Back to Journals » Drug Design, Development and Therapy » Volume 14
Network Pharmacology-Based Prediction and Verification of the Active Ingredients and Potential Targets of Zuojinwan for Treating Colorectal Cancer
Authors Huang S , Zhang Z , Li W , Kong F , Yi P , Huang J, Mao D, Peng W , Zhang S
Received 23 February 2020
Accepted for publication 2 June 2020
Published 14 July 2020 Volume 2020:14 Pages 2725—2740
DOI https://doi.org/10.2147/DDDT.S250991
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Siqi Huang,1 Zheyu Zhang,1 Wenqun Li,2 Fanhua Kong,3 Pengji Yi,1 Jianhua Huang,4 Dan Mao,1 Weijun Peng,1 Sifang Zhang1
1Department of Integrated Traditional Chinese & Western Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China; 2Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China; 3Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China; 4Hunan Academy of Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan 410013, People’s Republic of China
Correspondence: Sifang Zhang; Weijun Peng
Department of Integrated Traditional Chinese & Western Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China
Tel +86-73185292111
Email [email protected]; [email protected]
Background: Zuojinwan (ZJW), a famous Chinese medicine formula, has been widely used to treat colorectal cancer (CRC). However, its bioactive compounds, potential targets, and molecular mechanism remain largely elusive.
Aim: A network pharmacology-based strategy combined with molecular docking studies and in vitro validation were employed to investigate bioactive compounds, potential targets, and molecular mechanism of ZJW against CRC.
Materials and Methods: Bioactive compounds and potential targets of ZJW, as well as related genes of CRC, were acquired from public databases. Important ingredients, potential targets, and signaling pathways were determined through bioinformatics analysis, including protein–protein interaction (PPI), the Gene Ontology (GO), and the Kyoto Encyclopedia of Genes and Genomes (KEGG). Subsequently, molecular docking and cell experiments were performed to further verify the findings.
Results: A total of 36 bioactive ingredients of ZJW and 163 gene targets of ZJW were identified. The network analysis revealed that quercetin, baicalein, wogonin, beta-sitosterol, and isorhamnetin may be candidate agents. The AKT1, JUN, CDKN1A, BCL2L1, and NCOA1 could become potential drug targets. The KEGG indicated that PI3K-AKT signaling pathway may play an important role in the effect of ZJW against CRC. Molecular docking suggested that quercetin, baicalein, and wogonin combined well with AKT1 and JUN. The in vitro experiment showed that quercetin, the most important ingredient of ZJW, could induce apoptosis of HCT116 cells through PI3K-Akt signaling pathway. This finding was congruent with the prediction obtained through the network pharmacology approach.
Conclusion: This study comprehensively illuminated the active ingredients, potential targets, and molecular mechanism of ZJW against CRC. It also provided a promising approach to uncover the scientific basis and therapeutic mechanism of traditional Chinese medicine (TCM) formula treating for disease.
Keywords: Zuojinwan, colorectal cancer, network pharmacology, PI3K/AKT signal pathway
Introduction
Colorectal cancer (CRC) is considered the third most common cancer among men and second among women, and the second most common cause of cancer death worldwide.1 The incidence and mortality of CRC is rapidly rising particularly in low- and middle-income countries, and it is estimated that the global burden of CRC increases by 60% over 2.2 million new case and 1.1 million cancer death by 2030.2,3 Despite recent advances in therapy and multidisciplinary care, patients with CRC continue to suffer from severe adverse actions, which impair prognosis and reduce the quality of life. More than 50% of CRC patients were diagnosed at an advanced stage, and the 5-year survival rate is <14%.4–6 Hence, the development of alternative therapies with low toxicity is important for providing more effective clinical therapy and alleviating adverse reactions.
