Back to Journals » Research and Reports in Tropical Medicine » Volume 13
Neurocysticercosis: A Review into Treatment Options, Indications, and Their Efficacy
Authors Hamamoto Filho PT , Rodríguez-Rivas R , Fleury A
Received 1 November 2022
Accepted for publication 14 December 2022
Published 29 December 2022 Volume 2022:13 Pages 67—79
DOI https://doi.org/10.2147/RRTM.S375650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Pedro Tadao Hamamoto Filho,1 Roberto Rodríguez-Rivas,2 Agnès Fleury3,4
1Department of Neurology, Psychology and Psychiatry, Botucatu Medical School, UNESP –Universidad de Estadual Paulista, Botucatu, Brazil; 2Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suarez, Ciudad de México, México; 3Departamento de Medicina Genómica y Toxicología Ambiental, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Ciudad de México, México; 4Clínica de Neurocisticercosis, Instituto Nacional de Neurología Y Neurocirugía Manuel Velasco Suarez, Ciudad de México, México
Correspondence: Agnès Fleury, Insurgentes Sur 3877 CP 14269 Barrio La Fama, Tlalpan, Ciudad de México, México, Tel +52 5556063822, Email [email protected]
Abstract: Neurocysticercosis, due to the localization of Taenia solium larvae in the Central Nervous System, is a neglected tropical disease still endemic in much of Latin America, Asia and sub-Saharan Africa. The therapeutic management of NC has gradually improved with the establishment of neuroimaging studies (CT and MRI) in endemic countries and with the demonstration of the efficacy of albendazole and praziquantel in the 1980s. But the morbidity and mortality of this preventable disease remain an unacceptable fact. In this scoping review, we will revise the different treatment options and their indications.
Keywords: neglected tropical disease, neurocysticercosis, treatment, review, T. solium
Introduction
Neurocysticercosis (NC), the most common parasitic disease of the Central Nervous System (CNS), is a heterogeneous disease in which treatment options depend primarily on the location of the parasites.
Caused by ingestion of embryonated eggs of the helminth Taenia solium, this disease is a marker of poverty, clearly linked to an environment with poor excreta disposal and free-range pigs. Therefore, it is a public health problem in low- and middle-income countries (LMIC), such as in Latin America, Asia, and sub-Saharan Africa.
T. solium cysticercosis was added by World Health Organization (WHO) to the list of major Neglected Tropical Diseases (NTDs) in 2010 with NTD roadmap goals of making available a validated strategy for control and elimination of T. solium taeniasis/cysticercosis and those interventions to be scaled up in selected countries by 2020.1 Indeed, according to the global burden of disease atlas of 2019, neurocysticercosis was estimated to be the cause of 1.37 million Disability-adjusted life years (DALYs) around the globe.2 In Latin America and the Caribbean, it is considered the neglected tropical disease with the highest burden.3 Unfortunately, as its diagnosis require neuroimaging studies not available to all in endemic countries, precise data on its epidemiology are lacking. In some countries, recent data shows a clear decrease in its incidence, although, in others, the activity of infection seems to be still very present.4–8
Life Cycle
The natural life cycle of the T. solium involves the pigs and the humans as hosts. Pigs become intermediate hosts by eating embryonated eggs (or gravid proglottids) from human feces in places where feces disposal is deficient. The parasite then hatches and invades the intestinal wall, enters the bloodstream and migrates to multiple tissues, such as the striatal muscle, where they become cysticerci. After human consumption of contaminated undercooked pork meat, the cysticerci hatch in the small intestine. At that moment, the tapeworm attaches itself to the intestinal wall through its scolex, a jaw-like organ bearing suckers and hooks.4
Humans can also become intermediate hosts by eating vegetables/fruits or drinking water contaminated by T. solium eggs or by human-to-human contagion. The same scenario as in the case of pork will occur, and the most affected organs will be the striatal muscle, subcutaneous tissues, central nervous system, and eyes. When located in the CNS, the disease is named neurocysticercosis.
Evolution of the Disease
Once in the CNS, the cysticerci can stay viable in their vesicular form for years or even decades if the host’s immune system tolerates them. This latency period, generally asymptomatic, seems to be much longer when parasites are in the extraparenchymal compartment than when they are inside the parenchyma.9 However, when the host’s inflammatory response begins, the cysticerci undergo different stages of involution. The first is the colloidal stage, where the inflammatory reaction around the cyst is visible and where the inner fluid turns turbid (granular stage). Gradually, the cysticerci calcify (calcified stage) or disappear, the inflammatory response diminishes and is sometimes replaced by gliotic changes. Externalization of cysticerci’s antigens by the remodeling of calcified lesions may produce perilesional edema, possibly associated with clinical manifestations.10
Clinic
NC is a highly heterogeneous disease, with its manifestation mainly depending on the localization of the parasite ranging from headache to epilepsy or hydrocephalus.11 It is also important to note that NC is frequently asymptomatic, as computed tomography (CT)-scan studies made in endemic communities have demonstrated.11
Depending on the location, two presentations are possible. In the parenchymal location, epilepsy is the most common manifestation, but headache, focal deficits, and cognitive and psychiatric symptoms may also be present. Because the parenchymal location is the most common, epilepsy is the primary clinical manifestation of NC, occurring in 60–90% of patients.12,13 When cysts are located in the extraparenchymal compartment, the main symptom is intracranial hypertension, it will occur in around 70% of patients.11 In this location, stroke syndromes have been described in around 3% of the patients, mostly related to the initiation of treatment and its respective induced inflammatory response.14 Focal neurological signs can present multiple phenotypes in around 20% of patients with neurocysticercosis.15 The most common of them are motor symptoms due to pyramidal tract lesions, Nevertheless, sensory or language disturbances, such as involuntary movements, can also occur. These manifestations can be slowly progressive and related to parenchymal cysts or large subarachnoid cysts compressing brain parenchyma.15
Diagnosis
The gold standard of diagnosis remains neuroimaging studies (CT-scan, magnetic resonance imaging - MRI).13 And this probably remains the main problem, in terms of successful diagnosis and treatment, because of the difficulties for the general population in endemic countries to access these technologies. CT scan is superior to MRI for calcified NC (the main form) and is also very efficient for diagnosing parenchymal cysts.16 For extraparenchymal cysts, MRI, in particular 3D sequences, is clearly superior allowing good visualization of cysts.17
Serological diagnosis tests are an additional tool that can be of relevance to help, mainly when doubts exist in neuroimages. Detection of antibodies and antigens can be done, and different techniques have been used, currently principally enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunoelectrotransfer blot (EITB).18 The presence of specific antigens is a sign of a viable infection, while antibodies are still present when the infection is inactive.19
Based on the evidence described, different sets of diagnostic criteria and proposals for particular settings have been produced.12,20–22
Treatment Options
Medical Treatments
Antihelminthic (AH)
Although more than 30 years have passed since anthelmintics were first used, no new molecules have proven effective, and we continue to use the drugs approved for this purpose in the 1980s.
