF1-OPG are diagnosed in adolescence or later years.34–37 The exact incidence of late-onset OPG is difficult to ascertain, as some of the children may have had existing OPGs that went undiagnosed until they develop symptoms in adolescence.35 It is worth noting that NF1-OPGs have several unique features relative to their sporadic counterparts, such as younger ages of onset, higher likelihood of bilateral involvement, more indolent clinical courses, and overall better prognoses.38,39
NF1-OPGs can arise within any segment of the visual pathway (Figure 1). Although the majority (>75%) occur in the anterior visual pathway (prechiasmatic optic nerves and optic chiasm), NF1-OPGs can also arise in the posterior visual pathway (optic tracts and optic radiations).15,40,41 In one large retrospective study of 149 children with NF1-OPG, 64.4% of tumors were located in the prechiasmatic optic nerves, 28.2% in the optic chiasm, and 7.4% in the optic tracts and radiations (post-chiasmatic regions).42 As tumor location partly dictates clinical symptoms and signs, the Dodge classification scheme was developed in the 1950s to parse OPGs anatomically into stage 1 (optic nerve), stage 2 (chiasmal), or stage 3 (nearby structures) tumors.43 A modified Dodge classification was later proposed by the PLAN working group to further subdivide the anatomical localization of OPGs and more accurately characterize the tumor, track their impact on visual and endocrinologic function, and assist in treatment decision making.41 In general, while tumors arising anywhere along the visual pathway have the potential to cause vision loss, those involving the optic nerves can also lead to strabismus, amblyopia, and proptosis of the affected eye.35,44 In contrast, NF1-OPGs affecting the optic chiasm often cause bi-temporal vision loss or endocrinologic abnormalities, such as precocious puberty due to hypothalamic involvement. Besides vision loss, strabismus, exophthalmos, and amblyopia are the most frequently documented ophthalmological symptoms/signs of NF1-OPG.37 Endocrinologic disorders are also common in children with NF1-OPG, especially those with hypothalamic involvement, and can range from precocious puberty, growth hormone hypersecretion or deficiency, diabetes insipidus, diencephalic syndrome, obesity with insulin resistance, and hypogonadism.44–47
Risk Factors for Vision Loss in NF1-OPG
Over the past decade, efforts have focused on the identification of risk factors for developing NF1-OPGs and associated visual impairment. While some studies of individuals with NF1-OPG report no clear genotype-phenotype correlation,48,49 other studies have suggested that the location of the germline NF1 gene mutation could be one risk factor for OPG development.50–53 These studies found that mutations in the 5′ end of the NF1 gene were associated with OPG development. Strikingly, other mutations are associated with a lack of OPG development, including the Arg1809 missense and Met991 in-frame deletion mutations.54–57 In contrast, individuals with mutations affecting codons 844–848 tend are more likely to harbor OPGs (50% of children).58
Other risk factors include race/ethnic background, birth weight, and allergic conditions. In this respect, Caucasians with NF1 are more likely to be diagnosed with OPG than those who identify as Black or Asian.59 High birth weight is associated with increased brain tumor incidence in children with NF1,60 whereas allergic conditions, like eczema and asthma, are associated with a reduced risk of OPG development.61
Risk factors for vision loss include tumor location, subject age, and sex. NF1-OPGs involving the posterior visual pathway (optic tracts and radiations) have a greater likelihood of vision loss.19,31,34,38 On the other hand, chiasmal location with hypothalamic involvement, along with young age of onset and older age at last endocrine evaluation, portend a higher risk for endocrine abnormalities.46,47 The presence of clinical symptoms at diagnosis also increases the likelihood that OPGs will eventually require treatment.35 Children younger than 2 or older than 8–10 years of age are more likely to experience vision loss.19,37,62,63 Similarly, young age may also predict relapse after treatment.31 Lastly, girls with NF1-OPGs involving the optic nerve are more 3–5 times more likely to require treatment for vision loss than boys.64,65
Screening and Monitoring
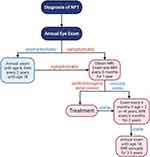
NF1-OPGs can exhibit highly variable clinical courses and visual outcomes. As such, not all children with an NF1-OPG will manifest symptoms or signs, and not all individuals with NF1-OPG-related vision loss will require treatment. For this reason, annual screening and longitudinal monitoring of individuals with NF1 for OPG-related signs and symptoms during childhood is crucial for prompt clinical decision making and treatment. Over the past two decades, consistent recommendations have been proposed by the NF1-OPG Task Force and the American Academy of Pediatrics (AAP; Figure 2): all children with NF1 should undergo ophthalmological exams annually by an experienced ophthalmologist, starting at 8–9 months of age until age 8, and then every two years until age 18.66,67 These annual exams should include detailed assessments of visual acuity using age-appropriate methods (eg, Teller acuity cards, eye chart with standard letters (HOTV) or shapes (Lea), and Snellen acuity cards), and include assessments of color vision, visual fields, ocular motility, and eye alignment. The eyelids, orbits, pupils, irises, and fundi should also be thoroughly examined. Children with NF1 should also undergo annual physical examinations with assessments of weight, height, growth velocity, and sexual development to monitor for signs of precocious puberty. If an individual with NF1 develops any of the above symptoms, especially reduced visual acuity, endocrine disorders, or new neurologic deficits, a brain MRI should be obtained to evaluate for the presence of an OPG. However, “screening” baseline MRIs of the brain to detect asymptomatic OPGs in individuals with NF1 is not recommended.35,62,67–69
Screening guidelines for NF1-OPG from the French NF Network align well with those from the NF1-OPG Task Force and AAP with respect to the need for annual ophthalmological examinations and MRI screening of asymptomatic children.70 In contrast, they recommend screening for OPG in asymptomatic children until 13, instead of 18, years of age. It is worth noting that both guidelines recommend screening well into the adolescent years in an effort to identify individuals with late-onset symptomatic OPGs.
