Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Ulcerated Cutaneous Melanoma: A Review of the Clinical, Histologic, and Molecular Features Associated with a Clinically Aggressive Histologic Phenotype
Authors Barricklow Z, DiVincenzo MJ, Angell CD , Carson WE
Received 7 May 2022
Accepted for publication 2 August 2022
Published 30 August 2022 Volume 2022:15 Pages 1743—1757
DOI https://doi.org/10.2147/CCID.S372287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Zoe Barricklow,1 Mallory J DiVincenzo,1,2 Colin D Angell,1 William E Carson1
1The Arthur G. James Cancer Hospital and Solove Research Institute, The Ohio, State University, Columbus, OH, USA; 2Department of Veterinary Biosciences, The Ohio State University, Columbus, OH, USA
Correspondence: William E Carson, The Ohio State University, N924 Doan Hall, 410 W. 10th Avenue, Columbus, OH, 43210, USA, Tel +1 614 293-6306, Fax +2 614 293-3465, Email [email protected]
Abstract: The presence of ulceration in melanoma is associated with poor clinical outcomes and is the third most powerful predictor of survival in the AJCC Melanoma Staging System after tumor thickness and mitotic activity. The aggressive biological behavior associated with ulceration has been hypothesized to be the result of an intrinsic biological attribute that favors dissemination and presents locally with the loss of epidermal integrity. Among the features of ulcerated melanoma, many show promise as potential prognostic tools, markers of differential immunogenicity and indicators of oncogenic drivers of invasion and metastasis. The incidence of ulcerated melanoma is greater in males, increases with age and with systemic inflammatory risk factors (diabetes, smoking, low vitamin D, elevated body mass index). Patients with ulcerated primary tumors seem to exclusively benefit from adjuvant interferon (IFN) therapy, which is likely the consequence of an altered tumor microenvironment. When ulceration is present, there is a higher density of macrophages and dendritic cells and enhanced expression of pro-inflammatory cytokines, such as IL-6. There is also an increased expression of proteins involved in tumor antigen presentation in ulcerated melanomas. Histologically, vascular density, vasculogenic mimicry and angiotropism are all significantly correlated with ulceration in melanoma. The presence of ulceration is associated with reduced protein expression of E-cadherin and PTEN and elevated levels of N-cadherin and the matrix metalloproteinases. Differential microRNA expression also holds promise as a potential prognostic biomarker of malignancy and disease spread within the setting of ulceration. However, the molecular and cellular differences associated with the ulcerated state are complex and further study will aid in determining how these differences can be harnessed to improve care for patients with melanoma.
Keywords: melanoma, ulcerated, review, treatment
Introduction
The presence of ulceration in melanoma is defined by the American Joint Committee on Cancer (AJCC) Melanoma Staging System as having three key features: a full thickness epidermal defect to the level of the basement membrane, evidence of a characteristic host response (fibrin, neutrophils), and thinning, effacement, or reactive hyperplasia of the surrounding epidermis.1,2 Ulceration is third most powerful predictor of survival in the AJCC Melanoma Staging System, with the first and second being tumor thickness and mitotic activity, respectively.2,3 While tumor thickness is also considered a prognostic criterion for melanoma, and thicker tumors are more commonly ulcerated, the presence of ulceration and the extent of ulceration are both independent predictive factors.2–6 The presence of ulceration may also reflect a highly proliferative phenotype, often associated with a higher mitotic rate, but not all thick or highly mitotic lesions are ulcerated.4,5,7 This suggests that processes other than cell proliferation may influence the development of ulceration.7–9 The aggressive biologic behavior associated with ulceration has been hypothesized to be a consequence of some intrinsic biological attribute of the tumor that favors dissemination and presents locally with the loss of epidermal integrity.5
The impact of ulceration in melanoma centers on its correlation with poor clinical outcomes. Thus, ulceration has consistently been considered an important indicator of prognosis. In the AJCC 8th Edition Melanoma Staging system, ulceration is included in the grading criteria for melanoma grades I–IV as a T-category criterion. Primary melanomas are thus classified as “a” or “b” indicating the absence or presence of ulceration, respectively.2–4 In 1980, Balch et al showed that survival in stage I melanoma is reduced from 80% to 55% if the primary tumor is ulcerated and from 53% to 12% in stage II melanoma (p < 0.001).10 The same study showed reduced progression free survival (PFS) and overall survival (OS) relative to non-ulcerated melanomas of the same thickness, further supporting an association of tumor ulceration with poor prognosis. Based on the prognostic differences associated with ulceration, it has been proposed that ulcerated melanoma represents a unique biologic entity, separate from its non-ulcerated counterpart.11
To date, there is no clear reason for the development of the ulcerated phenotype in melanoma, or a well-defined molecular explanation for its prognostic value. However, several studies have demonstrated significant molecular features of ulcerated melanomas as well as correlations with other diagnostic criteria that may aid in answering these questions. For example, gene expression patterns associated with the upregulation of certain pro-inflammatory cytokines are increased in ulcerated tumors compared to non-ulcerated tumors. In 2006, Winnepenninckx et al cited differences in gene expression profiles between ulcerated and non-ulcerated melanoma; an indicator of the biologic basis for the adverse impact of ulceration. Specifically, primary cutaneous ulcerated melanomas had enhanced expression of IL-6, a pro-inflammatory cytokine.11,12 This finding suggests that altered activation of inflammatory pathways in the tumor microenvironment is associated with the ulcerated phenotype.12–14 Melanomas with increased vascular density have a higher rate of ulceration,12–14 and lymphovascular invasion has also been identified as a feature significantly associated with the presence of ulceration.15 While these studies provide evidence of the unique features of ulcerated melanomas that carry potential prognostic and biologic significance, further characterization of the histologic, molecular, and immunologic differences is needed in the context of clinical data in order to improve care, prognostication and therapy.
Impact of Sex, Age and Race on Ulcerated Melanoma
In a study of 423 cases, primary melanoma tumor ulceration was found to occur with a higher frequency in males (30.4%) than females (14.4%) (p = 0.019).16 The Sunbelt Melanoma Trial was a multicenter, prospective randomized clinical study of over 1800 patients that evaluated the role of high-dose interferon alfa-2b therapy in patients with a single positive sentinel lymph node metastasis treated with a completion lymph node dissection. This study made similar observations in that men were more likely to have evidence of tumor ulceration with a diagnosis of primary cutaneous melanoma (p < 0.0001).17 However, other studies have indicated that sex, as well as anatomic location and degree of pigmentation, are not correlated with the occurrence of ulceration.18–20 The reason for these disparate findings could be based on differences in sample size. There is also evidence that ulcerated melanomas are more common with advancing age (50 years or older) and non-white race, according to an analysis from the National Cancer Database.21
Risk Factors and Comorbidities
Several associated conditions and comorbidities have been shown to correlate with the incidence of tumor ulceration in melanoma. One published study investigating the relationship between ulceration and systemic inflammatory factors found that the occurrence of tumor ulceration in cutaneous melanoma was associated with diabetes, smoking, low vitamin D levels, and higher body mass index (BMI) at the time of diagnosis.10 The association between low vitamin D and ulceration has been hypothesized to be the result of altered Wnt/β-catenin signaling.22 In 2017, von Schuckmann et al showed that patients who regularly used statins, aspirin or NSAIDs were less likely to be diagnosed with an ulcerated melanoma when incidence was adjusted for age, sex, thickness and mitotic rate (OR 0.68, 95% CI 0.46–1.00).23 While early epidemiologic studies suggested that statins may prevent melanoma development, more recent studies do not support this finding.24–27 Rather, it is suggested that the onset of statin therapy may be associated with more frequent medical surveillance.27
Histologic Features
Cell Morphology
Multiple specific histologic features of cutaneous melanoma have been investigated to determine their potential correlation with the presence of tumor ulceration, including cell morphology. Spindle cell morphology, defined by a fusiform cell shape and elongated nucleus, is generally associated with mesenchymal cells. Melanomas composed entirely of spindle-shaped cells are rare and are estimated to account for 3% to 14% of all melanoma cases.28 Melanomas in which only parts of the tumor are composed of spindle cells are seen more often, although data supporting this observation are limited. In one study, 7% of the included melanomas were composed entirely of spindle-shaped cells and were equally distributed between ulcerated and non-ulcerated melanomas. However, a larger number (29%) of melanomas included in the study exhibited partial spindle cell morphology, and overall, the presence of spindle cell morphology was shown to correlate significantly with tumor ulceration in melanoma (p = 0.021).28
Histological Type
Histologic type classification (nodular, superficial spreading, desmoplastic, or lentigo melanoma) has not been shown to be significantly associated with ulceration. However, differences in the frequency of ulceration have been detected. In a study of 423 melanoma patients, 18% of cases exhibited ulceration, with a high percentage of ulcerated tumors being categorized as nodular melanomas (47.8%) and desmoplastic melanomas (66.7%), relative to the frequencies observed in superficial spreading melanoma (9.2%) and lentigo malignant melanoma (7.7%).29 A separate study by Masback et al found that among a sample of 468 Swedish patients, there was a significant correlation between the presence of ulceration and nodular melanoma growth (p < 0.001).30 It is possible that the population being sampled may impact the histologic type in which ulceration is most commonly observed.