Albendazole (ABZ)
It is a broad-spectrum anthelmintic antiparasitic agent active against intestinal roundworms, lungworms and tapeworms. The study of ABZ for human medicine was initiated in 1979 and in 1987, Escobedo et al showed that the drug was effective in the treatment of NC.23 ABZ interferes with the glucose intake of parasites and depletes their glycogen stores. It also affects the production of adenosine triphosphate (ATP), which is the energy required for the survival of the helminth. It is poorly absorbed from the gastrointestinal tract, but after absorption it is rapidly converted in the liver in albendazole sulfoxide (ABZSO) that is the main active form of the drug and is distributed throughout the body.24 Administration of ABZ with a fatty meal significantly enhance its bioavailability, as its administration with grapefruit juice, whereas its administration with cimetidine decreases it. Administration of different antiepileptic drugs (phenytoin, carbamazepine, phenobarbital) with ABZ reduces the plasma concentration of ABZSO. On the other hand, when ABZ is administered simultaneously with dexamethasone, plasma levels of ABZSO increased significantly.25
The recommended doses to treat NC patients varied between 15 and 30 mg/kg/day, to be taken in 2 doses per day, during 8 to 10 days. 15 mg/kg/day is sufficient for parenchymal NC (Figure 1), while in case of extraparenchymal disease, 30 mg/kg/day has showed to be more efficient (Figures 2 and 3).26 It was demonstrated, in parenchymal and extraparenchymal locations, that treatment response is significantly associated with higher plasmatic concentrations of ABZSO. Main secondary effects are a transient increase of hepatic enzymes and reversible alopecia.27,28
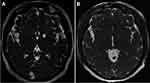 |
Figure 1 MRI-FIESTA sequences. Pre (A) and post ((B), 6 months after) cysticidal treatment of 3 cysts, one parenchymal, one located in a subarachnoid sulcus, and one in the Sylvian fissure. |
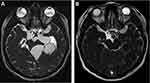 |
Figure 2 MRI-FIESTA sequences. Pre (A) and post ((B), 6 months after) cysticidal treatment of several racemose NC cysts located in basal subarachnoid cisterns. |
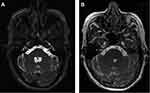 |
Figure 3 MRI-FIESTA axial sequences. Pre (A) and post ((B), 6 months after) cysticidal treatment of a NC cyst located in the fourth ventricle. |
Praziquantel (PZQ)
PZQ is mostly supplied in 600 mg tablets, and it has been used for human cysticercosis treatment since 1979.29 Although still not completely understood for the larvae, PZQ in adult worms causes a rapid muscular contraction and tegumental disruption that leads to the exposition of parasite antigens on the worm surface.24 PZQ is rapidly absorbed with a high inter-individual variation, and plasma peak concentration is reached 1.5−2h after administration. Administration of PZQ with a high carbohydrate meal enhances its bioavailability, and drugs that inhibit cytochrome P450 increase its plasma levels. Dexamethasone and different antiepileptic drugs (phenytoin, carbamazepine, phenobarbital, and primidone) reduce PZQ plasma levels. The recommended dose to treat NC patients is 50 mg/kg/day for 10 to 15 days.
Anti-Inflammatory Drugs
It is becoming increasingly clear that the inflammatory response against parasites is one of the major pathological mechanisms of NC.30 Before the use of anti-inflammatory drugs, the prognosis of NC patients was poor. For example, in patients with EP-NC complicated by hydrocephalus and requiring ventriculoperitoneal shunt (VPS) placement, the mortality rate was approximately 50%, with most deaths occurring within the first 2 years after placement.31 Similarly, in the early days of anthelmintic drug use, several reports highlighted the occurrence of serious adverse effects due to an exacerbated inflammatory response.32–34 The first publication showing the value of corticosteroids in NC patients was published in 1982.35 In 1996, the benefit of corticosteroids in extraparenchymal (EP)-NC patients with VPS was demonstrated,36 and currently corticosteroids, in case of anthelmintic administration or severe inflammation, are recommended in almost all types of NC.37 Because of the potential severity of inflammatory complications in patients with EP-NC (arachnoiditis, vasculitis, and secondary stroke), doses are generally higher than for parenchymal localization. It is relevant to mention that the possible dual role of corticosteroids on symptom control and parasite survival has been raised due to their immunosuppressive effects that prevent an appropriate host response.38,39 Despite this, due to the severity of possible inflammatory complications and the lack of non-immunosuppressive options, their use is currently necessary because the advantages outweigh the disadvantages. However, further research is needed to find other anti-inflammatory options and to define markers to identify which patients have a more inflammatory propensity to personalize treatment.38
In cases of corticosteroid resistance or severe adverse events due to their application, immunosuppressants, such as methotrexate, TNF-α inhibitor, or azathioprine, have been proposed by other researchers.40,41 These reports are currently anecdotal, and further studies are needed to assess their utility.