Once diagnosed with an OPG, either due to the development of symptoms or following incidental discovery, children with NF1-OPGs need close monitoring for the development or worsening of visual symptoms. Individuals should have ophthalmological exams and endocrinologic assessments every 3 months for the first year after diagnosis, given the fact that most decisions to initiate treatment are made within the first year.19 If vision and endocrine function remain stable, exams should be conducted every 6 months for individuals younger than 2 years or older than 8 years of age, then annually until 18 years of age.67,71 MRI should also be performed every 3 months for the first year after diagnosis and every 6 months for the next two years, especially for children with symptomatic OPGs or very young children in whom changes in vision cannot be confidently assessed.67 Annual ophthalmologic evaluations and neuroimaging then continue annually for another 3–5 years if no clinical changes are noted. Afterwards, the interval of neuroimaging can be further increased in the absence of clinical progression until 18 years of age.67
A significant number of children will require MRI under general anesthesia, where the risk of anesthesia-related adverse events is approximately 1%. These events include neurotoxicity and aspiration, which occur more often in children than in adults, especially those younger than 3 years of age.72,73 In addition to weighing the risk of MRI under anesthesia against the benefit of preventing lifelong disability due to visual loss, clinicians should also be aware of the rare phenomenon of spontaneous tumor involution on MRI, which has been reported to occur in the context of NF1-OPG.74–76
Complementary Assessments of Visual Function
Since many children with NF1 have co-morbid ADHD or autism spectrum symptomatology, other objective and quantitative methods have been developed to monitor for vision loss. The most commonly used assessments are optical coherence tomography (OCT) and visual evoked potentials (VEPs).77 VEPs present a set of standardized visual stimuli and measure elicited visual cortical response detected using scalp electrodes. Decreased amplitudes or prolonged latencies may indicate injury to the visual pathway from an OPG.67 While VEP has nearly 90% sensitivity at detecting the presence of an OPG, the specificity is much lower, and reports conflict on whether it correlates with vision loss.78–80 In addition, VEP requires patient cooperation for reliable results, which can be difficult in children with NF1 due to their young age and cognitive comorbidities.
OCT measures the thickness of the retinal nerve fiber layer (RNFL) and ganglion cell layer-inner plexiform layer complex (GCL-IPL), the thinning of which correlates with declining visual acuity in children with NF1-OPG.81–83 The advantage of OCT is its non-invasive nature and the automated measurements of RNFL and GCL-IPL thicknesses, which reduces inter-operator variability. RNFL thinning due to NF1-OPG may precede clinically measurable vision loss, making RNFL thickness an attractive biomarker for the early detection of vision loss.84 However, good fixation is still required for reliable segmentation of the retinal layers, which may be difficult for young children and individuals who already have poor central vision. For very young children, OCTs with accurate assessment of RNFL and GCL-IPL layers may require anesthesia, usually performed in coordinated with a sedated MRI. While this method is attractive, there is also inter-device and inter-protocol variability that may cloud the interpretation of changes measured by OCT.85,86
Treatment
Treatment is usually initiated after clinical and/or radiographic progression is observed. The clinical criteria for treatment are defined as a visual acuity decrease of 0.2 logMAR units or more, or new visual field defect.67,87 Tumor progression, especially in children whose visual acuity cannot be reliably assessed, may also be an indication for treatment.67,87 While surgical resection is rarely performed, debulking or enucleation may be necessary in children with large OPGs that compress the surrounding brain tissue or cause severe proptosis and disfiguration with little functional vision left in the affected eye.88,89 Surgical decompression or cerebrospinal fluid diversion may also be indicated if chiasmal OPGs cause compression of the third ventricle with associated hydrocephalus.23,25
The current first-line treatment for NF1-OPG is chemotherapy with vincristine and carboplatin.90,91 Hypersensitivity to carboplatin can occur, the risk of which increases with repeated exposure.92 For those individuals who develop carboplatin hypersensitivity, vinblastine has also been used as an alternative. Vinblastine has a more favorable side effect profile, but more clinical studies are needed to establish its efficacy for vision preservation and restoration in children with NF1-OPG.92 Other second-line chemotherapies include vinorelbine and temozolomide, as well as bevacizumab and irinotecan.93–97
Although chemotherapy is often effective at limiting tumor growth, only a minority of individuals will experience improvements in vision following treatment. In a large multicenter study of 115 children treated for NF1-OPG between 1997 and 2007, 32% had improved visual acuity following treatment, while 40% and 28% had no change or deterioration of visual acuity, respectively.19
Genetics of NF1-OPG
All children with NF1 are born with one functional NF1 allele and one non-functional NF1 allele harboring a germline loss-of-function mutation. The presence of a germline NF1 gene mutation alone is not sufficient for tumorigenesis. Optic glioma formation requires somatic loss of the remaining functional NF1 allele.98–100 This is consistent with the “two-hit” model of tumorigenesis, first proposed to explain the origin of retinoblastoma.101,102 Examination of NF1-associated pilocytic astrocytomas revealed that somatic inactivation of the second NF1 allele can occur through either loss of heterozygosity, loss-of-function mutations, or epigenetic modification.103–105 All of these events lead to undetectable levels of the NF1 protein (neurofibromin) in the tumor cells.106
Neurofibromin is a large 2818 amino acid protein that forms a functional homodimer.107,108 It contains a 300-amino-acid domain that binds to and negatively regulates the RAS proto-oncogene.3,109 By binding to RAS, neurofibromin functions as a GTPase-activating protein (GAP) and accelerates the conversion of RAS from its GTP-bound active form to an inactive GDP-bound form.110,111 NF1 loss-of-function mutations result in increased RAS activity and cell growth through hyperactivation of RAS downstream pathways (RAS-MEK-ERK or RAS-AKT-mTOR).112–114 Consistent with its role as a RAS-GAP, increased RAS activity is also detected in surgical specimens of human NF1-OPG.115,116
Insights from Preclinical Models
Because surgical resection or biopsy of NF1-OPGs is rarely performed, the majority of our understanding of these tumors derives from preclinical animal models. Genetically engineered mice are the most widely used and best characterized models of Nf1-OPG,117,118 although minipigs with germline NF1 mutations also develop OPGs.119 Because mice homozygous for Nf1 loss (Nf1null) die in utero, conditional transgenesis (Cre-LoxP) methods have been employed to develop mice in which somatic Nf1 loss could be targeted to specific cell types.117 Using this method, numerous Nf1-OPG mouse models have been generated.117,118,120 In these Nf1-OPG models, tumors arise in >95% of mice by 3–4 months of age, with loss of RGCs and thinning of the RNFL, similar to that observed in humans.81,121 Leveraging these murine strains, several key insights have been revealed relevant to improved patient risk assessment and treatment.
Tumor Cell of Origin
First, the likely cell of origin for murine Nf1-OPGs is a GFAP-, CD133-, and BLBP-expressing neural progenitor cell (NPC), rather than a NG2-expressing glial cell.118,122,123 Second, the NPCs capable of tumorigenesis are anatomically restricted to the subventricular zone of the third ventricle,124,125 where during normal development, they give rise to oligodendrocyte precursor cells that migrate into the optic nerve.126 The regional differences between NPCs in the third ventricle (Nf1-OPG cell of origin) and the lateral ventricle (unlikely Nf1-OPG cell of origin) result from increased expression of the mTOR complex protein, rictor, which controls Akt activation.124 Third, Nf1 somatic loss in Olig2-expressing oligodendrocyte precursor cells also induces Nf1-OPG formation, albeit with a longer latency (~6 months).123 To complement these studies in mice, human induced pluripotent stem cells (hiPSCs) harboring biallelic NF1 mutations have also been used to generate diverse types of cells in the central nervous system and investigated for their ability to form low grade gliomas.127 Consistent with the mouse model findings, hiPSC-derived neuroglial progenitors, but not astrocytes, generate tumors following transplantation into immunocompromised mice.