Traditional Chinese medicine (TCM), as a crucial complementary and alternative medicine, offers beneficial effects to patients with cancer and has been widely accepted worldwide.7 Previous studies demonstrated that traditional herbal medicine, as adjuvant therapy, combined with chemotherapy or radiotherapy, could enhance treatment effectiveness, alleviate adverse actions, improve quality of life, and prolong survival time.8–12 Owing to its effectiveness and fewer side effects, it is increasingly popular in Western countries. Zuojinwan (ZJW), a classical TCM formula, was first described in Danxi Xinfa for a long history.13 Besides, ZJW was also officially listed in the Chinese Pharmacopoeia.14 ZJW, consists of Coptis chinensis Franch (R. coptidis) and Evodia ruticarpa (A.Juss.) Hook.f. and Thomson (E. rutaecarpa) at a ratio of 6:1 (w/w), was initially employed in treating gastro-intestinal disorders in China.15–17 From bench to bedside, an increasing body of evidence suggests that ZJW exerts favorable anti-cancer effects. It has been demonstrated that ZJW significantly suppresses the growth of eight kinds of human cancer cell lines by inducing mitochondrial apoptosis.14 Besides, oral administration of ZJW notably inhibited tumor growth in HepG2 xenograft-bearing immunocompetent mice and breast cancer ZHENG model.18,19 Furthermore, ZJW extracts inhibit CRC cells growth and invasion, and enhance the sensitivity of chemotherapy of CRC cells and gastric cancer cells to chemotherapy.20–22 Similar to other TCM formulae, ZJW involves multiple components, targets, and pathways against tumors. However, its major bioactive ingredients and pharmacological molecular mechanism against CRC remain poorly understood.
Network pharmacology is a promising approach to uncover the scientific basis and therapeutic mechanism of TCM formulae, and identify new drugs.23 With the rapid development of bioinformatics and pharmacology, network pharmacology comprehensively investigates interrelationships of drugs, targets and diseases, and shows the network of drug-targets-disease in a visual way. This approach clearly observes the effects of drugs on disease,24 is consistent with the TCM theory that emphasizes synergy between Chinese medicine.25 Besides, new important bioactive compound of TCM formula could be found based on network pharmacology.
In the present study, we aim to explore and verify the molecular mechanisms and pathways of the main bioactive ingredients of ZJW in the treatment of CRC based on a network pharmacology with molecular docking and in vitro validation. The workflow is shown in Figure 1.
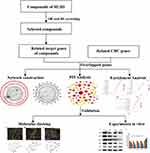 |
Figure 1 Workflow for network pharmacology-based prediction and validation of anti-CRC mechanism. |
Materials and Methods
Data Preparation
Composition of ZJW
All ingredients of ZJW were acquired from three public databases including Traditional Chinese Medicines for Systems Pharmacology Database and Analysis Platform (TCMSP, available online: http://tcmspw.com/tcmsp.php), TCM Database@Taiwan (http://tcm.cmu.edu.tw/), and A Bioinformation Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM, available online: http://bionet.ncpsb.org/batman-tcm/index.php/home/index/result/jobId/batman-I2019-04-16-17745-1555420409.html) and related studies.
Pharmacokinetic ADME Prediction
The TCMSP database was used to select all compounds based on absorption, distribution, metabolism, and excretion (ADME) criteria. In this study, oral bioavailability (OB) and drug-likeness (DL) were adopted to identify the bioactive ingredients of ZJW. OB described the percentage of an oral dose capable of producing pharmacological activity.26 DL was applied to determine the similarity of a compound to conventional drugs and its physicochemical properties.27 Only compounds that had related targets and met the ADME criteria (ie, OB threshold≥30% and DL threshold≥0.18) were included in the study for subsequent research.
Prediction Targets of Compounds
The TCMSP and DRUGBANK (https://www.drugbank.ca/) databases, a unique bioinformatics resource which combines detailed drug data with comprehensive drug target in formation, were utilized to predict the targets of all ingredients.28 The Uniprot database (https://www.uniprot.org/), which provides a comprehensive, high-quality, and free source of protein sequence and functional information, was applied to convert protein target names to their corresponding gene symbols. The predicted targets of CRC were collected from the GeneCards (https://www.genecards.org/, version 4.9.0) and OMIM (http://www.omim.org/, updated April 23, 2019) databases.
Bioinformatics Analysis
Protein–Protein Interaction (PPI) Data
The overlapping gene of compounds and CRC were considered as hub genes and analyzed using online STRING (https://string db.org/cgi/network.pl?taskId=wJDcOUv6JCzz, version 11.0, updated January 19, 2019) to obtain the PPI, with the species limited to “Homo sapiens” and a confidence score >0.990. The TSV format file, which was downloaded from the STRING database, was imported into the Cytoscape3.7.1 for analysis. Cytoscape 3.7.1, which is widely applied to network pharmacology research, was used to construct and visualize the network. Furthermore, it provides a basic set of characters for data integration, analysis, and visualization for complicated network analysis. Two key topological parameters (ie, degree and betweenness centrality) characterize the most important nodes in the network; higher quantitative values of topological parameters indicate greater importance of the node.