Antiepileptic Drugs
Epilepsy is the most common symptom of NC, mainly when the location is in the parenchyma.11 Studies in the late 20th or early 21st century showed that NC was the leading cause of late-onset epilepsy in endemic countries.36,42,43 More recent studies seem to show a decrease in its importance, although more information is needed to confirm these results.44,45
Epilepsy seems well controlled by antiepileptic drugs (AE), although studies focusing on this point and precise estimations are lacking. It is important to remember that since endemic countries are poor countries, the treatment gap (lack or irregularity of treatment, insufficient dosage) due to economic factors is high. This is a crucial aspect to consider when evaluating treatment efficacy.46 One study in Brazil evaluating causes of intractable epilepsy in 512 patients found calcified cysticerci lesions in 27% of patients but only in 1.56%, this image was the unique neuroimaging feature. In the other cases, calcified NC was associated with other lesions, principally mesial temporal sclerosis.47 The authors conclude that NC is rarely the cause of intractable epilepsy. However, the question of a causal link between mesial temporal sclerosis with NC exists, and further evidence is needed to support these conclusions.48
The most used drugs are carbamazepine, phenytoin, phenobarbital and valproic acid. There is no evidence that treatment should be different from other epilepsy causes. One study asserts no difference in efficacy between carbamazepine and levetiracetam,49 although some methodological aspects of this study were raised after its publication.50 A recent study in children with solitary viable parenchymal cysts showed similar efficacy with fewer adverse effects of lacosamide compared to oxcarbazepine.51 Frequently the main argument for deciding which drug to use should be the availability and cost of treatment.
Surgical Treatments
The surgical management of NC has evolved in the last two decades in terms of indication and microsurgical techniques. Previously, many patients with increased intracranial pressure (IPC) were managed surgically, irrespective of the location of the parasites, especially in cases of a pseudotumoral form of NC. The prognosis was poor, with high rates of morbidity and mortality.52 The change of the clinical and epidemiologic pictures of NC,53 with a lower proportion of cases with giant parenchymal cysts and cysticercosis encephalitis, changed the surgical indications of NC as well.
According to the last guidelines for the diagnosis and treatment of NC, currently, surgical treatment is mainly reserved for intraventricular cysts (strength of recommendation strong, level of confidence moderate). However, there are no published high-quality trials supporting that surgery is superior to medical treatment in this location.54 The main advantage of surgical removal of the cysts is that their complete removal dismisses the need for post-operative cysticidal treatment and sometimes, the need for permanent ventricle-peritoneal shunts for hydrocephalus.55,56
Classical Extraction
Nowadays, the surgical role for parenchymal cysts is of exception, reserved for cysts with a significant mass effect, since most patients can be safely and effectively managed with anthelmintics and anti-inflammatories. Besides, the surgical routes for some locations may add neurological deficits in case of damage to the normal tissue.
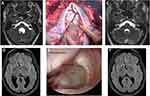
Cysts located in the ventricular system and the basal subarachnoid space could eventually be removed using classical neurosurgical approaches as long as the cysts do not have inflammatory reactions around them and are free-floating (Figure 4A–C). Therefore, optimal pre-operative imaging is necessary to support the surgical removal of cysts, ie, a 3-dimensional acquisition to check the precise location of the cysts and gadolinium enhancement acquisitions to check any inflammatory reactions surrounding the cysts.17 Besides, an excellent microsurgical armamentarium is ideal: a surgical microscope with depth of view control, micro scissors, micro dissectors, and bipolar forceps. The lack of adequate pre-operative workup and surgical tools may add risk for the surgical treatment, and it must be prudent, especially in low-income settings.
For spinal NC, microsurgical removal of cysts plays an important role when there are signs of spinal compression leading to neurological deficits. A recent systematic review on the treatment of spinal NC suggested that the combination of surgical to medical treatment provides superior outcomes than surgery or medical treatment alone.57
Endoscopic Surgery
The use of neuro-endoscopes added a significant improvement to the management of several diseases, including NC. This is a minimally invasive surgery in which a once burr-hole gives access to most parts of the ventricles, allowing for cysts removal and treatment of hydrocephalus with a third ventriculostomy (Figure 4D–F).
There are several well-illustrated case series demonstrating the benefits and efficacy of endoscopic surgery for cyst removal.58,59 In cases of free-floating cysts in the lateral ventricles, continuous irrigation allows for recovery of the cysts, which would be hardly achieved with conventional craniotomy approaches.
For endoscopic surgery, two major concerns should be considered: the risk of bleeding and manipulation of the cerebral aqueduct. Despite the low risk of bleeding with well-experienced surgeons, it must be remembered that hemostasis is more difficult in endoscopic surgery than in traditional microsurgery, especially in cases of large-vessels damage. And regarding the manipulation of the cerebral aqueduct, it may occur during attempts to reach the fourth ventricle through this thin work channel, posing the risk of post-operative ocular abnormalities and coma. This risk is significantly reduced by using flexible endoscopes.60
Surgery of Epilepsy
As stated before, pharmacological treatment of seizures in NC is the first choice, and most patients seem to have a good response in terms of seizure control if AE treatment is taken correctly. Besides, antiparasitic treatment of NC also has a satisfactory long-term effect on seizure evolution.61 Therefore, the role of surgery must be reserved for cases refractory to medical treatment.