Immune Tumor Microenvironment
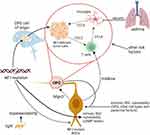
While biallelic Nf1 loss in the tumor cells of origin is necessary, it is not sufficient by itself, for tumorigenesis. In this regard, bi-allelic Nf1 loss in susceptible NPCs does not result in Nf1-OPG formation.128 Nf1 loss in third ventricle NPCs must be coupled with a germline Nf1 mutation in the non-cancerous cells in order for tumors to form.118 Examination of these non-neoplastic cells reveals the presence of monocytes (resident microglia) and T cells that form an immune microenvironment through the production of soluble chemokines129–131 (Figure 3).
The importance of microglia to glioma progression was revealed by genetic and pharmacologic studies, resulting in delayed optic glioma formation and reduced Nf1-OPG growth, respectively.132,133 Tumor-associated microglia produce a key growth factor for optic glioma tumor cells (Ccl5), such that its inhibition blocks Nf1-OPG growth.134,135 Following recruitment by the tumor cells (Ccl2 chemoattraction), T cells regulate microglia function and Ccl5 production through the elaboration of another cytokine (Ccl4).131,135 However, in contrast to RAS/RAS pathway inhibition, where cessation of drug treatment results in continued tumor growth,121,136 blocking T cell infiltration or microglia Ccl5 production during tumorigenesis has durable effects on Nf1-OPG growth following the cessation of treatment.137
Because T cells likely infiltrate the tumor from the systemic circulation, they may also serve as bellwethers of systemic disease. In Nf1-OPG mice, experimental asthma induction causes T cells to express decorin, which blocks microglial expression of Ccl5 and suppresses optic glioma formation.138 This experimental finding provides a mechanistic explanation for the reduced risk of OPG development in children with NF1 and asthma.61 In addition to suggesting potential immune therapies, the ability of T cells to function as sentinels of systemic disease make them possible convergence points for other NF1-OPG risk factors.
Neurons as Key Drivers of NF1-OPG
Neurons can also participate in NF1-OPG pathobiology. First, they can be the targets of OPG-induced damage. In this regard, Nf1-mutant retinal ganglion cells (RGCs) whose axons course through the optic nerve, have smaller growth cones, shorter neurites, and increased death due to impaired generation of intracellular cyclic AMP (cAMP).139 The importance of intracellular cAMP to the intrinsic vulnerability of Nf1-mutant neurons is underscored by the finding that pharmacologic cAMP elevation in Nf1-OPG mice rescues RGC death in vivo.139
Second, Nf1-mutant RGCs also play key roles in the initiation and progression of NF1-OPG. Increased neuronal activity in response to light stimulation (visual experience) is critical for Nf1 optic glioma initiation, such that rearing Nf1-OPG mice in the dark blocks tumor formation.140 This activity-dependent initiation of optic glioma by Nf1-mutant RGCs is mediated through neuronal shedding of neuroligin-3 (Nlgn3), a potent growth factor for gliomas. Additionally, Nf1 mutation also increases RGC neurons baseline excitability, acting at the level of the hyperpolarization activated cyclic nucleotide gated potassium channel (HCN), such that HCN pharmacological targeting with lamotrigine blocks optic glioma progression.141 In contrast to light-induced regulation of Nf1-mutant neuronal excitability, this HCN-controlled effect is mediated by Nf1-mutant neuronal production of midkine, which stimulates T cells to produce Ccl4 and activate the immune axis supportive of tumor growth. Taken together, these findings establish Nf1-mutant RGCs as active drivers of optic glioma initiation and growth, as well as targets of optic glioma-related vision loss, making them important cells to consider in future clinical trials.
Challenges and Future Prospects
Risk Assessment
Recent studies using NF1-mutant hiPSCs and Nf1-GEM models with patient-derived germline NF1 gene mutations demonstrate that not all germline NF1 gene mutations are functionally equivalent.130,141–143 In these experiments, mice with different germline Nf1 gene mutations exhibit a wide range of tumor phenotypes, including no tumors, reduced tumor penetrance, and faster tumor growth. These mice could be used to identify serum biomarkers for glioma formation and growth, and provide unique opportunities to perform preclinical studies on mouse strains with different tumor characteristics. The use of mice coupled with human epidemiologic studies may reveal additional predictive risk factors for precision medicine.144
Screening and Monitoring
In addition to OCT and MRI, which are now an integral part of monitoring children with NF1-OPGs, several new tools are being evaluated. These include tractography or diffusion tensor imaging (DTI) and volumetric MRI, which are techniques that map white matter tracts and calculate the volume of OPGs, respectively. Decreased functional anisotropy and increased diffusion on tractography may be associated with decreased visual acuity in individuals with NF1-OPG.145–149 Similarly, larger tumor size on volumetric MRI correlates with greater retinal nerve fiber layer thinning.150 DTI and volumetric MRI share some of the disadvantages of traditional brain MRI, such as the need for sedation in young patients and a relatively high cost. Additionally, these protocols call for experienced operators, which may also limit their widespread use. Further studies on the threshold of changes necessary to influence treatment decisions and the utility of these radiographic assessments for monitoring treatment response remain to be addressed.
Treatment
While carboplatin/vincristine has long been the primary first-line therapy for NF1-OPG, numerous molecularly-targeted therapies that block RAS pathway signaling have entered clinical trials (Table 1). Among them, MEK inhibitors have emerged as particularly promising drugs. MEK inhibition effectively suppressed tumor growth in animal models of Nf1-OPG,151 and in early clinical trials on NF1- and non-NF1-associated OPGs (selumetinib and trametinib).152–155 While these results are encouraging, it should be noted that more than a third of patients in two of these trials reported significant adverse events (mainly dermatological, gastrointestinal, and hematological sequelae) that were dose limiting.153,154 Disease rebound has also been observed in some individuals after withdrawal of MEK inhibition.153 In addition to MEK inhibitors, one immunomodulatory therapy (Poly-ICLC) is currently being evaluated.156–160
 |
Table 1 Active Clinical Trials for NF1-OPG |
Due to the limited vision restorative potential of the current mainstream cytotoxic agents, there is a pressing need to develop alternative treatment strategies to promote vision preservation and restoration. While no neuroprotective or vision-restorative agents are currently approved for NF1-OPG, a few promising candidates are in preclinical or early clinical trials. Topical nerve growth factor (NGF) delivery to the eye was shown to improve visual fields in a small cohort of individuals with NF1-OPG who completed chemotherapy.161 Future compounds could be considered, including those that elevate neuronal cyclic AMP or attenuate neuronal hyperexcitability (eg, lamotrigine).139,141 Other strategies are also being considered, such as viral delivery of pro-survival transcription factors and transplantation of stem cell-derived human RGCs.162–165
Platforms for Future Research
To ensure that future research continues to offer valuable insights into the molecular determinants of tumor initiation, progression, and vision loss, and that preclinical studies of novel therapeutic interventions have good chances of efficacy, there is a great need for additional experimental platforms that capture the inherent clinical heterogeneity seen in children with NF1-OPG. These platforms should include multiple small-animal models harboring different patient-derived germline mutations, as well as additional animal models in which optic gliomas arise within the optic tracts and optic radiations. Moreover, while the visual system of mice is easily accessible and well characterized, it may not accurately represent the complex structure and function of the human visual system. In this respect, the use of NF1 genetically engineered pig models that share many similarities with humans with respect to ocular anatomy and development of the visual system, could be employed.166,167 Other animals with highly developed visual systems, such as ferrets, are amendable to CRISPR/Cas9-mediated genomic engineering, and could be developed as future models of NF1-OPG.168
Disclosure
Y.T. is funded by a R25 grant from the NINDS (R25-NS090978). D.H.G. is funded by grants from the NINDS (R35-NS07211), National Cancer Institute (R01-CA258384, R01-CA261939), and the Gilbert Family Foundation Vision Restoration Initiative. The authors report no other conflicts of interest in this work.