Enrichment Analysis
Based on the R-package clusterProfiler, the Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were applied to the target proteins. A p<0.001 denoted statistical significance. The KEGG database, which was established in 1995 by the Kanehisa laboratory in the Bioinformatics Center of Kyoto University, is typically used in network pharmacology to annotate the pathways of target genes.
Network Construction
Construction of the network was performed as follows: (1) herbs-bioactive compound-target network of ZJW (C-T network); (2) herbs-bioactive compound-disease-target network of treatment with ZJW for CRC (C-D-T); (3) PPI network of treatment with ZJW for CRC; and (4) bioactive compound-pathway-target (C-P-T) network.
Validation
Molecular Docking
Molecular docking, which combines known protein with small compounds, is a crucial technology of network pharmacology. Molecular docking was performed using the Molecular Operating Environment (MOE) (v2015.10) software to validate compound-target interaction.29 The 3D structure of protein was obtained from the Protein Data Bank (PDB) (http://www.rcsb.org/) and then imputed it into MOE to construct mating pocket of molecular docking after deleting water molecules, preparing protein structure and minimizing energy. The mating pocket was performed molecular docking with compounds whose structure was downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/).
Cell Experiments
HCT116 and HT29 cells, a human colon cancer cell line, were purchased from GeneChem Co., Ltd. (Shanghai, China). The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 µg/mL ampicillin, and 100 µg/mL streptomycin, at 37°C with 5% CO2 and 95% air.
In brief, HCT116 and HT29 cells were seeded in 96-well plates at 4×103 cells/well and treated with different concentrations of quercetin (0, 100, 150, 200, and 250 μΜ) for 24, 48, and 72 h. The Cell Counting Kit-8 (CCK-8) assay was utilized to detected cell proliferation at 450 nm wavelength. The Graphpad prism 7.0 software was used to analyze and plot the data.
HCT116 and HT29 cells were incubated in 6-well plates at 37°C and treated with quercetin at different concentrations (0, 100, 150, and 200μΜ) for 72 h. Subsequently, cell apoptosis was measured through flow cytometry with the Annexin V-fluorescein isothiocyanate/propidium iodide (Annexin V-FITC/PI) apoptosis detection kit (40302ES20; Yeasen Biotechnology Co. Ltd., Shanghai, China) according to the manufacturer instruction. Briefly, treated cells were collected and washed twice with cold phosphate-buffered saline, resuspended in 400 µL 1×binding buffer with 5 µL Annexin V-FITC and 5 µL PI staining solution, and incubated for 15 min in the dark at room temperature. Next, 400 µL 1×binding buffer was added and mixed. Finally, cell apoptosis was determined using flow cytometry (Guava PCA; Millipore, USA), and data were analyzed using the software FlowJo 7.6 (De Novo Software, Los Angeles, CA, USA).
Total protein was obtained from treated cells in radioimmunoprecipitation assay buffer (P0013B; Beyotime, Shanghai, China) and centrifuged at 12,000 × g and 4°C for 15 min. Proteins were separated via sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). After blocking in 5% bovine serum albumin (Gen-view Scientific Inc., USA) for 1.5 h at room temperature, the membranes were incubated with primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (Abclonal), PI3K (cell signal technique), AKT (cell signal technique), p-AKT (cell signal technique), BCL2 (Abclonal), and BAX (cell signal technique) at 4°C overnight. Next, the membranes were incubated with horseradish-peroxidase-conjugated secondary antibodies (proteintech) at room temperature for 1 h. Subsequently, the membranes were incubated with horseradish-peroxidase-conjugated secondary antibodies (proteintech) at room temperature for 1 h. The Enhanced Chemiluminescence Detection Kit (P10100; New Cell & Molecular Biotech Co., Ltd.) was used to detect and visualize protein bands.
Results
Data Statistics
Ingredients of ZJW
ZJW consists of R. coptidis and E. rutaecarpa. A total of 245 ingredients were recognized in ZJW, including 62 in R. coptidis and 183 in E. rutaecarpa. Among the 245 ingredients, eight were duplicated and thus eliminated, while the remaining 237 ingredients were eventually included in the analysis. According to a reference, three ingredients were included: alpha-bulnesene, aspartate, and nerolidol. The details are described in Table S1 (supplementary material).