Although ulceration in melanoma is an independent predictor of survival in melanoma, one study found that in acral melanoma, the prognostic value of ulceration depended on tumor thickness. In this study of 1053 patients with acral melanoma, ulceration was a significant prognostic indicator for tumors ≤1 mm. However, there was no association between ulceration and survival for intermediate/thick or stage III acral melanoma.31
Vascular Density and Vasculogenic Mimicry
Vascular density has been shown to be of prognostic value in malignant melanoma. It is hypothesized that vessel density is increased in tumors to subsequently increase oxygen and blood supply for support of tumor growth.32 A tendency toward vascularity in ulcerated melanoma was demonstrated by Storr et al in a study of 417 patients, wherein melanoma vascularity was evaluated via immunohistochemistry (IHC) for CD34 using a semi-quantitative technique. It was demonstrated that a high CD34+ microvessel density was associated with ulcerated lesions and infiltration of CD68+ macrophages.14
Vasculogenic mimicry, or the ability of cancer cells to mimic vasculogenic-like patterned structures to obtain nutrients and oxygen, has also been shown to aid in providing microcirculation in aggressive melanomas independent of the presence of endothelial cells.33 The acquisition of gene expression characteristic of endothelial cells is termed endothelial trans-differentiation, and CD31 and CD34 are two endothelial markers that have been used to measure this process. A study by Pisacane et al examining 45 cutaneous melanoma samples by immunohistochemistry (IHC) and found that 87.5% and 68% of cutaneous ulcerated melanomas were CD31-positive and CD34-positive, respectively. In comparison, 68% and 27% of non-ulcerated melanomas that were CD31-positive and CD34-positive, respectively (p = 0.03).33 Endothelial trans-differentiation is associated with the development of intravascular niches of disseminated melanoma cells at metastatic sites. This event may occur more commonly in ulcerated tumors.34
Angiotropism and Vascular Invasion
Angiotropism is defined as tumor cells closely associated with abluminal vascular surfaces without intravasation.35 When observed in clinical specimens, angiotropism of melanoma cells has been termed “extravascular migratory metastasis”.36,37 Angiotropism has been shown to be significantly correlated with the presence of ulceration in primary melanomas when assessed in H&E stained tissue sections (p = 0.0331).38 The amount of angiotropism observed has been shown to vary significantly based on location within ulcerated lesions through semi-quantitative analysis (p < 0.0001). Angiotropism was predominantly observed in the superficial and most ulcerated part of the tumor compared with the non-ulcerated part of the tumor (86% vs 28%, respectively). Angiotropism has also correlated strongly with the presence of neutrophils. The dense infiltration of neutrophils near vessels can be detected by immunohistochemistry for CD34 and CD66 in double-stained tissue sections.39 Neutrophils have also been shown to contribute to the adhesion of tumor cells to the endothelium.40 Therefore, concurrent observation of tumor angiotropism and neutrophilic infiltrate may contribute to extravascular migratory metastasis and the increased likelihood for intravasation of tumor cells into the circulation. This relationship could be a possible explanation for the increased risk of both regional lymph node metastasis and systemic spread in ulcerated melanoma.
In addition to angiotropism, lymphovascular invasion (LVI) is a feature that aligns with increased metastatic potential. LVI has been examined for its correlation with the presence of ulceration in melanoma. In a study with 246 samples, Rose et al found the presence of LVI, as detected via immunohistochemistry for podoplanin (lymphatic vessel marker detected with D2-40 monoclonal antibody) and/or CD34, was significantly associated with ulceration (p < 0.0001). Previous studies have found conflicting results regarding the clinical value of IHC-detected LVI and its association with disease-free survival (DFS) or OS on univariate analysis.41 While there is a correlation between LVI and ulceration in cutaneous melanoma, causation between the variables has yet to be proven.42,43
Patterns of Gene Expression, Associated Mutations, and Molecular Signatures
Efforts to characterize the gene expression profile, associated oncogenic mutations, and molecular signatures of ulcerated melanoma have been made by several groups. Identification of unique molecular features associated with ulcerated tumors has revealed multiple pathways by which affected patients may harbor an increased risk of metastasis and recurrence, and thus may hold prognostic value. Following is a discussion of genes that have been investigated in the context of melanomas exhibiting ulceration.
Matrix Metalloproteinases
Matrix metalloproteinase (MMP) function to degrade extracellular matrix proteins and have been shown to play a role in modulation of melanoma development, progression and metastasis. Vihinen et al found that serum MMP-8 was increased in melanoma patients with primary tumor ulceration.44 Elevated expression of MMP-1 and MMP-3 in melanoma metastases is associated with shorter disease-free survival (median 17.0 vs 11.2 months, p = 0.0383).45 MMP-1 promoter single nucleotide polymorphisms (SNPs) have been shown to have a significant association with a susceptibility to the development of cutaneous melanoma. Two SNPs in the MMP-1 promoter have been specifically associated with tumor ulceration in melanoma. SNP −422A > T (rs475007) and SNP −755T > G (rs498186) were identified using a dominant genetic model. However, this study showed no association between MMP-1 promoter SNPs and clinical outcome, suggesting only a moderate effect of these SNPs on melanoma progression.46
Cadherins
E-cadherins are essential for cell-to-cell adhesion in epithelial tissues. Loss of E-cadherin expression is recognized as an early event in epithelial-mesenchymal transition, and is associated with development of tumor metastasis in other cancers.47 Bonnelykke-Behrndtz et al found that ulceration status in melanoma was significantly linked with the loss of E-cadherin expression when estimated as a global score (E-cadherin expression across the entire tumor) and as a weak-spot score (1 x 1 mm area with the lowest E-cadherin expression). However, E-cadherin expression was portrayed as being heterogeneous throughout the tumor. This study showed that the loss of E-cadherin was most prominent within superficial and ulcerated part of the melanoma, while a study by Lade-Keller et al reported loss of E-cadherin specifically in the invasive zone.29,39 These studies differed in their methods of analyzing E-cadherin expression, with one using a quantitative computer-assisted analysis to divide the area of membrane expression by the area of region of interest to estimate E-cadherin expression, and the other using a semi-quantitative scoring system to assign a percentage range of positive staining tumor cells. These differences in the approach to analysis of IHC expression may have contributed to these differential findings.