References
1. Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east wales. I. prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26(11):704–711. doi:10.1136/jmg.26.11.704
2. Hattori S, Maekawa M, Nakamura S. Identification of neurofibromatosis type I gene product as an insoluble GTPase-activating protein toward ras p21. Oncogene. 1992;7(3):481–485.
3. Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356(6371):713–715. doi:10.1038/356713a0
4. Legius E, Messiaen L, Wolkenstein P, et al. Revised diagnostic criteria for neurofibromatosis type 1 and legius syndrome: an international consensus recommendation. Genet Med. 2021;23(8):1506–1513. doi:10.1038/s41436-021-01170-5
5. Denckla MB, Hofman K, Mazzocco MM, et al. Relationship between T2-weighted hyperintensities (unidentified bright objects) and lower IQs in children with neurofibromatosis-1. Am J Med Genet. 1996;67(1):98–102. doi:10.1002/(SICI)1096-8628(19960216)67:1<98::AID-AJMG17>3.0.CO;2-K
6. Leschziner GD, Golding JF, Ferner RE. Sleep disturbance as part of the neurofibromatosis type 1 phenotype in adults. Am J Med Genet A. 2013;161A(6):1319–1322. doi:10.1002/ajmg.a.35915
7. Mbarek O, Marouillat S, Martineau J, Barthélémy C, Müh JP, Andres C. Association study of the NF1 gene and autistic disorder. Am J Med Genet. 1999;88(6):729–732. doi:10.1002/(SICI)1096-8628(19991215)88:6<729::AID-AJMG26>3.0.CO;2-Q
8. Johnson NS, Saal HM, Lovell AM, Schorry EK. Social and emotional problems in children with neurofibromatosis type 1: evidence and proposed interventions. J Pediatr. 1999;134(6):767–772. doi:10.1016/s0022-3476(99)70296-9
9. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6(3):185–194. doi:10.1076/chin.6.3.185.3155
10. Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65(7):1037–1044. doi:10.1212/01.wnl.0000179303.72345.ce
11. Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108(1):193–198. doi:10.1038/bjc.2012.535
12. Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34(17):1978–1986. doi:10.1200/JCO.2015.65.3576
13. Landry JP, Schertz KL, Chiang YJ, et al. Comparison of cancer prevalence in patients with neurofibromatosis type 1 at an academic cancer center vs in the general population from 1985 to 2020. JAMA Netw Open. 2021;4(3):e210945. doi:10.1001/jamanetworkopen.2021.0945
14. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi:10.1093/neuonc/noab106
15. Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114(5):788–792. doi:10.1016/s0022-3476(89)80137-4
16. Lund AM, Skovby F. Optic gliomas in children with neurofibromatosis type 1. Eur J Pediatr. 1991;150(12):835–838. doi:10.1007/BF01955002
17. Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. doi:10.1016/s0022-3476(94)70122-9
18. Czyzyk E, Jóźwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18(7):471–478. doi:10.1177/08830738030180070401
19. Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. doi:10.1093/neuonc/nos076
20. Mehlan J, Schüttauf F, Salamon JM, Kordes U, Friedrich RE, Mautner VF. Manifestations and treatment of adult-onset symptomatic optic pathway glioma in neurofibromatosis type 1. Anticancer Res. 2019;39(2):827–831. doi:10.21873/anticanres.13181
21. Lee AG, Dutton J. A practice pathway for the management of gliomas of the anterior visual pathway: an update and an evidence-based approach. Neuro-Ophthalmol. 1999;22:139–155. doi:10.1076/noph.22.3.139.3722
22. Ahn Y, Cho BK, Kim SK, et al. Optic pathway glioma: outcome and prognostic factors in a surgical series. Childs Nerv Syst. 2006;22(9):1136–1142. doi:10.1007/s00381-006-0086-7
23. Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. doi:10.1002/ana.21107
24. Liao C, Zhang H, Liu Z, et al. The visual acuity outcome and relevant factors affecting visual improvement in pediatric sporadic chiasmatic-hypothalamic glioma patients who received surgery [published correction appears in Front Neurol. 2022 May 17;13:914268]. Front Neurol. 2020;11:766. doi:10.3389/fneur.2020.00766
25. Hill CS, Khan M, Phipps K, Green K, Hargrave D, Aquilina K. Neurosurgical experience of managing optic pathway gliomas. Childs Nerv Syst. 2021;37(6):1917–1929. doi:10.1007/s00381-021-05060-8
26. Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. doi:10.1200/JCO.2005.03.8349
27. Cappelli C, Grill J, Raquin M, et al. Long-term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. 1998;79(4):334–338. doi:10.1136/adc.79.4.334
28. Tsang DS, Murphy ES, Merchant TE. Radiation therapy for optic pathway and hypothalamic low-grade gliomas in children. Int J Radiat Oncol Biol Phys. 2017;99(3):642–651. doi:10.1016/j.ijrobp.2017.07.023
29. Rakotonjanahary J, Gravier N, Lambron J, et al. Long-term visual acuity in patients with optic pathway glioma treated during childhood with up-front BB-SFOP chemotherapy-analysis of a French pediatric historical cohort. PLoS One. 2019;14(3):e0212107. doi:10.1371/journal.pone.0212107
30. Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol. 2014;116(2):341–347. doi:10.1007/s11060-013-1300-6
31. Kotch C, Avery R, Getz KD, et al. Risk factors for treatment-refractory and relapsed optic pathway glioma in children with neurofibromatosis type 1. Neuro Oncol. 2022;24(8):1377–1386. doi:10.1093/neuonc/noac013
32. Lewis RA, Gerson LP, Axelson KA, Riccardi VM, Whitford RP. von Recklinghausen neurofibromatosis. II. incidence of optic gliomata. Ophthalmology. 1984;91(8):929–935. doi:10.1016/s0161-6420(84)34217-8
33. Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 optic pathway glioma task force. Ann Neurol. 1997;41(2):143–149. doi:10.1002/ana.410410204
34. Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131(4):442–445. doi:10.1016/s0002-9394(00)00852-7
35. King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A(2):95–99. doi:10.1002/ajmg.a.20211
36. Listernick R, Ferner RE, Piersall L, Sharif S, Gutmann DH, Charrow J. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology. 2004;63(10):1944–1946. doi:10.1212/01.wnl.0000144341.16830.01
37. Friedrich RE, Nuding MA. Optic pathway glioma and cerebral focal abnormal signal intensity in patients with neurofibromatosis type 1: characteristics, treatment choices and follow-up in 134 affected individuals and a brief review of the literature. Anticancer Res. 2016;36(8):4095–4121.
38. Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28(4):262–270. doi:10.1016/s0887-8994(02)00628-8
39. Mandiwanza T, Kaliaperumal C, Khalil A, Sattar M, Crimmins D, Caird J. Suprasellar pilocytic astrocytoma: one national centre’s experience. Childs Nerv Syst. 2014;30(7):1243–1248. doi:10.1007/s00381-014-2374-y
40. Guillamo JS, Créange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–160. doi:10.1093/brain/awg016
41. Taylor T, Jaspan T, Milano G, et al. Radiological classification of optic pathway gliomas: experience of a modified functional classification system. Br J Radiol. 2008;81(970):761–766. doi:10.1259/bjr/65246351
42. Prada CE, Hufnagel RB, Hummel TR, et al. The use of magnetic resonance imaging screening for optic pathway gliomas in children with neurofibromatosis type 1. J Pediatr. 2015;167(4):851–856.e1. doi:10.1016/j.jpeds.2015.07.001
43. Grill J, Laithier V, Rodriguez D, Raquin MA, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159(9):692–696. doi:10.1007/s004310000531
44. Parness-Yossifon R, Listernick R, Charrow J, Barto H, Zeid JL. Strabismus in patients with neurofibromatosis type 1-associated optic pathway glioma. J AAPOS. 2015;19(5):422–425. doi:10.1016/j.jaapos.2015.06.003
45. Sani I, Albanese A. Endocrine long-term follow-up of children with neurofibromatosis type 1 and optic pathway glioma. Horm Res Paediatr. 2017;87(3):179–188. doi:10.1159/000458525
46. Santoro C, Perrotta S, Picariello S, et al. Pretreatment endocrine disorders due to optic pathway gliomas in pediatric neurofibromatosis type 1: multicenter study. J Clin Endocrinol Metab. 2020;105(6):dgaa138. doi:10.1210/clinem/dgaa138
47. Gil Margolis M, Yackobovitz-Gavan M, Toledano H, et al. Optic pathway glioma and endocrine disorders in patients with and without NF1. Pediatr Res. 2023;93(1):233–241. doi:10.1038/s41390-022-02098-5
48. Hutter S, Piro RM, Waszak SM, et al. No correlation between NF1 mutation position and risk of optic pathway glioma in 77 unrelated NF1 patients. Hum Genet. 2016;135(5):469–475. doi:10.1007/s00439-016-1646-x
49. Melloni G, Eoli M, Cesaretti C, et al. Risk of optic pathway glioma in neurofibromatosis type 1: no evidence of genotype-phenotype correlations in a large independent cohort. Cancers. 2019;11(12):1838. doi:10.3390/cancers11121838
50. Sharif S, Upadhyaya M, Ferner R, et al. A molecular analysis of individuals with neurofibromatosis type 1 (NF1) and optic pathway gliomas (OPGs), and an assessment of genotype-phenotype correlations. J Med Genet. 2011;48(4):256–260. doi:10.1136/jmg.2010.081760
51. Bolcekova A, Nemethova M, Zatkova A, et al. Clustering of mutations in the 5’ tertile of the NF1 gene in Slovakia patients with optic pathway glioma. Neoplasma. 2013;60(6):655–665. doi:10.4149/neo_2013_084
52. Anastasaki C, Morris SM, Gao F, Gutmann DH. Children with 5’-end NF1 gene mutations are more likely to have glioma. Neurol Genet. 2017;3(5):e192. doi:10.1212/NXG.0000000000000192
53. Xu M, Xiong H, Han Y, et al. Identification of mutation regions on NF1 responsible for high- and low-risk development of optic pathway glioma in neurofibromatosis type I. Front Genet. 2018;9:270. doi:10.3389/fgene.2018.00270
54. Upadhyaya M, Huson SM, Davies M, et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet. 2007;80(1):140–151. doi:10.1086/510781
55. Pinna V, Lanari V, Daniele P, et al. p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur J Hum Genet. 2015;23(8):1068–1071. doi:10.1038/ejhg.2014.243
56. Santoro C, Maietta A, Giugliano T, et al. Arg(1809) substitution in neurofibromin: further evidence of a genotype-phenotype correlation in neurofibromatosis type 1. Eur J Hum Genet. 2015;23(11):1460–1461. doi:10.1038/ejhg.2015.93
57. Rojnueangnit K, Xie J, Gomes A, et al. High incidence of Noonan syndrome features including short stature and pulmonic stenosis in patients carrying NF1 missense mutations affecting p.Arg1809: genotype-phenotype correlation. Hum Mutat. 2015;36(11):1052–1063. doi:10.1002/humu.22832
58. Koczkowska M, Chen Y, Callens T, et al. Genotype-phenotype correlation in NF1: evidence for a more severe phenotype associated with missense mutations affecting NF1 codons 844–848. Am J Hum Genet. 2018;102(1):69–87. doi:10.1016/j.ajhg.2017.12.001
59. Abadin SS, Zoellner NL, Schaeffer M, Porcelli B, Gutmann DH, Johnson KJ. Racial/ethnic differences in pediatric brain tumor diagnoses in patients with neurofibromatosis type 1. J Pediatr. 2015;167(3):613–20.e202. doi:10.1016/j.jpeds.2015.04.076
60. Johnson KJ, Zoellner NL, Gutmann DH. Peri-gestational risk factors for pediatric brain tumors in neurofibromatosis type 1. Cancer Epidemiol. 2016;42:53–59. doi:10.1016/j.canep.2016.03.005
61. Porcelli B, Zoellner NL, Abadin SS, Gutmann DH, Johnson KJ. Associations between allergic conditions and pediatric brain tumors in neurofibromatosis type 1. Fam Cancer. 2016;15(2):301–308. doi:10.1007/s10689-015-9855-3
62. Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111(3):568–577. doi:10.1016/j.ophtha.2003.06.008
63. Dodgshun AJ, Elder JE, Hansford JR, Sullivan MJ. Long-term visual outcome after chemotherapy for optic pathway glioma in children: site and age are strongly predictive. Cancer. 2015;121(23):4190–4196. doi:10.1002/cncr.29649
64. Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014;75(2):309–316. doi:10.1002/ana.24093
65. Fisher MJ, Loguidice M, Gutmann DH, et al. Gender as a disease modifier in neurofibromatosis type 1 optic pathway glioma. Ann Neurol. 2014;75(5):799–800. doi:10.1002/ana.24157
66. Hersh JH; American Academy of Pediatrics Committee on Genetics. Health supervision for children with neurofibromatosis. Pediatrics. 2008;121(3):633–642. doi:10.1542/peds.2007-3364
67. de Blank PMK, Fisher MJ, Liu GT, et al. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol. 2017;37(Suppl 1):S23–S32. doi:10.1097/WNO.0000000000000550