Acquiring Compounds by ADME Screening and C-T Network Analysis
Two ADME-related models (ie, OB and DL) were utilized to screen all compounds with related targets. In total, after removing two duplicated compounds (ie, berberine and quercetin), 36 compounds (OB≥30% and DL≥0.18) were included in the study (13 in R. coptidis and 25 in E. rutaecarpa). The results are listed in Table 1. The C-T network includes 226 nodes and 587 edges shown in Figure 2. The pink ellipse represents 188 potential target genes of compounds. The blue triangle, red v and yellow diamond stand for compounds of E. rutaecarpa, R. coptidis and common compounds of E. rutaecarpa and R. coptidis, respectively. Furthermore, higher degrees indicated larger sizes, which present important nodes in the network. Quercetin, baicalein, wogonin, beta-sitosterol, and isorhamnetin, which exhibited higher degrees, play major roles in the effect of ZJW against CRC.
 |
Table 1 Bioactive Compounds of ZJW |
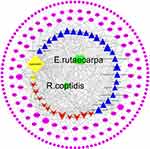 |
Figure 2 C-T network of ZJW. Pink ellipse represents genes. The blue triangle, red v and yellow diamond stand for compounds ZJW. The green diamond stand for E. rutaecarpa and R. coptidis. |
Potential Target Genes and C-D-T Network Analysis
After removing duplication values and transferring gene symbol, 188 potential target genes of compounds were collected from the TCMSP and DRUGBANK databases. CRC was used as a keyword for the search, and a total of 7481 related target genes for CRC were collected from the GeneCards and OMIM databases. Among 188 of compounds and 7481 CRC-related target genes, there were 163 overlapping related target genes, considered as the hub genes for subsequent research. The results are shown in Table S2 (see supplementary material). The C-D-T network contains 202 nodes and 786 edges (Figure 3). The red circle stands for gene, while the blue, pink, and navy-blue triangles stand for compounds of ZJW. The green diamond stands for CRC, E. rutaecarpa, and R. coptidis. The size of the shape is proportional to the degree. Higher degrees indicated that the compounds or genes play more important anti-CRC roles.
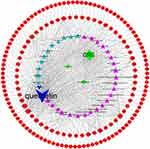 |
Figure 3 C-D-T network of ZJW. The red circle stands for gene, blue, pink and navy-blue triangle stand for compounds of ZJW. The green diamond stand for CRC, E. rutaecarpa and R. coptidis. |
Bioinformatics Analysis
Analysis on CRC-Related PPI
The 163 CRC-related hub genes were submitted to STRING to obtain PPI data. The network was subsequently constructed and visualized using Cytoscape 3.7.1. As shown in Figure 4, the PPI network included 69 nodes and 76 edges. Higher degrees indicated larger node sizes; red indicates the highest degree, and yellow represents the lowest degree. The nodes with the top five degrees were AKT1, JUN, CDKN1A, BCL2L1, and NCOA1. These nodes may play a pivotal role in the PPI network for the anti-CRC effect.
 |
Figure 4 PPI network of anti-CRC-related protein. Higher degrees indicated larger node sizes, and red indicates higher degree, and yellow represents the lowest degree. |
Enrichment Analyses
The R-package clusterProfiler was used to elucidate the biological process (BP), cell composition (CC), and molecular function (MF) annotation of the 163 hub target proteins. There were 902 GO terms, including 828 of BP, 24 of CC, and 50 of MF. Most GO terms of BP were related to response to lipopolysaccharide, response to molecule of bacterial origin, response to oxygen levels, response to oxidative stress, response to reactive oxygen species, response to steroid hormone, reactive oxygen species metabolic process, response to hypoxia, response to antibiotic, response to decreased oxygen levels, etc. The main terms of CC were associated with membrane raft, membrane microdomain, membrane region, caveola, transcription factor complex, etc. MF enrichment was mainly involved in nuclear receptor activity, transcription factor activity, direct ligand-regulated sequence-specific DNA binding, steroid hormone receptor activity, cytokine receptor binding, DNA-binding transcription activator activity, RNA polymerase II-specific, etc. The top 20 terms of BP, CC, and MF, which were ranked according to their adjusted p-value, are shown in Figure 5. Low-adjusted p-values and red color indicated greater enrichment of the GO terms.