Surgery for NC-related epilepsy can be classified as lesionectomy and mesial temporal lobe surgery. Lesionectomy is the resection of the epileptogenic lesion (which may be simply a scar tissue from previous destroyed cyst) and it should be performed only after extensive pre-operative investigation of the epileptogenic zone and, preferably, with the aid of intraoperative electrocorticography for accurate resection of the area with abnormal discharges.62
Regarding mesial temporal lobe surgery, there is a current extensive discussion in the literature on the relationship between NC and mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS). Many authors argue that this association is merely a casual relationship, while others point to causal factors determining MTLE-HS in patients with NC.48,63 Besides, MTLE-HS can be a differential diagnosis in patients with seizures non-concordant with NC localization. Despite this discussion, it seems clear that mesial temporal lobe surgery is an effective option for drug-resistant seizures, providing long-term seizure control and cessation of anti-epileptic drugs. Surgical possibilities include anterior temporal lobectomy and selective amygdalohippocampectomy, and the option may take in consideration the location of the NC lesion in the temporal lobe as well as the surgeon’s experience.63,64
Ventricle-Peritoneal Shunts (VPS) Placement
Hydrocephalus is one of the most serious complications of NC and may be present in up to 70% of the patients with EP-NC.11 It is also the most common cause of raised intracranial pressure among patients with NC. Inflammation and mechanical obstruction are the two main mechanisms responsible for developing hydrocephalus in NC. Inflammation causes epididymitis and arachnoiditis that compromise the cerebrospinal fluid (CSF) flow in the ventricles and the subarachnoid space. Mechanic obstruction occurs either by direct cyst entrapment within the narrowest points of the CSF flow or due to inflammatory scars in the ventricular system.30,31 It is also possible that an increase in the CSF viscosity related to high protein levels and obstruction of the arachnoid villi contribute to the development of hydrocephalus. Therefore, if cyst removal is enough to restore the CSF flow by unblocking the ventricular system, no further interventions are needed.
However, in many cases, inflammatory reactions remain within the CSF compartments, even without viable cysts. In these cases, a VPS is needed to alleviate the increased ICP and its related symptoms (ie, headache, nausea, vomiting, blurred vision) (Figure 5). It is relevant to note that some report points to the fact that VPS in NC is at higher risk of infection and malfunction, and mortality in patients with NC-induced hydrocephalus is higher and directly related to the number of VPS revisions.65 It has been proposed that post-shunt treatment with Albendazole reduces the risk of further shunt malfunction.66 However, it is possible that the role of Albendazole in these cases is related to the elimination of remaining cysts and, therefore, the resolution of the cause of hydrocephalus.
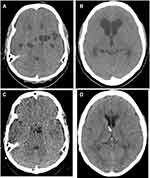 |
Figure 5 CT-scans of a patient with subarachnoid cysts and hydrocephalus. Before (A and B) and after (C and D) placement of a VPS and administration of cysticidal treatment. |
Endoscopic Third Ventriculostomy (ETV)
Besides the removal of free-floating cysts inside the ventricular systems, endoscopic approaches are also helpful in treating obstructive hydrocephalus. The inflammatory reactions targeted to eliminate the cysts may cause epididymitis that obstructs the narrowest points of CSF flow, such as the cerebral aqueduct, causing hydrocephalus. Therefore, ETV provides an alternative route for the CSF flow: directly from the third ventricle towards the subarachnoid space (Figure 6).58 Such as in other intraventricular diseases, it is recommended that the approach to intraventricular lesions includes an ETV, providing an internal CSF diversion. The combination of cyst removal and ETV is a less invasive procedure than conventional craniotomies and avoids shunt placement with all related complications.67
 |
Figure 6 MRI-FLAIR sequences [before (A), after (C)] and intraoperative view (B) of a patient in whom a third endoscopic ventriculostomy was performed to treat hydrocephalus. |
Besides ETV, endoscopic approaches can also be helpful to treat isolated hydrocephalus of a single lateral ventricle with septostomy and dilatation of the foramen of Monro using foraminotomy.68
Treatment of Parenchymal Cysticercosis
Parenchyma is the more frequent location for NC. Although several factors are probably involved in differences in prevalence between endemic countries (ie, infection pressure), this location seems to exist between 70% of the patients.9 As said before, this form is the more benign, as symptoms (mainly epilepsy) are controlled by AH drugs.
Treatment with anthelminthic drugs is now largely accepted in the case of symptomatic vesicular parenchymal cysts, as shown in the last guidelines (strength of recommendation strong, level of confidence moderate).37 Doses of ALB and PZQ are those mentioned before, and their use is significantly associated with complete radiological resolution (RR 2.52, 95% CI 1.65 to 3.85).69 Two controlled studies performed by the same research group showed that the combination of both drugs (ABZ 15 mg/kg/day and PZQ 50 mg/kg/day for ten days) was significantly more efficient than ABZ alone in patients with three or more cysts.70,71 This increased efficacy seems to be related to a significant increase in albendazole sulfoxide plasmatic concentration.72 Corticosteroids are recommended one day before and during the anthelminthic treatment, although optimal doses and duration are unknown, as they will depend on the number, size, and location of parasites.37 The addition of AE drugs in case of epilepsy is necessary. Still, the need for prophylactic AE treatment in addition to AH without previous seizures is not demonstrated.73 Particular attention must be provided if multiple (>10) cysts are present. In these cases, hospitalization of the patient and high doses of corticosteroids is necessarily associated with anthelminthic drugs. No information comparing therapeutic schemes is available, but it is possible to avoid a generalized inflammatory response with several cycles of lower doses of anthelminthic instead of a single cycle with high doses.12
The treatment of asymptomatic vesicular cysts is controversial because the evidence for specific management is lacking. The fact that calcified parasites are frequently diagnosed in asymptomatic patients without a history of seizures or other neurological symptoms shows that the process of parasite involution is frequently asymptomatic. However, it is also clear that this process, occurring without anti-inflammatory treatment, can generate seizures. To treat or not to treat, both attitudes are understandable; however, if an observation strategy is chosen, radiological follow-up every 6 months is recommended.