68. Varan A, Batu A, Cila A, et al. Optic glioma in children: a retrospective analysis of 101 cases. Am J Clin Oncol. 2013;36(3):287–292. doi:10.1097/COC.0b013e3182467efa
69. Kinori M, Armarnik S, Listernick R, Charrow J, Zeid JL. Neurofibromatosis type 1-associated optic pathway glioma in children: a follow-up of 10 years or more. Am J Ophthalmol. 2021;221:91–96. doi:10.1016/j.ajo.2020.03.053
70. Bergqvist C, Servy A, Valeyrie-Allanore L, Ferkal S, Combemale P, Wolkenstein P. NF France network. neurofibromatosis 1 french national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. 2020;15(1):37. PMID: 32014052; PMCID: PMC6998847. doi:10.1186/s13023-020-1310-3
71. Packer RJ, Iavarone A, Jones DTW, et al. Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference. Neuro Oncol. 2020;22(6):773–784. doi:10.1093/neuonc/noaa036
72. Bjur KA, Payne ET, Nemergut ME, Hu D, Flick RP. Anesthetic-related neurotoxicity and neuroimaging in children: a call for conversation. J Child Neurol. 2017;32(6):594–602. doi:10.1177/0883073817691696
73. Salerno S, Granata C, Trapenese M, et al. Is MRI imaging in pediatric age totally safe? A critical reprisal. Radiol Med. 2018;123(9):695–702. doi:10.1007/s11547-018-0896-1
74. Schmandt SM, Packer RJ, Vezina LG, Jane J. Spontaneous regression of low-grade astrocytomas in childhood. Pediatr Neurosurg. 2000;32(3):132–136. doi:10.1159/000028917
75. Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119(4):516–529. doi:10.1001/archopht.119.4.516
76. Lama G, Esposito Salsano M, Grassia C, et al. Neurofibromatosis type 1 and optic pathway glioma. A long-term follow-up. Minerva Pediatr. 2007;59(1):13–21.
77. North K, Cochineas C, Tang E, Fagan E. Optic gliomas in neurofibromatosis type 1: role of visual evoked potentials. Pediatr Neurol. 1994;10(2):117–123. doi:10.1016/0887-8994(94)90043-4
78. Falsini B, Ziccardi L, Lazzareschi I, et al. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol. 2008;88(1):87–96. doi:10.1007/s11060-008-9537-1
79. Kelly JP, Weiss AH. Detection of tumor progression in optic pathway glioma with and without neurofibromatosis type 1. Neuro Oncol. 2013;15(11):1560–1567. doi:10.1093/neuonc/not120
80. Bowman R, Walters B, Smith V, et al. Visual outcomes and predictors in optic pathway glioma: a single centre study [published online ahead of print, 2022 May 13]. Eye. 2022. doi:10.1038/s41433-022-02096-1
81. Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–9.e2. doi:10.1016/j.ajo.2010.08.046
82. Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014;55(3):1402–1408. doi:10.1167/iovs.13-13119
83. Avery RA, Cnaan A, Schuman JS, et al. Longitudinal change of circumpapillary retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2015;160(5):944–952.e1. doi:10.1016/j.ajo.2015.07.036
84. Parrozzani R, Clementi M, Kotsafti O, et al. Optical coherence tomography in the diagnosis of optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54(13):8112–8118. doi:10.1167/iovs.13-13093
85. Barkana Y, Burgansky-Eliash Z, Gerber Y, et al. Inter-device variability of the stratus optical coherence tomography. Am J Ophthalmol. 2009;147(2):260–266. doi:10.1016/j.ajo.2008.08.008
86. Yang H, Lee HS, Bae HW, Seong GJ, Kim CY, Lee SY. Effect of image quality fluctuations on the repeatability of thickness measurements in swept-source optical coherence tomography. Sci Rep. 2020;10(1):13897. doi:10.1038/s41598-020-70852-y
87. Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–S24. doi:10.1212/01.wnl.0000435745.95155.b8
88. Astrup J. Natural history and clinical management of optic pathway glioma. Br J Neurosurg. 2003;17(4):327–335. doi:10.1080/02688690310001601216
89. Doganis D, Pourtsidis A, Tsakiris K, et al. Optic pathway glioma in children: 10 years of experience in a single institution. Pediatr Hematol Oncol. 2016;33(2):102–108. doi:10.3109/08880018.2016.1155101
90. Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi:10.3171/jns.1997.86.5.0747
91. Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. doi:10.1002/cncr.29987
92. Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103(12):2636–2642. doi:10.1002/cncr.21091
93. Cappellano AM, Petrilli AS, da Silva NS, et al. Single agent vinorelbine in pediatric patients with progressive optic pathway glioma. J Neurooncol. 2015;121(2):405–412. doi:10.1007/s11060-014-1652-6
94. Gururangan S, Fisher MJ, Allen JC, et al. Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9(2):161–168. doi:10.1215/15228517-2006-030
95. Okada K, Yamasaki K, Tanaka C, Fujisaki H, Osugi Y, Hara J. Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Jpn J Clin Oncol. 2013;43(11):1073–1079. doi:10.1093/jjco/hyt124
96. Zhukova N, Rajagopal R, Lam A, et al. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019;8(1):40–50. doi:10.1002/cam4.1799
97. Green K, Panagopoulou P, D’Arco F, et al. A nationwide evaluation of bevacizumab-based treatments in paediatric low-grade glioma in the UK: safety. Efficacy, visual morbidity and outcomes [published online ahead of print, 2022 Oct 14]. Neuro Oncol. 2022:noac223. doi:10.1093/neuonc/noac223
98. Maertens O, Prenen H, Debiec-Rychter M, et al. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet. 2006;15(6):1015–1023. doi:10.1093/hmg/ddl016
99. Brems H, Park C, Maertens O, et al. Glomus tumors in neurofibromatosis type 1: genetic, functional, and clinical evidence of a novel association [published correction appears in Cancer Res. 2009 Oct 15;69(20):8216. Messia, Ludwine [corrected to Messiaen, Ludwine]]. Cancer Res. 2009;69(18):7393–7401. doi:10.1158/0008-5472.CAN-09-1752
100. Laycock-van Spyk S, Thomas N, Cooper DN, Upadhyaya M. Neurofibromatosis type 1-associated tumours: their somatic mutational spectrum and pathogenesis. Hum Genomics. 2011;5(6):623–690. doi:10.1186/1479-7364-5-6-623
101. Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–823. doi:10.1073/pnas.68.4.820
102. Wang LH, Wu CF, Rajasekaran N, Shin YK. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem. 2018;51(6):2647–2693. doi:10.1159/000495956