 |
Figure 5 BP, CC, MF of GO analysis. |
The KEGG pathway annotation indicated that 149 of the 163 target genes were enriched and yielded 97 pathways (p< 0.001). It verified CRC-related pathways including the PI3K-AKT signaling pathway, MAPK signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, etc. Furthermore, the top 20 KEGG pathways with high counts are shown in Figure 6. The results distinctly suggested that the PI3K-AKT signaling pathway, which enriched a larger count among CRC-related pathways, played a key role in the treatment of CRC. The C-P-T network, containing 150 nodes and 762 edges, was constructed (Figure 7). The red circle nodes denote genes, pink triangle nodes denote compounds of ZJW, blue diamond nodes denote pathways, and green octagon nodes denote E. rutaecarpa and R. coptidis.
 |
Figure 6 Top 20 KEGG pathway with high count. |
Validation
Molecular Docking
Molecular docking was applied to validate the binding action mode of AKT1 and JUN with quercetin, wogonin, and baicalein, respectively, according to the network analysis. The results revealed that AKT1 interacted with quercetin, baicalein, and wogonin, as shown in Figure 8A–C, respectively. As shown in Figure 8A, the structure of quercetin could form two hydrogen bonds and one π-π bond with Ile290, Gln79, and Trp80 in AKT1. In Figure 8B, the structure of baicalein could form one hydrogen bond, one H-π bond, and two π-H bonds with Gln79, Trp80, Leu210, and Asp292 in AKT1. As shown in Figure 8C, one hydrogen bond was formed between wogonin and AKT1.
 |
Figure 8 (A–C) represent AKT1 interacted action mode with quercetin, baicalein and wogonin respectively. |
The results showed that JUN interacted with quercetin, wogonin, and baicalein, as shown in Figure 9A–C, respectively. As shown in Figure 9A, the structure of quercetin could form a hydrogen bond and a π-H bond with Ser144 and Val150 in JUN, respectively. Similarly, in Figure 9B, a hydrogen bond and a π-H bond were formed to bind baicalein with Glu243 and Gly143 in JUN, respectively. Wogonin interacted with Gly143 and Val150 in JUN through two π-H bonds (Figure 9C).
 |
Figure 9 (A–C) represent JUN interacted action mode with quercetin, baicalein and wogonin respectively. |
In conclusion, there is an interplay between ingredients and protein targets with different bonds. The binding energy was counted to assess the matching degree of ingredients with proteins. The detail binding energy score is shown in Table 2. Lower binding energy indicated greater stability. The results suggested that the ingredients could bind to the active site of protein targets.
 |
Table 2 The Binding Energy Score of Ingredients in ZJW Binding with AKT1 and JUN. AKT1, RAC-Alpha Serine/Threonine-Protein Kinase; JUN, Transcription Factor AP-1 |
Quercetin Suppressed the Proliferation of CRC Cells
CCK-8 assays were performed to determine the anti-cancer effect of treatment with quercetin at different concentrations (0, 100, 150, 200, and 250 µM) for 24, 48, and 72 h in HCT116 and HT29 cells. As shown in Figure 10A and B, the viability of HCT116 and HT29 cells was markedly reduced by quercetin in a dose- and time-dependent manner. The IC50 values for quercetin were 225.7, 186.3, and138.6 µM in HCT116 cells; 221.6, 185.4, 157.4 µM in HT29 cells for 24, 48, and 72 h, respectively.
Quercetin Promoted Apoptosis of CRC Cells
The effect of quercetin on the apoptosis of HCT116 and HT29 cells was detected through flow cytometric analysis. After treatment with 0, 100, 150, and 200 µM of quercetin for 72 h, CRC cells were stained with Annexin V-FITC and PI to determine the degree of apoptosis. Figure 11 shows that the percentage of apoptotic cells was notably increased in a dose-dependent manner after treatment with quercetin, strongly indicating that quercetin induced apoptosis in HCT116 and HT29 cells.
Quercetin Inhibits the PI3K/AKT Pathway in CRC Cells
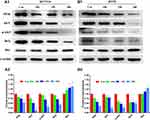
We further explored the molecular mechanism of quercetin involved in inducing apoptosis in HCT116 and HT29 cells. The PI3K/AKT pathway, which is a key pathway associated with the effect of ZJW against CRC, was analyzed through Western blotting. As shown in Figure 12, the protein levels of PI3K, AKT, p-AKT, and BCL2 were significantly decreased in a dose-dependent manner. In contrast, the protein levels of BAX were significantly increased. The results demonstrated that quercetin induced apoptosis in HCT116 and HT29 cells via the PI3K/AKT pathway.