Regarding degenerating (colloidal) cysts, recent guidelines recommend anthelminthic treatment (Strength of this recommendation: weak. Level of confidence: moderate).37 In these cases, degeneration and resolution of the lesions is in process, and the parasites are metabolically inactive and with no evidence of antigen production. As discussed in a recent meta-analysis, evidence still needs to be provided regarding what attitude is the best.74 If anthelminthic treatment is chosen, ABZ and PZQ are recommended at the same doses as vesicular forms. Two Indian studies evaluate the combination of both drugs. Results are contrasting since, compared with ABZ alone, the first study showed a non-significant increase in efficacy, while the second found a significant increase in efficacy. AE treatment is recommended in patients with seizures and might be withdrawn 6 months after the last seizure if resolution without calcification occurs. Corticosteroids are also recommended; their use seems to have a beneficial effect, both on seizure reduction and cyst resolution.74–76
A particular case is the presence of multiple degenerating parasites (cysticercosis encephalitis). In this case, anthelminthic is contraindicated in the acute phase, where measures to decrease intracranial hypertension will be the only ones to be performed.12
Calcified cysts required only symptomatic treatment, mainly AE. Duration of treatment is still not demonstrated, but it might be at least 2 years after the last seizure, although evidence is scarce. In inflammatory reactions around calcification, it is not demonstrated that corticosteroid administration can improve symptomatology.37,73
As mentioned before, the place of surgery in these cases is now limited to large cysts with significant mass effect, and surgery for epilepsy in the cases where it is demonstrated that calcification (or MTLE-HS possibly linked to NC) represents the epileptic foci.
A flow-chart for treatment of parenchymal NC is shown in Figure 7.
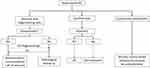 |
Figure 7 Treatment flow-chart for parenchymal NC. |
Treatment of Extraparenchymal Cysticercosis
This topic is probably the one that still has the most unknowns. Evidence for this observation is that in the last guidelines, on the 10 recommendations, evidence has low quality in 70% of them, and the strength is weak in 50% of them.37
However, several points are clear. Inflammation is the main pathogenic mechanism and should be treated adequately. As an important heterogeneity regarding the intensity of inflammatory reactions between patients exists, an evaluation before treatment is needed. We propose to perform a lumbar puncture before treatment (if no intracranial hypertension exists, or after its correction by VPS if exists) to evaluate cytochemical characteristics and an intracranial Doppler or an angio scan to evaluate presence of vasculitis. Angio scan might be preferred as false positive diagnosis of vasculitis due to mass effect of cyst on arteries could occur with intracranial Doppler. In case of vasculitis, anthelminthic treatment should be avoided as long as it persists. The use of corticosteroids is required in this case, but also, if no vasculitis is detected as anthelminthic will increase the inflammatory reaction. Doses might be high (0.4 mg/kg/day, beginning before anthelminthic and during all the treatment), and the velocity of tapering will depend on the intensity of the inflammatory reaction.
The efficacy of anthelminthic is lower than in the case of parenchymal cysts. The reasons are not completely clear, although the fact that parasites in this location lie in an acellular medium (cerebrospinal fluid) makes difficult the development of an efficient immune reaction necessary for the destruction of parasites. To increase the efficacity of anthelminthic, two strategies are used; to increase the doses; indeed, it was shown that the use of 30 mg/kg is significantly more efficient than the use of 15 mg/kg/day.26 The other strategy is to increase the period of administration of anthelmintics. Some authors argue that their administration should be continued until signs of degeneration are visible on imaging.77
Surgery for extraparenchymal cysticercosis depends on the localization of cysts. For the basal subarachnoid location, surgery poses considerable risk of injury to cranial nerves, large vessels, and the brain parenchyma. As mentioned before, a preoperative MRI is important to assess signals of active inflammation, which prevents surgical removal of cysts. Surgery should be reserved for cases with raised ICP or compression of structures with risk of permanent deficits (such as optic nerve compression with visual worsening).78
For ventricle location, surgical removal of cysts can be as effective as medical treatment. The advantages of surgery in these cases rely on the possibility of treating hydrocephalus simultaneously, reducing the need for a definite VP shunt, and on the possibility of no further anti-helminthic agents needed in cases of removal of all cysts.55
A flow-chart for treatment of extraparenchymal NC is shown in Figure 8.
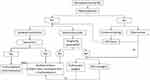 |
Figure 8 Treatment flow-chart for extraparenchymal NC. |
Conclusions
In conclusion, although great strides have been made in the treatment of NC, much work remains to be done to know the best management of several conditions. However, based on current knowledge, the following recommendations can be made: 1) for vesicular parenchymal NC, anthelminthics should be used in combination with corticosteroid to avoid deteriorating clinical symptoms. 2) For degenerative parenchymal cysts, doubts remain about the best management – if anthelminthics or simple follow-up. 3) Calcified cysts require only symptomatic treatment. 4) For extraparenchymal NC, attention should be driven to treat hydrocephalus with surgical options (endoscopic third ventriculostomy or VP shunt), and vasculitis (with corticosteroids). 5) Surgical removal of cysts (mainly with endoscopic techniques) is a very good option in cases of ventricular cysts, with the advantage of no further treatment if all cysts are removed. 6) In cases of basal subarachnoid cysts and ventricular cysts not amenable to endoscopic removal, anthelminthics combined with corticosteroids should be used with higher doses or prolonged duration.
Abbreviations
NC, neurocysticercosis; AH, anthelmintic; ALB, albendazole; PZQ, praziquantel; ABZSO, Albendazole sulfoxide.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Fact sheet on taeniasis/cysticercosis (updated February 2018). Wkly Epidemiol Record. 2018;93:630–632.
2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222.