103. Kluwe L, Hagel C, Tatagiba M, et al. Loss of NF1 alleles distinguish sporadic from NF1-associated pilocytic astrocytomas. J Neuropathol Exp Neurol. 2001;60(9):917–920. doi:10.1093/jnen/60.9.917
104. Gutmann DH, James CD, Poyhonen M, et al. Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology. 2003;61(10):1397–1400. doi:10.1212/wnl.61.10.1397
105. Gutmann DH, McLellan MD, Hussain I, et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23(3):431–439. doi:10.1101/gr.142604.112
106. Gutmann DH, Donahoe J, Brown T, James CD, Perry A. Loss of neurofibromatosis 1 (NF1) gene expression in NF1-associated pilocytic astrocytomas. Neuropathol Appl Neurobiol. 2000;26(4):361–367. doi:10.1046/j.1365-2990.2000.00258.x
107. Sherekar M, Han SW, Ghirlando R, et al. Biochemical and structural analyses reveal that the tumor suppressor neurofibromin (NF1) forms a high-affinity dimer. J Biol Chem. 2020;295(4):1105–1119. doi:10.1074/jbc.RA119.010934
108. Young LC, Goldstein de Salazar R, Han SW, et al. Destabilizing NF1 variants act in a dominant negative manner through neurofibromin dimerization. Proc Natl Acad Sci U S A. 2023;120(5):e2208960120. doi:10.1073/pnas.2208960120
109. Bollag G, McCormick F, Clark R. Characterization of full-length neurofibromin: tubulin inhibits Ras GAP activity. EMBO J. 1993;12(5):1923–1927. doi:10.1002/j.1460-2075.1993.tb05841.x
110. Ballester R, Marchuk D, Boguski M, et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63(4):851–859. doi:10.1016/0092-8674(90)90151-4
111. Xu GF, Lin B, Tanaka K, et al. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990;63(4):835–841. doi:10.1016/0092-8674(90)90149-9
112. Gutmann DH, Loehr A, Zhang Y, Kim J, Henkemeyer M, Cashen A. Haploinsufficiency for the neurofibromatosis 1 (NF1) tumor suppressor results in increased astrocyte proliferation. Oncogene. 1999;18(31):4450–4459. doi:10.1038/sj.onc.1202829
113. Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–2760. doi:10.1158/0008-5472.CAN-04-4058
114. Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR [published correction appears in Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16119]. Proc Natl Acad Sci U S A. 2005;102(24):8573–8578. doi:10.1073/pnas.0503224102
115. Lau N, Feldkamp MM, Roncari L, et al. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. J Neuropathol Exp Neurol. 2000;59(9):759–767. doi:10.1093/jnen/59.9.759
116. Fisher MJ, Jones DTW, Li Y, et al. Integrated molecular and clinical analysis of low-grade gliomas in children with neurofibromatosis type 1 (NF1). Acta Neuropathol. 2021;141(4):605–617. doi:10.1007/s00401-021-02276-5
117. Zhu Y, Romero MI, Ghosh P, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–876. doi:10.1101/gad.862101
118. Bajenaru ML, Hernandez MR, Perry A, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63(24):8573–8577.
119. Isakson SH, Rizzardi AE, Coutts AW, et al. Genetically engineered minipigs model the major clinical features of human neurofibromatosis type 1. Commun Biol. 2018;1:158. doi:10.1038/s42003-018-0163-y
120. Zhu Y, Harada T, Liu L, et al. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132(24):5577–5588. doi:10.1242/dev.02162
121. Toonen JA, Ma Y, Gutmann DH. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction [published correction appears in Neuro Oncol. 2017 Jun 1;19(6):876–877]. Neuro Oncol. 2017;19(6):808–819. doi:10.1093/neuonc/now267
122. Hegedus B, Dasgupta B, Shin JE, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1(4):443–457. doi:10.1016/j.stem.2007.07.008
123. Solga AC, Toonen JA, Pan Y, et al. The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget. 2017;8(29):47206–47215. doi:10.18632/oncotarget.17589
124. Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24(20):2317–2329. doi:10.1101/gad.1957110
125. Lee DY, Gianino SM, Gutmann DH. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. doi:10.1016/j.ccr.2012.05.036
126. Ono K, Yasui Y, Rutishauser U, Miller RH. Focal ventricular origin and migration of oligodendrocyte precursors into the chick optic nerve. Neuron. 1997;19(2):283–292. doi:10.1016/s0896-6273(00)80939-3
127. Anastasaki C, Chatterjee J, Cobb O, et al. Human induced pluripotent stem cell engineering establishes a humanized mouse platform for pediatric low-grade glioma modeling. Acta Neuropathol Commun. 2022;10(1):120. doi:10.1186/s40478-022-01428-2
128. Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22(14):5100–5113. doi:10.1128/MCB.22.14.5100-5113.2002
129. Simmons GW, Pong WW, Emnett RJ, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. doi:10.1097/NEN.0b013e3182032d37
130. Toonen JA, Anastasaki C, Smithson LJ, et al. NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet. 2016;25(9):1703–1713. doi:10.1093/hmg/ddw039
131. Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro Oncol. 2019;21(10):1250–1262. doi:10.1093/neuonc/noz080
132. Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. doi:10.1093/hmg/ddm059
133. Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. doi:10.1002/ana.23813
134. Solga AC, Pong WW, Kim KY, et al. RNA sequencing of tumor-associated microglia reveals Ccl5 as a stromal chemokine critical for neurofibromatosis-1 glioma growth. Neoplasia. 2015;17(10):776–788. doi:10.1016/j.neo.2015.10.002
135. Pan Y, Xiong M, Chen R, et al. Athymic mice reveal a requirement for T-cell-microglia interactions in establishing a microenvironment supportive of Nf1 low-grade glioma growth. Genes Dev. 2018;32(7–8):491–496. doi:10.1101/gad.310797.117
136. Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. doi:10.1158/0008-5472.CAN-07-5916
137. de Andrade Costa A, Chatterjee J, Cobb O, et al. Immune deconvolution and temporal mapping identifies stromal targets and developmental intervals for abrogating murine low-grade optic glioma formation. Neurooncol Adv. 2021;4(1):vdab194. doi:10.1093/noajnl/vdab194