The latter study by Lade-Keller et al also demonstrated a significant association between ulceration and high N‐cadherin, low E‐cadherin and low PTEN expression. The ratio of E-cadherin to N-cadherin was termed the “cadherin switch profile” and was found to be of prognostic value.29 The cadherin switch profile predicted poor melanoma‐specific survival (HR 2.5, 95% CI 1.3–4.6, p = 0.005) and poor distant‐metastasis‐free survival (HR 2.2, 95% CI 1.2–4.0, p = 0.01). Both survival measures were independent of Breslow thickness, ulceration, and tumor stage in multivariate analysis (HR 1.96, 95% CI 1.0–3.7, p = 0.04; and HR 2.1, 95% CI 1.1–3.9, p = 0.02, respectively). However, the presence of ulceration was significantly associated with high N-cadherin, low E-cadherin and low PTEN expression, and with the cadherin switch profile.29
BRAF
Other groups have demonstrated an association between melanoma tumor ulceration status with loss of E-cadherin expression in conjunction with BRAF mutational status. In a study of 68 samples, loss of E-cadherin expression and BRAF mutation individually were associated with ulceration in primary cutaneous melanoma (p = 0.05 and p = 0.02, respectively). There was also an association between BRAF mutation and E-cadherin loss even after controlling for confounding variables. These results suggest mutant BRAF may repress E-cadherin expression, thus indicating a role for BRAF in mediating epithelial-mesenchymal transition and the facilitation of metastasis.48 In another study of 232 cases of primary melanoma, overall levels of BRAF protein expression were examined. High BRAF expression was detected in 85% of patients with ulceration at the time of diagnosis compared with 49.5% of patients without ulceration (p = 0.000015). Also, univariate analysis demonstrated that BRAF expression, tumor thickness, presence of ulceration and AJCC stage were all significantly associated with overall and 5-year disease-specific survival in patients diagnosed with primary melanoma.49 In a study of 71 patients with ulcerated cutaneous melanoma, patients with high proportion of mutant BRAF alleles (>35%) had shorter recurrence-free survival (HR 2.44, 95% CI 1.23–4.84, p = 0.011).50 While targeted therapies for BRAF mutations have shown to increase recurrence-free survival in patients with stage III malignant melanoma, clinical benefit was consistent regardless of ulceration status.51
PTEN
Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene on chromosome 10 that is frequently mutated in melanoma and functions in regulation of the cell cycle, cellular adhesion, migration and apoptosis.52 Loss of PTEN expression in melanoma has also been shown to correlate with reduced T cell infiltration and immune evasion.53 A switch from the expression of E-cadherin to N-cadherin (discussed above as a feature of ulcerated tumors) has been associated with downregulation of PTEN. Lade-Keller et al have shown a significant association between the presence of ulceration and low PTEN expression (p = 0.003). Lade-Keller et al also found reduced or absent PTEN expression significantly predicted poor overall relapse‐free survival in melanoma (HR 2.0, 95% CI 1.2–3.2, p = 0.006), a finding not independent of Breslow thickness, ulceration, or tumor stage in multivariate analyses (p > 0.05).29 Mikhail et al found an association between reduced PTEN expression and ulceration but no association with other progression markers or survival. Sample size and time to follow-up may have contributed to the lack of an association with outcome measures in this study.54 However, Slipicevic et al found no association between reduced PTEN expression and primary tumor thickness, relapse‐free survival, or overall survival.55
The discrepancies across multiple studies regarding PTEN expression, ulceration status, and progression markers or survival may be due to differences in staining and/or cohort characteristics. Lade-Keller et al examined a larger cohort with a longer clinical follow-up compared to other studies. Notably, Mikhail et al assessed nuclear staining and Slipicevic et al assessed cytoplasmic staining, while Lade-Keller et al analyzed both cellular compartments.29,54–56 While altered PTEN expression appears to be a feature associated with tumor ulceration, a cause for its dysregulated expression was not identified in these studies. De Unamuno Bustos et al have shown aberrant methylation of PTEN was associated with increased mitotic rate (OR 2.2, 95% CI 1.0–4.6, p = 0.021) and ulceration (OR 5.8, 95% CI 2.5–13.1, p < 0.001).56 Thus, PTEN methylation status may be altered in ulcerated tumors. Taken together, these findings suggest loss of PTEN expression may be a feature of ulcerated tumors that contributes to activation of PI3K/Akt signaling, but this relationship requires examination of other factors contributing to the regulation of this signaling pathway.
HLA Class I and MX1
Major Histocompatibility Complex (MHC) I (HLA Class I) and MX Dynamin Like GTPase 1 (MX1) are proteins involved in tumor antigen presentation.57,58 A study by Verver et al conducted IHC analysis for MHC I, MHC II, and MX1 in two retrospective cohorts of melanoma patients. Cohort 1 was diagnosed with a primary cutaneous melanoma (n = 172, 49% ulcerated melanoma). Cohort 2 was diagnosed with metastatic melanoma and underwent lymph node resection (n = 98, 44% ulcerated melanoma). Primary and metastatic ulcerated melanomas were found to exhibit higher basal expression of MHC class I molecules compared with non-ulcerated melanomas, independent of Breslow thickness, histologic type and lymphocytic infiltrate. Primary ulcerated melanomas also exhibited higher baseline levels of MX1 at the border of tumor beds compared to non-ulcerated melanomas (50.6% (42/83) and 31.3% (25/80), respectively, p = 0.01). Moderate or strong peri-tumoral MX1 expression was independently associated with ulcerated melanoma in multivariable logistic regression analysis (p = 0.02). MX1 and MHC molecules are both regulated by interferon (IFN) signaling, and therefore the differences in expression of MHC class I and MX1 in ulcerated tumors indicate that the composition of the tumor microenvironment may be altered when ulceration is present. The altered tumor microenvironment presents an opportunity for immune-based therapies and helps to explain why ulcerated primary melanomas seem to exclusively benefit from adjuvant IFN.57
Maspin
Maspin is a member of the serpin family of protease inhibitors that may act either as a tumor suppressor or promoter, depending on the cell type under study and its subcellular localization. In a study by Martinoli et al, nuclear maspin expression in melanoma samples (n = 152) was significantly associated with melanoma thickness (p < 0.0001), mitotic rate (p < 0.0001), and the presence of ulceration (p < 0.002), while no significant association was detected between ulceration status and cytoplasmic mapsin expression.59 These findings suggest a tumor-promoting effect of nuclear maspin and potential prognostic utility in melanoma based on its subcellular site of expression.