3. Flisser A, Sarti E, Lightowlers M, Schantz P. Neurocysticercosis: regional status, epidemiology, impact and control measures in the Americas. Acta Trop. 2003;87:43–51. doi:10.1016/S0001-706X(03)00054-8
4. Hernández-Chea RD, Morales-Ramírez P, Hernández M, et al. Taenia solium taeniasis/cysticercosis in Guatemala: a prevalent public health problem? Pathog Glob Health. 2022;1–9. doi:10.1080/20477724.2022.2083757
5. Tellez-Zenteno JF, Hernandez-Ronquillo L. Epidemiology of neurocysticercosis and epilepsy, is everything described? Epilepsy Behav. 2017;76:146–150. doi:10.1016/j.yebeh.2017.01.030
6. Rodríguez-Rivas R, Flisser A, Norcia LF, et al. Neurocysticercosis in Latin America: current epidemiological situation based on official statistics from four countries. PLoS Negl Trop Dis. 2022;16:e0010652. doi:10.1371/journal.pntd.0010652
7. Stelzle D, Schmidt V, Keller L, et al. Characteristics of people with epilepsy and Neurocysticercosis in three Eastern African countries-a pooled analysis. PLoS Negl Trop Dis. 2022;16:e0010870. doi:10.1371/journal.pntd.0010870
8. Stelzle D, Makasi C, Schmidt V, et al. Epidemiological, clinical and radiological characteristics of people with neurocysticercosis in Tanzania-a cross-sectional study. PLoS Negl Trop Dis. 2022;16:e0010911. doi:10.1371/journal.pntd.0010911
9. Hamamoto Filho PT, Singh G, Winkler AS, Carpio A, Fleury A. Could differences in infection pressure be involved in cysticercosis heterogeneity? Trends Parasitol. 2020;36:826–834. doi:10.1016/j.pt.2020.07.003
10. Nash TE, Pretell EJ, Lescano AG, et al.; Cysticercosis Working Group in Peru. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 7;2008:1099–1105. doi:10.1016/S1474-4422(08)70243-6
11. Marcin Sierra M, Arroyo M, Cadena Torres M, et al. Extraparenchymal neurocysticercosis: demographic, clinicoradiological, and inflammatory features. PLoS Negl Trop Dis. 2017;11:E0005646. doi:10.1371/journal.pntd.0005646
12. Del Brutto OH, Garcia HH. The many facets of disseminated parenchymal brain cysticercosis: a differential diagnosis with important therapeutic implications. PLoS Negl Trop Dis. 2021;15:e0009883. doi:10.1371/journal.pntd.0009883
13. Carpio A, Fleury A, Romo ML, et al. New diagnostic criteria for neurocysticercosis: reliability and validity. Ann Neurol. 2016;80:434–442. doi:10.1002/ana.24732
14. Del Brutto OH. Stroke and vasculitis in patients with cysticercosis. In: Caplan LR, editor. Uncommon Causes of Stroke. New York, NY, USA: Cambridge University Press; 2008:53–58.
15. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011;9:123–133. doi:10.1586/eri.10.150
16. Zhao JL, Lerner A, Shu Z, Gao XJ, Zee CZ. Imaging spectrum of neurocysticercosis. Radiol Infect Dis. 2015;1:94–102. doi:10.1016/j.jrid.2014.12.001
17. Carrillo Mezo R, Lara García J, Arroyo M, Fleury A. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop. 2015;152:60–65. doi:10.1016/j.actatropica.2015.08.017
18. Hernández M, Astudillo OG, Diego G, et al. Immunodiagnosis of human neurocysticercosis: comparative performance of serum diagnostic tests in Mexico. Parasitol Res. 2019;118:2891–2899. doi:10.1007/s00436-019-06425-4
19. Fleury A, Hernández M, Avila M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78:970–974. doi:10.1136/jnnp.2006.107243
20. Del Brutto OH, Nash TE, White AC, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202–210. doi:10.1016/j.jns.2016.11.045
21. Gabriël S, Blocher J, Dorny P, et al. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis. 2012;6:e1851. doi:10.1371/journal.pntd.0001851
22. Garg RK. Diagnostic criteria for neurocysticercosis: some modifications are needed for Indian patients. Neurol India. 2004;52:171–177.
23. Escobedo F, Penagos P, Rodrígez J, Sotelo J. Albendazole therapy for neurocysticercosis. Arch Intern Med. 1987;147:738–741. doi:10.1001/archinte.1987.00370040120021
24. Jung H, Cárdenas G, Sciutto E, Fleury A. Medical treatment for neurocysticercosis: drugs, indications and perspectives. Curr Top Med Chem. 2008;8:424–433. doi:10.2174/156802608783790811
25. Jung H, Hurtado M, Medina MT, Sanchez M, Sotelo J. Dexamethasone increases plasma levels of albendazole. J Neurol. 1990;237:279–280. doi:10.1007/BF00314741
26. Göngora-Rivera F, Soto-Hernández JL, González Esquivel D, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology. 2006;66:436–438. doi:10.1212/01.wnl.0000195887.63124.dc
27. Osorio R, Carrillo-Mezo R, Romo ML, et al. Factors associated with cysticidal treatment response in extraparenchymal neurocysticercosis. J Clin Pharmacol. 2019;59:548–556. doi:10.1002/jcph.1346
28. Arroyo G, Bustos JA, Lescano AG, et al; Cysticercosis Working Group in Peru. Albendazole sulfoxide plasma levels and efficacy of antiparasitic treatment in patients with parenchymal neurocysticercosis. Clin Infect Dis. 2019;69:1996–2002. doi:10.1093/cid/ciz085
29. Sotelo J, Escobedo F, Rodriguez-Carbajal J, Torres B, Rubio-Donnadieu F. Therapy of parenchymal brain cysticercosis with praziquantel. N Engl J Med. 1984;310:1001–1007. doi:10.1056/NEJM198404193101601
30. Hamamoto Filho PT, Fragoso G, Sciutto E, Fleury A. Inflammation in neurocysticercosis: clinical relevance and impact on treatment decisions. Expert Rev Anti Infect Ther. 2021;19:1503–1518. doi:10.1080/14787210.2021.1912592
31. Sotelo J, Marin C. Hydrocephalus secondary to cysticercotic arachnoiditis. A long-term follow-up review of 92 cases. J Neurosurg. 1987;66:686–689. doi:10.3171/jns.1987.66.5.0686
32. Woo E, Yu YL, Huang CY. Cerebral infarct precipitated by praziquantel in neurocysticercosis--a cautionary note. Trop Geogr Med. 1988;40:143–146.