138. Chatterjee J, Sanapala S, Cobb O, et al. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat Commun. 2021;12(1):7122. doi:10.1038/s41467-021-27455-6
139. Brown JA, Gianino SM, Gutmann DH. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J Neurosci. 2010;30(16):5579–5589. doi:10.1523/JNEUROSCI.3994-09.2010
140. Pan Y, Hysinger JD, Barron T, et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature. 2021;594(7862):277–282. doi:10.1038/s41586-021-03580-6
141. Anastasaki C, Mo J, Chen JK, et al. Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nat Commun. 2022;13(1):2785. doi:10.1038/s41467-022-30466-6
142. Anastasaki C, Woo AS, Messiaen LM, Gutmann DH. Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning. Hum Mol Genet. 2015;24(12):3518–3528. doi:10.1093/hmg/ddv103
143. Anastasaki C, Wegscheid ML, Hartigan K, et al. Human iPSC-derived neurons and cerebral organoids establish differential effects of germline NF1 gene mutations. Stem Cell Rep. 2020;14(4):541–550. doi:10.1016/j.stemcr.2020.03.007
144. Morris SM, Gupta A, Kim S, Foraker RE, Gutmann DH, Payne PRO. Predictive modeling for clinical features associated with neurofibromatosis type 1. Neurol Clin Pract. 2021;11(6):497–505. doi:10.1212/CPJ.0000000000001089
145. de Blank PM, Berman JI, Liu GT, Roberts TP, Fisher MJ. Fractional anisotropy of the optic radiations is associated with visual acuity loss in optic pathway gliomas of neurofibromatosis type 1. Neuro Oncol. 2013;15(8):1088–1095. doi:10.1093/neuonc/not068
146. Yeom KW, Lober RM, Andre JB, et al. Prognostic role for diffusion-weighted imaging of pediatric optic pathway glioma. J Neurooncol. 2013;113(3):479–483. doi:10.1007/s11060-013-1140-4
147. Hales PW, Smith V, O’Hare P, et al. In vivo assessment of tumour invasion of the visual pathway in optic pathway glioma patients using multi-shell diffusion tensor MRI.
148. de Blank P, Fisher MJ, Gittleman H, Barnholtz-Sloan JS, Badve C, Berman JI. Validation of an automated tractography method for the optic radiations as a biomarker of visual acuity in neurofibromatosis-associated optic pathway glioma. Exp Neurol. 2018;299(Pt B):308–316. doi:10.1016/j.expneurol.2017.06.004
149. Pajavand AM, Sharifi G, Anvari A, et al. Case report: chemotherapy indication in a case of neurofibromatosis type 1 presenting optic pathway glioma: a one-year clinical case study using differential tractography approach. Front Hum Neurosci. 2021;15:620439. doi:10.3389/fnhum.2021.620439
150. Avery RA, Mansoor A, Idrees R, et al. Optic pathway glioma volume predicts retinal axon degeneration in neurofibromatosis type 1. Neurology. 2016;87(23):2403–2407. doi:10.1212/WNL.0000000000003402
151. Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 2015;17(6):843–853. doi:10.1093/neuonc/nou329
152. Perreault S, Larouche V, Tabori U, et al. A Phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19(1):1250. doi:10.1186/s12885-019-6442-2
153. Selt F, van Tilburg CM, Bison B, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 2020;149(3):499–510. doi:10.1007/s11060-020-03640-3
154. Fangusaro J, Onar-Thomas A, Poussaint TY, et al. A Phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a pediatric brain tumor consortium study. Neuro Oncol. 2021;23(10):1777–1788. doi:10.1093/neuonc/noab047
155. Hanzlik E, Archambault B, El-Dairi M, et al. Use of trametinib in children and young adults with progressive low-grade glioma and glioneuronal tumors. J Pediatr Hematol Oncol. 2023;45(4):e464–e470. doi:10.1097/MPH.0000000000002598
156. Butowski N, Chang SM, Junck L, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05). J Neurooncol. 2009;91(2):175–182. doi:10.1007/s11060-008-9693-3
157. Rosenfeld MR, Chamberlain MC, Grossman SA, et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12(10):1071–1077. doi:10.1093/neuonc/noq071
158. Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi:10.1158/1078-0432.CCR-09-3106
159. Hartman LL, Crawford JR, Makale MT, et al. Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. J Pediatr Hematol Oncol. 2014;36(6):451–457. doi:10.1097/MPH.0000000000000047
160. Pollack IF, Jakacki RI, Butterfield LH, et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol. 2016;18(8):1157–1168. doi:10.1093/neuonc/now026
161. Falsini B, Chiaretti A, Rizzo D, et al. Nerve growth factor improves visual loss in childhood optic gliomas: a randomized, double-blind, phase II clinical trial. Brain. 2016;139(Pt 2):404–414. doi:10.1093/brain/awv366
162. Boczek T, Cameron EG, Yu W, et al. Regulation of neuronal survival and axon growth by a perinuclear cAMP compartment. J Neurosci. 2019;39(28):5466–5480. doi:10.1523/JNEUROSCI.2752-18.2019
163. Zhang KY, Tuffy C, Mertz JL, et al. Role of the internal limiting membrane in structural engraftment and topographic spacing of transplanted human stem cell-derived retinal ganglion cells. Stem Cell Rep. 2021;16(1):149–167. doi:10.1016/j.stemcr.2020.12.001
164. Luo Z, Chang KC, Wu S, et al. Directly induced human retinal ganglion cells mimic fetal RGCs and are neuroprotective after transplantation in vivo. Stem Cell Rep. 2022;17(12):2690–2703. doi:10.1016/j.stemcr.2022.10.011
165. Tian F, Cheng Y, Zhou S, et al. Core transcription programs controlling injury-induced neurodegeneration of retinal ganglion cells [published correction appears in Neuron. 2023 Feb 1;111(3):444]. Neuron. 2022;110(16):2607–2624.e8. doi:10.1016/j.neuron.2022.06.003
166. Ruiz-Ederra J, García M, Hernández M, et al. The pig eye as a novel model of glaucoma. Exp Eye Res. 2005;81(5):561–569. doi:10.1016/j.exer.2005.03.014
167. van Zyl T, Yan W, McAdams A, et al. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc Natl Acad Sci U S A. 2020;117(19):10339–10349. doi:10.1073/pnas.2001250117
168. Yu M, Sun X, Tyler SR, et al. Highly efficient transgenesis in ferrets using CRISPR/Cas9-mediated homology-independent insertion at the ROSA26 locus. Sci Rep. 2019;9(1):1971. doi:10.1038/s41598-018-37192-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.