PHIP Copy Number
Pleckstrin homology domain interacting protein (PHIP) has previously shown utility as a marker and mediator of melanoma metastasis. Bezrookove et al found that high PHIP copy number (determined by fluorescence in situ hybridization) was significantly associated with ulceration status by univariate logistic regression analysis (n = 238, p = 0.004). The combined impact of increased PHIP copy number and tumor vascularity on ulceration status was highly significant. A 2018 study by this group confirmed the role of PHIP copy number in melanoma progression and the association between copy number and ulceration status.60 Correlation between PHIP levels and ulceration suggests a role for the IGF1R pathway in ulceration, given the known involvement of PHIP in this signal transduction pathway.61
CDKN2A
CDKN2A, also known as cyclin dependent kinase inhibitor 2A, is a tumor suppressor that functions as a regulator of the cell cycle. CDKN2A mutations are associated with an increased risk for cutaneous melanoma and other cancers. One study showed that patients with wild-type CDKN2A have a greater prevalence of ulceration in superficial spreading melanomas than carriers of CDKN2A germline mutations (p = 0.036). This association was not maintained for all invasive cutaneous melanomas or nodular melanomas.62 A separate study investigated survival rates in multiple primary melanoma cases from a Swedish population based on family history (familial vs sporadic) and CDKN2A status (mutated vs wild type – wt). The three cohorts were familial mutated (fam-CDKN2Amut), familial wild-type (fam-CDKN2Awt), and sporadic wild-type (spor-CDKN2Awt). When adjusting for age at diagnosis of second melanoma, sex and T classification of the thicker of the first 2 melanomas, the fam-CDKN2Amut cohort had significantly worse overall survival from melanoma compared to the fam-CDKN2Awt and the spor-CDKN2Awt cohorts.63 These disparate results indicate that further investigation is needed to ascertain the importance of CDKN2A in mediating the ulcerated state in melanoma.
Skp2
S-phase kinase protein 2, an F-box protein, targets cell cycle regulators via ubiquitin-mediated degradation. Chen et al found that increased cytoplasmic Skp2 expression was correlated with the presence of ulceration status in melanoma (p = 0.005).64 However, cytoplasmic Skp2 expression was not found to be an independent prognostic marker for melanoma patient survival by multivariate Cox proportional hazard analysis.
Beclin 1 and LC3
Beclin 1 and LC3 are mammalian autophagic genes typically altered in other cancer types, with Beclin 1 acting as a tumor suppressor. Melanoma ulceration has been shown to correlate with Beclin 1 non-cytoplasmic expression and inversely correlate with cytoplasmic LC3 protein expression.65 While the contribution of autophagy to the development and progression of melanoma is complex, altered expression patterns of autophagy-associated genes such as Beclin 1 and LC3 suggest that this pathway helps to promote the ulcerated state.
EGFR
Epidermal growth factor receptor (EGFR) is a transmembrane receptor tyrosine kinase that is overexpressed in several types of cancer, and its downstream signaling has been shown to facilitate cancer cell proliferation and migration.66,67 However, in melanoma, there is conflicting evidence regarding the connection between EGFR expression and ulceration status. In primary ulcerated melanoma, EGFR expression was found to be downregulated compared to non-ulcerated tumors.13 Consistent with this finding, Lee et al found that in distant metastatic melanoma, the lack of EGFR expression was significantly correlated with ulceration.66 Of the 12 patients with ulcerated tumors, only one patient had an EGFR positive tumor.66 However, in a study by Katunaríc et al, the presence of ulceration was correlated with an increase in membrane EGFR in nodular melanoma.63 This discrepancy may be due to the fact that Katunaríc et al focused exclusively on nodular melanomas and suggests that histological type needs to be considered when trying to understand the role of EGFR in melanoma.67
Next-Generation Sequencing
Advanced molecular diagnostic therapies, such as next-generation sequencing (NGS), have been used to identify de novo somatic cancer cell mutations.68,69 To date, there has been relatively little research on ulcerated melanoma tumors using NGS. de Unamuno Bustos et al conducted a study of mutations in cutaneous melanoma samples using a custom melanoma-specific sequencing panel that covered the coding regions of 35 melanoma-related genes. NF1 mutations were more frequent in ulcerated tumors compared to non-ulcerated tumors (p = 0.003). Tert promoter mutations were significantly associated with melanoma ulceration (p = 0.010), but they were not independently associated in multivariate analysis. A 2015 study using NGS found no statistical significance between mutations in 275 specific epigenetic genes and the presence/absence of ulceration among 6 patient samples with ulcerated melanoma tumors.70 Additional efforts in the personalized medicine of cutaneous melanoma may reveal new therapeutic targets.69
microRNAs
microRNAs are a group of small noncoding RNA molecules (~ 22 bp in length) that negatively regulate expression of specific target genes and have been identified as contributors to cancer progression via dysregulated gene expression. Altered expression of specific microRNAs is associated with melanoma progression and metastasis, but there has been a limited examination of microRNAs in the context of tumor ulceration.71–73 In particular, significantly lower expression of microRNA-145-5p and microRNA-203-3p was found by RT-PCR in cases with Breslow thickness >1 mm, ulceration and mitotic rate ≥1/mm2 (all p values <0.02).71 In a separate study using next-generation sequencing, decreased let-7b microRNA precursor expression significantly correlated with invasive depth, Clark’s level, ulceration, and AJCC stage.72 A study by Lin et al used RT-PCR to show that high microRNA-106b expression was correlated with Breslow thickness, tumor ulceration, and advanced clinical stage (p = 0.002, p = 0.002, p < 0.001, respectively).73 Multivariate regression analysis of the clinicopathological patient prognosis factors showed that the status of microRNA-106b expression was an independent prognostic factor for overall survival (HR 2.09, 95% CI 1.11–10.26, p = 0.02). Therefore, overexpression of microRNA-106b might play a role in melanoma progression.73
While these studies highlight a subset of microRNAs of potential interest for their role in ulcerated melanoma, these findings each demonstrate correlation of expression patterns with other tumor features concurrently, and therefore may not be specific to ulceration alone. Additionally, each study identified a unique microRNA or subset of microRNAs associated with ulceration relative to the others. This is in part due to the limited number of microRNAs evaluated in two of the three studies using qPCR. Furthermore, although microRNAs exert their functions through regulation of target gene expression, no studies thus far have examined correlations between microRNA expression and expression of their target genes in ulcerated melanoma. Thus, further efforts to characterize the expression of microRNAs and their target genes together are needed to elucidate the role of this group of molecules in the development and progression of melanoma in patients with ulcerated tumors and is an active area of investigation by our group. Dr. DiVincenzo from our group used NanoString human microRNA assay platform to determine microRNA expression profiles. Nine microRNAs (including hsa-microRNA-4286, hsa-microRNA-4488, and hsa-microRNA-1469) demonstrated at least a 2-fold change up or down in expression between ulcerated and non-ulcerated melanoma tumors. Additionally, restoring microRNA-1469 expression resulted in a significant reduction in melanoma cell migration compared to untransfected and microRNA-scramble transfected controls in CHL1, MEL39 and A375 cell lines.74 Transfection of a microRNA-1469 mimic resulted in a significant reduction in the migratory and invasive capacity of the CHL1 and MEL39 melanoma cell lines (p < 0.0332), as well as the invasive capacity of the A375 melanoma cell line (p < 0.0021). These findings suggest that loss of microRNA-1469 expression may aid in migration and invasion of adjacent tissues in the context of ulceration.75
Tumor Microenvironment and Immunogenicity
The inflammatory and immune microenvironment associated with ulcerated melanomas has unique features when compared to non-ulcerated tumors. As an ulcerated region of the skin is inherently associated with these tumors, a high degree of tumor inflammation is not unexpected.12,15 Elements identified as features specific to ulcerated tumors include a higher density of macrophages and microvessels. In addition, ulcerated melanomas exhibit enhanced expression of genes encoding cytokines, such as IL-6, as well as other inflammatory and wound-healing factors.12,15 In addition to altered influx of inflammatory cell populations into the tumor and cytokine expression, multiple immune cellular populations have been identified as being present at increased numbers in ulcerated melanomas.