Discussion
In the present study, a network pharmacology-based approach, molecular docking and in vitro experimental validation were performed to reveal bioactive compounds and the molecular mechanism of ZJW for treating CRC. A total of 36 bioactive compounds and 163 protein targets were selected from public databases. The PPI network showed the interaction of each protein and indicated that AKT1, JUN, CDKN1A, BCL2L1, and NCOA1 may play key roles in the effect of ZJW against CRC. The GO enrichment analysis annotated the function of protein targets from BP, CC, and MF. The KEGG pathway enrichment analysis revealed that 149 of the 163 target proteins significantly (p<0.001) enriched 97 cancer-related signal pathways, including the PI3K-AKT signaling pathway, MAPK signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, etc. Molecular docking suggested that important ingredients combined well with target proteins. The cell experiments verified that the key ingredient quercetin inhibited proliferation and induced apoptosis by suppressing the PI3K-AKT signaling pathway.
In recent years, naturally bioactive compounds are attracting considerable attention as anti-cancer agents, owing to their high therapeutic value and low systemic toxicity.30 Previous studies demonstrated that berberine and evodiamine, the major active compounds of ZJW, exhibited anti-cancer effects (colon cancer, breast cancer, liver cancer, lung cancer, CRC, etc.) by inhibiting proliferation, and inducing apoptosis and autophagic cell death.31–35 However, to the best of our knowledge, this is the first study to report that quercetin, which shows the highest degree among all compounds, is the most important compound of ZJW in the treatment of CRC. Quercetin belongs to the group of polyphenolic compounds termed flavonoids and is abundantly distributed in plants, foods, and beverages.36,37 Previous evidence showed that quercetin exerted its anti-cancer effects by suppressing proliferation and metastasis, and inducing apoptosis and autophagy.38–41 Chuang et al demonstrated that quercetin enhanced the anti-cancer activity of trichostatin A by upregulating the p300 protein in lung cancer.42 Lu et al showed that quercetin inhibited the migration and invasion of HCCLM3 cells by downregulating the expression of p-AKT1, Matrix Metalloproteinase (MMP) MMP-2, and MMP-9.43 Refolo et al revealed that quercetin suppressed proliferation and apoptosis was mediated by CB1 receptor.44 Moreover, quercetin enhances hypoxia-mediated apoptosis in HCT116 cells through AMPK-related signaling pathway.45,46 Furthermore, quercetin increased the sensitivity of MSI CRC cells to 5-FU.47
The PPI results indicated that AKT1, JUN, BCL2L, CDKN1A, and NCOA1, especially AKT1, may play a key role in the anti-CRC process. The KEGG results indicated that the PI3K-AKT signaling pathway, which exhibited the highest gene count enrichment among the cancer-related signal pathways, plays a critical role in the effect of ZJW against CRC. AKT1, belonging to the AKT family, regulates biological functions, such as cell survival, proliferation, metabolism, and growth to affect cancer growth.48,49 AKT is involved in the treatment of numerous types of cancer via various signaling pathways, especially the PI3K-AKT signaling pathway. Accumulating evidence indicates that the PI3K/AKT signaling pathway, which is one of the most frequently activated signal-transduction pathways in cancer, is involved in cell cycle, cell proliferation, growth, migration, angiogenesis, and apoptosis.50–53 Liu et al demonstrated that aloperine induced apoptosis and arrested the cell cycle in hepatocellular carcinoma cells through the PI3K-AKT signaling pathway.54 Hossan et al verified that cerberin inhibited cell proliferation, migration, and survival in pancreatic, triple-negative breast, and non-small cell lung cancer through the PI3K/AKT signaling pathway.55
Hence, molecular docking and in vitro experiments were performed to further verify the molecular mechanism of quercetin which possesses the highest degree and may be the most important bioactive compound of ZJW for treating for CRC. The results of molecular docking showed that quercetin interacted with AKT1 through three bonds, indicating that AKT1 may be a key action target for the effect of quercetin against CRC. Moreover, the cell experiments demonstrated that quercetin for the treatment of CRC suppressed the proliferation and induced apoptosis in HCT116 cell by inhibiting the PI3K-AKT signaling pathway with the downregulation of PI3K, AKT, and BCL2.