33. Bang OY, Heo JH, Choi SA, Kim DI. Large cerebral infarction during praziquantel therapy in neurocysticercosis. Stroke. 1997;28:211–213. doi:10.1161/01.STR.28.1.211
34. Fong GC, Cheung RT. Caution with praziquantel in neurocysticercosis. Stroke. 1997;28:1648–1649. doi:10.1161/str.28.8.1648/a
35. Minguetti G, Ferreira MV. Effect of corticoids in the acute phase of neurocysticercosis: preliminary note. Arq Neuropsiquiatr. 1982;40:77–85. doi:10.1590/S0004-282X1982000100008
36. Suastegui Roman RA, Soto-Hernandez JL, Sotelo J. Effects of prednisone on ventriculoperitoneal shunt function in hydrocephalus secondary to cysticercosis: a preliminary study. J Neurosurg. 1996;84:629–633. doi:10.3171/jns.1996.84.4.0629
37. White AC, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2018;98:945–966. doi:10.4269/ajtmh.18-88751
38. Toledo A, Osorio R, Matus C, et al. A. human extraparenchymal neurocysticercosis: the control of inflammation favors the host…but also the parasite. Front Immunol. 2018;9:2652. doi:10.3389/fimmu.2018.02652
39. Palomares-Alonso F, Toledo A, Palencia Hernández G, Jung-Cook H, Fleury A. Effect of dexamethasone on albendazole cysticidal activity in experimental cysticercosis by Taenia crassiceps in BALB/c mice: in vitro and in vivo evaluation. Exp Parasitol. 2020;208:107801. doi:10.1016/j.exppara.2019.107801
40. Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44:549–553. doi:10.1086/511040
41. Nash TE, Ware JM, Coyle CM, Mahanty S. Etanercept to control inflammation in the treatment of complicated neurocysticercosis. Am J Trop Med Hyg. 2019;100:609–616. doi:10.4269/ajtmh.18-0795
42. Medina MT, Rosas E, Rubio-Donnadieu F, Sotelo J. Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Arch Intern Med. 1990;150:325–327. doi:10.1001/archinte.1990.00390140065014
43. Debacq G, Moyano LM, Garcia HH, et al. Systematic review and meta-analysis estimating association of cysticercosis and neurocysticercosis with epilepsy. PLoS Negl Trop Dis. 2017;11:e0005153. doi:10.1371/journal.pntd.0005153
44. Arteaga-Rodríguez C, Menine-Kubis M, Teixeira-Arteaga CB, Hernández-Fustes OJ. Clinical characteristics of patients with epilepsy attending primary health care. Rev Neurol. 2022;75:7–12. doi:10.33588/rn.7501.2022036
45. Langa I, Padama F, Nhancupe N, et al. The burden of T. solium cysticercosis and selected neuropsychiatric disorders in Mocuba district, Zambézia province, Mozambique. PLoS Negl Trop Dis. 2022;16:E0010606. doi:10.1371/journal.pntd.0010606
46. Stelzle D, Kaducu J, Schmidt V, et al. Characteristics of people with epilepsy in three Eastern African countries - a pooled analysis. BMC Neurol. 2022;22:321. doi:10.1186/s12883-022-02813-z
47. Velasco TR, Zanello PA, Dalmagro CL, et al. Calcified cysticercotic lesions and intractable epilepsy: a cross-sectional study of 512 patients. J Neurol Neurosurg Psychiatry. 2006;77:485–488. doi:10.1136/jnnp.2005.078675
48. Secchi TL, Brondani R, Bragatti JA, Bizzi JWJ, Bianchin MM. Evaluating the association of calcified neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis in a large cohort of patients with epilepsy. Front Neurol. 2022;12:769356. doi:10.3389/fneur.2021.769356
49. Santhosh AP, Kumar Goyal M, Modi M, et al. Carbamazepine versus levetiracetam in epilepsy due to neurocysticercosis. Acta Neurol Scand. 2021;143:242–247. doi:10.1111/ane.13355
50. Panda PK, Sharawat IK. Carbamazepine or levetiracetam: which one is better in neurocysticercosis? Acta Neurol Scand. 2022;145(4):484–485. doi:10.1111/ane.13424
51. Sharawat IK, Panda PK, Kumar V, Sherwani P. Comparative efficacy and safety of lacosamide and oxcarbazepine for seizure control in children with newly diagnosed solitary neurocysticercosis. J Trop Pediatr. 2022;68:fmac032. doi:10.1093/tropej/fmac032
52. Colli BO, Carlotti CG, Assirati JA, Machado HR, Valença M, Amato MC. Surgical treatment of cerebral cysticercosis: long-term results and prognostic factors. Neurosurg Focus. 2002;12:e3. doi:10.3171/foc.2002.12.6.4
53. Hamamoto Filho PT, Zanini MA, Fleury A. Hydrocephalus in neurocysticercosis: challenges for clinical practice and basic research perspectives. World Neurosurg. 2019;126:264–271. doi:10.1016/j.wneu.2019.03.071
54. Carpio A, Fleury A, Kelvin EA, Romo ML, Abraham R, Tellez-Zenteno J. New guidelines for the diagnosis and treatment of neurocysticercosis: a difficult proposal for patients in endemic countries. Expert Rev Neurother. 2018;18:743–747. doi:10.1080/14737175.2018.1518133
55. Nash TE, Ware JM, Mahanty S. Intraventricular neurocysticercosis: experience and long-term outcome from a tertiary referral center in the United States. Am J Trop Med Hyg. 2018;98:1755–1762. doi:10.4269/ajtmh.18-0085
56. Paiva ALC, Araujo JLV, Ferraz VR, et al. Surgical treatment of neurocysticercosis. Retrospective cohort study and an illustrative case report. Sao Paulo Med J. 2017;135:146–149. doi:10.1590/1516-3180.2016.0304171216