Tumor Infiltrating Lymphocytes
While the presence and quantity of tumor infiltrating lymphocytes (TILs) has not been found to significantly differ between ulcerated and non-ulcerated melanomas, TILs have been found to be predictive of different clinical outcomes.76 Specifically, the level of TILs based on quantification of CD2 expression within the tumor using immunofluorescence has been shown to correlate with clinical outcome when ulceration is present in melanoma but is not prognostic in non-ulcerated tumors.72 In patients with TIL-rich ulcerated melanoma, there was significantly improved recurrence-free survival and overall survival compared to patients with TIL-deficient ulcerated melanomas. In those patients with non-ulcerated tumors, mitotic index of cancer cells was found to be the most predictive variable for overall survival.76 Falkenius et al also found statistically significant higher presence of TILs in ulcerated tumors without recurrence compared to ulcerated tumors with recurrence. However, a better predictor of outcome was found to be the combination of the presence of TILs, wildtype BRAF/low proportion of mutated alleles and low Ki67 expression.50 These findings provide further evidence that the tumor microenvironment is altered when ulceration is present.
Dendritic Cells
Dendritic cells (DCs) have been examined for their role in mediating the clinical outcomes associated with tumor ulceration in melanoma. DC populations in the sentinel lymph nodes of patients with ulcerated primaries were severely immunosuppressed relative to those from patients with non-ulcerated primaries. Lower density of LAMP+ mature DCs were associated with ulceration presence (p = 0.0005).77 This association was also observed in tumor-free sentinel nodes associated with ulcerated primaries.11,77 In another study evaluating the prognostic and predictive value of DCs in the progression of cutaneous malignant melanoma, it was suggested that a higher number of CD207/langerin+ DCs at the border of the tumor favored disease progression. There was a significantly higher number of peritumoral CD207/langerin+ cells in ulcerated invasive melanoma samples in comparison with tumors without ulceration.78
Cytokine Profile
Cytokines are small proteins involved in the regulation of immune cell function. Chemokines are a family of cytokines involved in the chemotaxis and homing of leukocytes. IL-8 (CXCL8) is a chemokine expressed by melanoma. In one study of patients with malignant melanoma (n = 125), circulating levels of IL-8 were significantly increased in melanoma patients compared with healthy controls. Elevated serum concentrations of IL-8 were associated with advanced disease stages and tumor burden.79 Vihinen et al suggested that the chemokine profile of an ulcerated tumor would aid in the prediction of melanoma metastases.80 The relationship between IL-8 (CXCL8) and ulceration in the setting of melanoma has not been investigated. As mentioned above, Winnepenninckx et al found gene expression profiles for primary cutaneous ulcerated melanomas expressed enhanced IL6, a gene encoding cytokines. Genes were chosen if their expression was associated with 4-year distant metastasis-free survival. IL6 was the only gene that encoded for a cytokine in the study.12
Response to Therapy
The value of primary tumor ulceration as a predictive marker of therapeutic response has been evaluated in several different contexts. In those clinical studies in which ulceration status has been examined relative to therapeutic response, significant differences in outcome have been identified that are highlighted below. With the introduction of new therapeutic strategies such as immune checkpoint inhibition and targeted therapies with known benefits, inclusion of primary tumor ulceration status as a feature for examination relative to outcome measures may prove valuable for treatment planning.
Interferons
The interferons (IFN) are a group of cytokines that can directly impact tumor cell growth or indirectly induce the immune system to eliminate tumor cells. The efficacy of IFN adjuvant therapy for melanoma has been debated due to inconsistent results between studies, but overall, randomized control trials have shown modest efficacy.80,81 Multiple studies have demonstrated that patients with ulcerated primary tumors benefit significantly from adjuvant IFN therapy.11,80,82 In a meta-analysis performed by Eggermont et al, two Phase III adjuvant trials, EORTC 18952 and 18991, compared IFN/PEG-IFN to observation alone. The impact of treatment was greater in the ulcerated group (n = 849) compared with the non-ulcerated group (n = 1336) for recurrence-free survival (RFS, p = 0.02), distant metastasis-free survival (DMFS, p < 0.001) and overall survival (OS, p < 0.001). The greatest risk reductions were observed in patients with ulceration and stage IIb/III-N1 tumors, with an estimated hazard ratio (HR) for RFS, DMFS, and OS of 0.69 (p = 0.003), 0.59 (p < 0.0001) and 0.58 (p < 0.0001), respectively.83 Ulceration was the most important determinant for PEG-IFN treatment utility. In patients with non-ulcerated primary melanomas, efficacy of adjuvant interferon therapy was uniformly absent. In the long-term follow-up analysis of EORTC 18991, the impact on the ulcerated patient population was preserved. In sentinel node positive patients with an ulcerated primary tumor, hazard ratio reductions of 30–40% at 7.6 years median follow-up were observed.83
Post-hoc analysis of the Sunbelt trial results regarding the impact of ulceration of the primary tumor on adjuvant IFN-treatment efficacy in sentinel node positive patients demonstrated that a significant improvement of disease-free survival was observed only in IFN-treated patients with an ulcerated primary.78,84 While IFN therapy use in melanoma has declined since the completion of these trials, the results indicate a clear difference in the tumor immune response when ulceration is present in the primary tumor. The impact of ulceration should therefore be considered in the evaluation of any melanoma immune-based treatment.11
Tumor Vaccines
Ulceration of melanoma tumors has been associated with clinical benefit from adjuvant vaccination with tumor-specific antigenic peptides in primary melanoma.85 In a study of 41 patients receiving a series of injections consisting of 4 antigenic peptides derived from MAGE-A3, NA17, gp100 and tyrosinase. Multivariate analysis assessing prognostic factors suggested that the ulceration of the primary tumor was associated with a clinical benefit following vaccination (HR 0.295, 95% CI 0.09–0.97), but the specific measures for improved clinical outcomes were not defined.85 In a 2021 study of Seviprotimut-L, a polyvalent melanoma vaccine, recurrence-free survival was greatest in patients <60 years old (HR 0.324, 95% CI 0.121–0.864) and those with ulcerated primary melanomas (HR 0.493, 95% CI 0.255–0.952).86 Thus, further trials examining efficacy of novel tumor vaccines for treatment of melanoma may benefit from stratification of patient groups by ulceration status or use of ulceration as an inclusion criterion.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICI), such as ipilimumab (targets CTLA-4), nivolumab and pembrolizumab (target PD-1) are novel therapeutic antibodies that boost T cell immune responses and have been demonstrated to improve overall survival in patients with advanced melanoma.87 Few studies have examined ulceration status of the primary tumor for correlation with outcome or disease progression. A study of 385 patients treated with ipilimumab for stage IV melanoma found that ulceration status of the primary tumor was not predictive of patients’ response to ipilimumab. However, in this study cohort, ulceration was associated with an increased risk of death (adjusted HR 1.47, 95% CI 0.95-2.26).87 A Phase 3 trial evaluating adjuvant ipilimumab at a dose of 10 mg per kilogram in patients who had undergone complete resection of stage III melanoma found the survival benefit of ipilimumab over placebo was generally consistent between patients with ulcerated and non-ulcerated primary tumors.88 In contrast, a meta-analysis investigating pembrolizumab and ipilimumab usage found higher RFS in ulcerated malignant melanoma (MM) compared to non-ulcerated MM (pembrolizumab: HR 0.52, 95% CI 0.35–0.79 vs 0.68; 95% CI 0.45–1.05, ipilimumab: HR 0.64, 95% CI 0.44–0.94, vs 0.80, 95% CI 0.54–1.20).51
Conclusions
Among the studies examining the features of ulcerated melanoma, many show promise as potential prognostic tools, markers of differential immunogenicity and indicators of oncogenic drivers of invasion and metastasis for future research. Several notable associations in relation to ulcerated melanoma tumors have been identified (Figure 1, Table 1). The incidence of ulcerated melanoma is greater in males, in patients 50 years or older, and in individuals that are non-white. Risk factors such as diabetes, smoking, low vitamin D, and elevated body mass index have also been associated with an increased occurrence of ulcerated melanoma. Histologically, spindle cell morphology has been shown to correlate significantly with tumor ulceration, along with vascular density, vasculogenic mimicry, and angiotropism. Molecular data have shown that ulceration status is correlated with proteins involved in epithelial-mesenchymal transition, including high N-cadherin, low E-cadherin and low PTEN. Ulceration status is also correlated with proteins associated with antigen presentation (increased MHC I and MX1), autophagy (increased non-cytoplasmic Beclin 1 and decreased cytoplasmic LC3), and cell cycle (increased cytoplasmic Skp2). Differential microRNA expression holds promise as a potential prognostic biomarker of malignancy and disease spread within the setting of ulceration. Specifically, microRNA-106-5b has been shown to be increased in ulcerated melanoma and microRNA-145-5p, microRNA-203-3p, microRNA-let-7b and microRNA-1469 has been shown to be decreased. Additionally, adjuvant IFN therapy has shown increased efficacy with respect to survival outcomes in patients with ulcerated melanomas, which is likely the consequence of an altered tumor microenvironment. When ulceration is present, there is a higher density of macrophages and dendritic cells and enhanced expression of pro-inflammatory cytokines, such as IL-6. However, the molecular and cellular differences associated with the ulcerated state and their clinical implications in melanoma patients are complex. Further study of this unique clinical and histologic presentation will aid in determining how these differences can be harnessed to improve care for patients with melanoma.
 |
Table 1 Features Correlated with Ulcerated Cutaneous Melanoma |
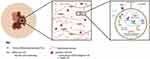 |
Figure 1 Features correlated with ulcerated cutaneous melanoma. Note: Created with BioRender.com. |
Funding
This work was supported in part by Grant Number 5T32 CA933840 (to MJD) and Grant Number 1T32 GM139784-01A1 (to CDA) from the National Institute of Health, P30 CA016058, National Cancer Institute, Bethesda, MD to the Comprehensive Cancer Center, The Ohio State University, Columbus, OH, and the Pelotonia Institute of Immuno-oncology (PIIO) at The Ohio State University. This research was also supported by Award Number UM1CA186712 from the National Cancer Institute and The John B. and Jane T. McCoy Chair in Cancer Research Endowment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Spatz A, Cook MG, Elder DE, Piepkorn M, Ruiter DJ, Barnhill RL. Interobserver reproducibility of ulceration assessment in primary cutaneous melanomas. Eur J Cancer. 2003;39(13):1861–1865. doi:10.1016/S0959-8049(03)00325-3
2. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi:10.3322/caac.21409
3. Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) and beyond. Ann Surg Oncol. 2018;25(8):2105–2110. doi:10.1245/s10434-018-6513-7
4. Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199. doi:10.1200/JCO.2009.23.4799
5. Haydu LE, Murali R, Bonenkamp JJ, Thompson JF, Scolyer RA, Scolyer RA. Prognostic importance of the extent of ulceration in patients with clinically localized cutaneous melanoma. Ann Surg. 2012;255(6):1165–1170. doi:10.1097/SLA.0b013e31824c4b0b
6. Portelli F, Galli F, Cattaneo L, et al. The prognostic impact of the extent of ulceration in patients with clinical stage I–II melanoma: a multicentre study of the Italian Melanoma Intergroup (IMI). Br J Dermatol. 2021;184(2):281–288. doi:10.1111/bjd.19120
7. Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American joint committee on cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199. doi:10.1200/JCO.2010.31.5812
8. Gogas H, Eggermont AMM, Hauschild A, et al. Biomarkers in melanoma. Ann Oncol. 2009;20:vi8–vi13. doi:10.1093/annonc/mdp251
9. Spatz A, Stock N, Batist G, van Kempen LC. The biology of melanoma prognostic factors. Discov Med. 2010;10(50):87–93.
10. Newton‐Bishop JA, Davies JR, Latheef F, et al. 25‐Hydroxyvitamin D2/D3 levels and factors associated with systemic inflammation and melanoma survival in the leeds melanoma cohort. Int J Cancer. 2015;136(12):2890–2899. doi:10.1002/ijc.29334
11. Eggermont AMM, Spatz A, Lazar V, Robert C. Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol. 2012;24(2):137–140. doi:10.1097/CCO.0b013e32834fcb0d
12. Winnepenninckx V, Lazar V, Michiels S, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98(7):472–482. doi:10.1093/jnci/djj103
13. Storr SJ, Safuan S, Mitra A, et al. Objective assessment of blood and lymphatic vessel invasion and association with macrophage infiltration in cutaneous melanoma. Mod Pathol. 2012;25(4):493–504. doi:10.1038/modpathol.2011.182
14. Jewell R, Elliott F, Laye J, et al. The clinicopathological and gene expression patterns associated with ulceration of primary melanoma. Pigment Cell Melanoma Res. 2015;28(1):94–104. doi:10.1111/pcmr.12315
15. Egger ME, Gilbert JE, Burton AL, et al. Lymphovascular invasion as a prognostic factor in melanoma. Am Surg. 2011;77(8):992–997. doi:10.1177/000313481107700816
16. Avilés-Izquierdo JA, Lázaro-Ochaita P. Histological ulceration as a prognostic factor in cutaneous melanoma: a study of 423 cases in Spain. Clin Transl Oncol. 2012;14(3):237–240. doi:10.1007/s12094-012-0790-6
17. Scoggins CR, Ross MI, Reintgen DS, et al. Gender-related differences in outcome for melanoma patients. Ann Surg. 2006;243(5):693. doi:10.1097/01.sla.0000216771.81362.6b
18. Balch CM, Wilkerson JA, Murad TM, Soong S, Ingalls AL, Maddox WA. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45(12):3012–3017. doi:10.1002/1097-0142(19800615)45:12<3012::AID-CNCR2820451223>3.0.CO;2-O
19. McGovern VJ, Shaw HM, Milton GW, McCarthy WH. Ulceration and prognosis in cutaneous malignant melanoma. Histopathology. 1982;6(4):399–407. doi:10.1111/j.1365-2559.1982.tb02737.x
20. Callender GG, Egger ME, Burton AL, et al. Prognostic implications of anatomic location of primary cutaneous melanoma of 1 mm or thicker. Am J Surg. 2011;202(6):659–665. doi:10.1016/j.amjsurg.2011.06.048
21. Boczar D, Sisti A, Restrepo DJ, et al. National analysis of patients with ulcerated melanoma in the United States. Anticancer Res. 2020;40(2):1055–1058. doi:10.21873/anticanres.14042
22. Davies J, Muralidhar S, Randerson‐Moor J, et al. Ulcerated melanoma: systems biology evidence of inflammatory imbalance towards pro‐tumourigenicity. Pigment Cell Melanoma Res. 2022;35(2):252–267. doi:10.1111/pcmr.13023
23. von Schuckmann LA, Smith D, Hughes MCB, et al. Associations of statins and diabetes with diagnosis of ulcerated cutaneous melanoma. J Invest Dermatol. 2017;137(12):2599–2605. doi:10.1016/j.jid.2017.07.836
24. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279(20):1615–1622. doi:10.1001/jama.279.20.1615
25. Bonovas S, Nikolopoulos G, Filioussi K, Peponi E, Bagos P, Sitaras NM. Can statin therapy reduce the risk of melanoma? A meta-analysis of randomized controlled trials. Eur J Epidemiol. 2010;25(1):29–35. doi:10.1007/s10654-009-9396-x
26. Curiel-Lewandrowski C, Nijsten T, Gomez ML, Hollestein LM, Atkins MB, Stern RS. Long-term use of nonsteroidal anti-inflammatory drugs decreases the risk of cutaneous melanoma: results of a United States case–control study. J Invest Dermatol. 2011;131(7):1460–1468. doi:10.1038/jid.2011.58
27. Jagtap D, Rosenberg CA, Martin LW, et al. Prospective analysis of association between use of statins and melanoma risk in the women’s health initiative. Cancer. 2012;118(20):5124–5131. doi:10.1002/cncr.27497
28. Xu Z, Shi P, Yibulayin F, Feng L, Zhang H, Wushou A. Spindle cell melanoma: incidence and survival, 1973–2017. Oncol Lett. 2018;16(4):5091–5099. doi:10.3892/ol.2018.9247