57. Barrie U, Badejo O, Aoun SG, et al. Systematic review and meta-analysis of management strategies and outcomes in adult spinal neurocysticercosis. World Neurosurg. 2020;138:504–511.e8. doi:10.1016/j.wneu.2020.03.093
58. Torres-Corzo JG, Tapia-Pérez JH, Vecchia RR, Chalita-Williams JC, Sánchez-Aguilar M, Sánchez-Rodríguez JJ. Endoscopic management of hydrocephalus due to neurocysticercosis. Clin Neurol Neurosurg. 2010;112:11–16. doi:10.1016/j.clineuro.2009.08.022
59. Zhenye L, Chuzhong L, Xuyi Z, et al. Ventriculoscopic approach for intraventricular neurocysticercosis: a single neurosurgical center’s experience. World Neurosurg. 2017;107:853–859. doi:10.1016/j.wneu.2017.08.059
60. Bergsneider M. Endoscopic removal of cysticercal cysts within the fourth ventricle. J Neurosurg. 1999;91:340–345. doi:10.3171/jns.1999.91.2.0340
61. Garcia HH, Del Brutto OH; Cysticercosis Working Group in Peru. Antiparasitic treatment of neurocysticercosis - the effect of cyst destruction in seizure evolution. Epilepsy Behav. 2017;76:158–162. doi:10.1016/j.yebeh.2017.03.013
62. Tan YT, Zhang SJ, Shu K, Lei T, Niu HQ. Microsurgical treatment of epilepsy with parenchymal neurocysticercosis. Curr Med Sci. 2019;39:984–989. doi:10.1007/s11596-019-2132-1
63. Espino PH, Couper RG, Burneo JG. An update on neurocysticercosis-related epilepsy. Clin Neurol Neurosurg. 2022;213:107139. doi:10.1016/j.clineuro.2022.107139
64. Bianchin MM, Velasco TR, Wichert-Ana L, Dos Santos AC, Sakamoto AC. Understanding the association of neurocysticercosis and mesial temporal lobe epilepsy and its impact on the surgical treatment of patients with drug-resistant epilepsy. Epilepsy Behav. 2017;76:168–177. doi:10.1016/j.yebeh.2017.02.030
65. Agapejev S, Pouza AF, Bazan R, Faleiros AT. Clinical and evolutive aspects of hydrocephalus in neurocysticercosis. Arq Neuropsiquiatr. 2007;65(3a):674–680. doi:10.1590/S0004-282X2007000400025
66. Kelley R, Duong DH, Locke GE. Characteristics of ventricular shunt malfunctions among patients with neurocysticercosis. Neurosurgery. 2002;50:757–762. doi:10.1097/00006123-200204000-00014
67. Suri A, Goel RK, Ahmad FU, Vellimana AK, Sharma BS, Mahapatra AK. Transventricular, transaqueductal scope-in-scope endoscopic excision of fourth ventricular neurocysticercosis: a series of 13 cases and a review. J Neurosurg Pediatr. 2008;1:35–39. doi:10.3171/PED-08/01/035
68. Goel RK, Ahmad FU, Vellimana AK, et al. Endoscopic management of intraventricular neurocysticercosis. J Clin Neurosci. 2008;15:1096–1101. doi:10.1016/j.jocn.2007.10.004
69. Monk EJM, Abba K, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. 2021;6:CD000215. doi:10.1002/14651858.CD000215.pub5
70. Garcia HH, Gonzales I, Lescano AG, et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomized controlled trial. Lancet Infect Dis. 2014;14:687–695. doi:10.1016/S1473-3099(14)70779-0
71. Garcia HH, Lescano AG, Gonzales I, et al. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis. 2016;62:1375–1379. doi:10.1093/cid/ciw134
72. Garcia HH, Lescano AG, Lanchote VL, et al. Pharmacokinetics of combined treatment with praziquantel and albendazole in neurocysticercosis. Br J Clin Pharmacol. 2011;72:77–84. doi:10.1111/j.1365-2125.2011.03945.x
73. Walton D, Castell H, Collie C, et al. Antiepileptic drugs for seizure control in people with neurocysticercosis. Cochrane Database Syst Rev. 2021;11:CD009027. doi:10.1002/14651858.CD009027.pub4
74. Abraham A, Bustos JA, Carabin H, et al. The effectiveness of anti-inflammatory and anti-seizure medication for individuals with single enhancing lesion neurocysticercosis: a meta-analysis and expert group-based consensus recommendations. PLoS Negl Trop Dis. 2021;15:e0009193. doi:10.1371/journal.pntd.0009193
75. Kaur S, Singhi P, Singhi S, Khandelwal N. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: a randomized, placebo-controlled double blind trial. Pediatr Infect Dis J. 2009;28:403–406. doi:10.1097/INF.0b013e31819073aa
76. Singh K, Saini AG, Khandelwal N, Singhi P. Efficacy of combination therapy of albendazole and praziquantel vs albendazole monotherapy in children with persistent neurocysticercosis: a randomized controlled trial. J Child Neurol. 2022;37:366–372. doi:10.1177/08830738221077762
77. Nash TE, O’Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S. Natural history of treated subarachnoid neurocysticercosis. Am J Trop Med Hyg. 2020;102:78–89. doi:10.4269/ajtmh.19-0436
78. Cuellar-Hernandez JJ, Valadez-Rodriguez A, Olivas-Campos R, et al. Intrasellar cysticercosis cyst treated with a transciliary supraorbital keyhole approach- a case report. Surg Neurol Int. 2020;11:436. doi:10.25259/SNI_755_2020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.