29. Lade‐Keller J, Riber‐Hansen R, Guldberg P, Schmidt H, Hamilton‐Dutoit SJ, Steiniche T. E‐to N‐cadherin switch in melanoma is associated with decreased expression of phosphatase and tensin homolog and cancer progression. Br J Dermatol. 2013;169(3):618–628. doi:10.1111/bjd.12426
30. Måsbäck A, Westerdahl J, Ingvar C, Olsson H, Jonsson N. Cutaneous malignant melanoma in southern Sweden 1965, 1975, and 1985: prognostic factors and histologic correlations. Cancer. 1997;79(2):275–283. doi:10.1002/(SICI)1097-0142(19970115)79:2<275::AID-CNCR11>3.0.CO;2-Y
31. Wei X, Wu D, Chen Y, et al. Prognostic value of ulceration varies across Breslow thicknesses and clinical stages in acral melanoma: a retrospective study. Br J Dermatol. 2022;186:977–987. doi:10.1111/bjd.21026
32. Vlaykova T, Muhonen T, Hahka‐Kemppinen M, Pyrhönen S, Jekunen A. Vascularity and prognosis of metastatic melanoma. Int J Cancer. 1997;74(3):326–329. doi:10.1002/(SICI)1097-0215(19970620)74:3<326::AID-IJC16>3.0.CO;2-9
33. Pisacane AM, Picciotto F, Risio M. CD31 and CD34 expression as immunohistochemical markers of endothelial transdifferentiation in human cutaneous melanoma. Anal Cell Pathol. 2007;29(1):59–66. doi:10.1155/2007/486579
34. Li X, Karras P, Torres R, et al. Disseminated melanoma cells transdifferentiate into endothelial cells in intravascular niches at metastatic sites. Cell Rep. 2020;31(11):107765. doi:10.1016/j.celrep.2020.107765
35. Barnhill R, Dy K, Lugassy C. Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol. 2002;119(3):705–706. doi:10.1046/j.1523-1747.2002.01871.x
36. Lugassy C, Vernon SE, Busam K, et al. Angiotropism of human melanoma: studies involving in transit and other cutaneous metastases and the chicken chorioallantoic membrane: implications for extravascular melanoma invasion and metastasis. Am J Dermatopathol. 2006;28(3):187. doi:10.1097/00000372-200606000-00001
37. Lugassy C, Wadehra M, Li X, et al. Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron. 2013;6(1):19–29. doi:10.1007/s12307-012-0128-5
38. Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109–113. doi:10.1038/nature13111
39. Bønnelykke-Behrndtz ML, Steiniche T, Nørgaard P, et al. Loss of E-cadherin as part of a migratory phenotype in melanoma is associated with ulceration. Am J Dermatopathol. 2017;39(9):672–678. doi:10.1097/DAD.0000000000000750
40. Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. doi:10.1016/j.immuni.2010.11.011
41. Rose AE, Christos PJ, Lackaye D, et al. Clinical relevance of detection of lymphovascular invasion in primary melanoma using endothelial markers D2-40 and CD34. Am J Surg Pathol. 2011;35(10):1441. doi:10.1097/PAS.0b013e31822573f5
42. Sahni D, Robson A, Orchard G, Szydlo R. The use of LYVE-1 antibody for detecting lymphatic involvement in patients with malignant melanoma of known sentinel node status. J Clin Pathol. 2005;58(7):715–721. doi:10.1136/jcp.2004.020123
43. Massi D, Puig S, Franchi A, et al. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case–control study. J Clin Pathol. 2006;59(2):166–173. doi:10.1136/jcp.2005.028431
44. Vihinen P, Koskivuo I, Syrjänen K, Tervahartiala T, Sorsa T, Pyrhönen S. Serum matrix metalloproteinase-8 is associated with ulceration and vascular invasion of malignant melanoma. Melanoma Res. 2008;18(4):268–273. doi:10.1097/CMR.0b013e3283090031
45. Nikkola J, Vihinen P, Vlaykova T, Hahka‐Kemppinen M, Kähäri V, Pyrhönen S. High expression levels of collagenase‐1 and stromelysin‐1 correlate with shorter disease‐free survival in human metastatic melanoma. Int J Cancer. 2002;97(4):432–438. doi:10.1002/ijc.1636
46. Liu H, Wei Q, Gershenwald JE, et al. Influence of single nucleotide polymorphisms in the MMP1 promoter region on cutaneous melanoma progression. Melanoma Res. 2012;22(2):169. doi:10.1097/CMR.0b013e32834fc46b
47. Petrova YI, Schecterson L, Gumbiner BM. Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell. 2016;27(21):3233–3244. doi:10.1091/mbc.E16-01-0058
48. Mitchell B, Leone DA, Feller JK, Yang S, Mahalingam M. BRAF and epithelial-mesenchymal transition in primary cutaneous melanoma: a role for Snail and E-cadherin? Hum Pathol. 2016;52:19–27. doi:10.1016/j.humpath.2015.12.030
49. Safaee Ardekani G, Jafarnejad SM, Khosravi S, Martinka M, Ho V, Li G. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169(2):320–328. doi:10.1111/bjd.12351
50. Falkenius J, Keskitalo J, Kanter L, et al. A biomarker panel predicts recurrence-free survival in ulcerated primary cutaneous melanoma. Acta Oncologica. 2022;61(1):14–21. doi:10.1080/0284186X.2021.1989719
51. Christofyllakis K, Pföhler C, Bewarder M, et al. Adjuvant therapy of high-risk (Stages IIC–IV) malignant melanoma in the post interferon-alpha era: a systematic review and meta-analysis. Front Oncol. 2021;10:3481. doi:10.3389/fonc.2020.637161
52. Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22(20):3113–3122. doi:10.1038/sj.onc.1206451
53. Cabrita R, Mitra S, Sanna A, et al. The role of PTEN loss in immune escape, melanoma prognosis and therapy response. Cancers. 2020;12(3):742. doi:10.3390/cancers12030742
54. Mikhail M, Velazquez E, Shapiro R, et al. PTEN expression in melanoma: relationship with patient survival, Bcl-2 expression, and proliferation. Clin Cancer Res. 2005;11(14):5153–5157. doi:10.1158/1078-0432.CCR-05-0397
55. Slipicevic A, Holm R, Nguyen MTP, Bøhler PJ, Davidson B, Flørenes VA. Expression of activated Akt and PTEN in malignant melanomas: relationship with clinical outcome. Am J Clin Pathol. 2005;124(4):528–536. doi:10.1309/YT58WWMTA6YR1PRV
56. de Unamuno Bustos B, Murria Estal R, Pérez Simó G, et al. Aberrant DNA methylation is associated with aggressive clinicopathological features and poor survival in cutaneous melanoma. Br J Dermatol. 2018;179(2):394–404. doi:10.1111/bjd.16254
57. Verver D, Poirier-Colame V, Tomasic G, et al. Upregulation of intratumoral HLA class I and peritumoral Mx1 in ulcerated melanomas. OncoImmunology. 2019;8(11):e1660121. doi:10.1080/2162402X.2019.1660121
58. Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi:10.1038/nm.3708
59. Martinoli C, Gandini S, Luise C, et al. Maspin expression and melanoma progression: a matter of sub-cellular localization. Mod Pathol. 2014;27(3):412–419. doi:10.1038/modpathol.2013.157
60. Bezrookove V, Nosrati M, Miller JR, et al. Role of elevated PHIP copy number as a prognostic and progression marker for cutaneous melanoma. Clin Cancer Res. 2018;24(17):4119–4125. doi:10.1158/1078-0432.CCR-18-0791
61. Bezrookove V, de Semir D, Nosrati M, et al. Prognostic impact of PHIP copy number in melanoma: linkage to ulceration. J Invest Dermatol. 2014;134(3):783–790. doi:10.1038/jid.2013.369
62. Gironi LC, Colombo E, Pasini B, et al. Melanoma-prone families: new evidence of distinctive clinical and histological features of melanomas in CDKN2A mutation carriers. Arch Dermatol Res. 2018;310(10):769–784. doi:10.1007/s00403-018-1866-0
63. Helgadottir H, Tuominen R, Olsson H, Hansson J, Höiom V. Cancer risks and survival in patients with multiple primary melanomas: association with family history of melanoma and germline CDKN2A mutation status. J Am Acad Dermatol. 2017;77(5):893–901. doi:10.1016/j.jaad.2017.05.050
64. Chen G, Cheng Y, Zhang Z, Martinka M, Li G. Cytoplasmic Skp2 expression is increased in human melanoma and correlated with patient survival. PLoS One. 2011;6(2):e17578. doi:10.1371/journal.pone.0017578
65. Miracco C, Cevenini G, Franchi A, et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol. 2010;41(4):503–512. doi:10.1016/j.humpath.2009.09.004
66. Lee KH, Suh HY, Lee MW, Lee WJ, Chang SE. Prognostic significance of epidermal growth factor receptor expression in distant metastatic melanoma from primary cutaneous melanoma. Ann Dermatol. 2021;33(5):432. doi:10.5021/ad.2021.33.5.432
67. Katunaric M, Jurišic D, Petkovic M, Grahovac M, Grahovac B, Zamolo G. EGFR and cyclin D1 in nodular melanoma: correlation with pathohistological parameters and overall survival. Melanoma Res. 2014;24(6):584–591. doi:10.1097/CMR.0000000000000123
68. Papadodima O, Kontogianni G, Piroti G, Maglogiannis I, Chatziioannou A. Genomics of cutaneous melanoma: focus on next-generation sequencing approaches and bioinformatics. J Transl Genet Genom. 2019;3:7.
69. de Unamuno Bustos B, Murria Estal R, Pérez Simó G, et al. Towards personalized medicine in melanoma: implementation of a clinical next-generation sequencing panel. Sci Rep. 2017;7(1):1–11. doi:10.1038/s41598-017-00606-w
70. Lee JJ, Sholl LM, Lindeman NI, et al. Targeted next-generation sequencing reveals high frequency of mutations in epigenetic regulators across treatment-naïve patient melanomas. Clin Epigenetics. 2015;7(1):1–17. doi:10.1186/s13148-015-0091-3
71. Valentini V, Zelli V, Gaggiano E, et al. miRNAs as potential prognostic biomarkers for metastasis in thin and thick primary cutaneous melanomas. Anticancer Res. 2019;39(8):4085–4093. doi:10.21873/anticanres.13566
72. Babapoor S, Wu R, Kozubek J, Auidi D, Grant-Kels JM, Dadras SS. Identification of microRNAs associated with invasive and aggressive phenotype in cutaneous melanoma by next-generation sequencing. Lab Invest. 2017;97(6):636–648. doi:10.1038/labinvest.2017.5
73. Lin N, Zhou Y, Lian X, Tu Y. Expression of microRNA-106b and its clinical significance in cutaneous melanoma. Genet Mol Res. 2015;14(4):16379–16385. doi:10.4238/2015.December.9.6
74. DiVincenzo MJ, Ren C, Suarez-Kelly L, et al. Determination of the expression patterns and functional implications of microRNAs in ulcerated cutaneous melanoma. Cancer Res. 2020;80:3696.
75. DiVincenzo MJ, Barricklow Z, Schwarz E, et al. Loss of miR-1469 expression mediates melanoma cell migration and invasion. PLoS One. 2021;16(9):e0256629. doi:10.1371/journal.pone.0256629
76. de Moll EH, Fu Y, Qian Y, et al. Immune biomarkers are more accurate in prediction of survival in ulcerated than in non-ulcerated primary melanomas. Cancer Immunol Immunother. 2015;64(9):1193–1203. doi:10.1007/s00262-015-1726-0
77. Elliott B, Scolyer RA, Suciu S, et al. Long-term protective effect of mature DC-LAMP+ dendritic cell accumulation in sentinel lymph nodes containing micrometastatic melanoma. Clin Cancer Res. 2007;13(13):3825–3830. doi:10.1158/1078-0432.CCR-07-0358
78. Dyduch G, Tyrak KE, Glajcar A, Szpor J, Okoń K. CD207+/langerin positive dendritic cells in invasive and in situ cutaneous malignant melanoma. Adv Dermatol Allergol. 2017;34(3):233. doi:10.5114/ada.2017.67845
79. Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19(2):577–583. doi:10.1200/JCO.2001.19.2.577
80. Vihinen PP, Pyrhönen SO, Kähäri VM. New prognostic factors and developing therapy of cutaneous melanoma. Ann Med. 2003;35(2):66–78. doi:10.1080/07853890310009980
81. Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30(31):3810–3818. doi:10.1200/JCO.2011.41.3799
82. Abdolvahab MH, Darvishi B, Zarei M, Majidzadeh-A K, Farahmand L. Interferons: role in cancer therapy. Immunotherapy. 2020;12(11):833–855. doi:10.2217/imt-2019-0217
83. Eggermont AMM, Suciu S, Testori A, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48(2):218–225. doi:10.1016/j.ejca.2011.09.028
84. Egger ME, Scoggins CR, McMasters KM. The sunbelt melanoma trial. Ann Surg Oncol. 2020;27(1):28–34. doi:10.1245/s10434-019-07828-4
85. Baurain J, Stas M, Hammouch F, et al. Association of primary melanoma ulceration and clinical benefit of adjuvant vaccination with tumor-specific antigenic peptides. J Clin Oncol. 2009;27(15_suppl):3022. doi:10.1200/jco.2009.27.15_suppl.3022
86. Slingluff CL, Lewis KD, Andtbacka R, et al. Multicenter, double-blind, placebo-controlled trial of seviprotimut-L polyvalent melanoma vaccine in patients with post-resection melanoma at high risk of recurrence. J Immunother Cancer. 2021;9:10. doi:10.1136/jitc-2021-003272
87. Rossfeld K, Hade EM, Gangi A, et al. Metastatic melanoma patients’ sensitivity to ipilimumab cannot be predicted by tumor characteristics. Int J Surg Oncol. 2017;2(9):e43. doi:10.1097/IJ9.0000000000000043
88. Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi:10.1056/NEJMoa1611299
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