The present study has several limitations. Firstly, the public databases investigated in the study are constantly updated; thus, some other bioactive ingredients and target genes may not have been included in our analysis. In addition, other signaling pathways (eg, MAPK, TNF, HIF-1 signaling pathways) may also be involved in the anti-tumor effect of ZJW. Further studies are warranted to examine the potential involvement of these pathways. Moreover, the quercetin could not really represent for ZJW, even though it was found the most important bioactive ingredient of ZJW in the treatment of CRC. Therefore, additional research is required to further explore the molecular mechanism of ZJW in the treatment of CRC in vitro and in vivo.
Conclusion
In the present study, we combined network pharmacology-based prediction, molecular docking, and in vitro experiments to verify the targets of ZJW and the mechanisms through which its main bioactive ingredients exert their effectively anti-CRC. We demonstrated that ZJW treated CRC by activating its targets, which play key roles in inhibiting cell proliferation and promoting apoptosis. Based on this multidisciplinary strategy, the present study provided a promising approach for the treatment of disease using TCM.
Abbreviations
ZJW, Zuojinwan; CRC, colorectal cancer; PPI, protein–protein interaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; TCM, traditional Chinese medicine; R. coptidis, Rhizoma coptidis; E. rutaecarpa, Evodia ruticarpa (A.Juss.) Hook.f. and Thomson; TCMSP, Traditional Chinese Medicines for Systems Pharmacology Database and Analysis Platform; BATMAN-TCM, Bioinformation Analysis Tool for Molecular mechANism of Traditional Chinese Medicine; ADME, absorption, distribution, metabolism, and excretion; OB, oral bioavailability; DL, drug-likeness; MOE, Molecular Operating Environment; PDB, Protein Data Bank; CCK-8, Cell Counting Kit-8; Annexin V-FITC/PI, Annexin V-fluorescein isothiocyanate/propidium iodide; BP, biological process; CC, cell composition; MF, molecular function.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 81273722, 81603,670) and Hunan Provincial Natural Science Foundation of China (No. 2017JJ3459, 2018JJ2595).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018.
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:10.1136/gutjnl-2015-310912
3. Benarba B, Pandiella A. Colorectal cancer and medicinal plants: principle findings from recent studies. Biomed Pharmacother. 2018;107:408–423. doi:10.1016/j.biopha.2018.08.006
4. McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. 2017;24(15):1537–1557. doi:10.2174/0929867324666170111152436
5. Huang S, Peng W, Mao D, et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evid Based Compl Alter Med. 2019;2019:8423037.
6. Kong F, Zou H, Liu X, et al. miR-7112-3p targets PERK to regulate the endoplasmic reticulum stress pathway and apoptosis induced by photodynamic therapy in colorectal cancer CX-1 cells. Photodiagnosis Photodyn Ther. 2020;29:101663.
7. Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8(5):1958–1975. doi:10.1002/cam4.2108
8. Mao D, Feng L, Huang S, Zhang S, Peng W, Zhang S. Meta-analysis of xihuang pill efficacy when combined with chemotherapy for treatment of breast cancer. Evid Based Compl Alter Med. 2019;2019:3502460.
9. Wang Q, Jiao L, Wang S, et al. Maintenance chemotherapy with Chinese herb medicine formulas vs. with placebo in patients with advanced non-small cell lung cancer after first-line chemotherapy: a multicenter, randomized, double-blind trial. Front Pharmacol. 2018;9:1233.
10. Tang YC, Zhang Y, Zhou J, et al. Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo. Int J Oncol. 2018;52(1):127–138. doi:10.3892/ijo.2017.4183
11. Zhang Q, Chen X, Luo Y, Ren H, Qiao T. Fuzi enhances anti-tumor efficacy of radiotherapy on lung cancer. J Cancer. 2017;8(19):3945–3951. doi:10.7150/jca.22162
12. Liu X, Tian S, Liu M, Jian L, Zhao L. Wogonin inhibits the proliferation and invasion, and induces the apoptosis of HepG2 and Bel7402 HCC cells through NFkappaB/Bcl-2, EGFR and EGFR downstream ERK/AKT signaling. Int J Mol Med. 2016;38(4):1250–1256. doi:10.3892/ijmm.2016.2700
13. Wang J, Zhang T, Zhu L, Ma C, Wang S. Anti-ulcerogenic effect of Zuojin Pill against ethanol-induced acute gastric lesion in animal models. J Ethnopharmacol. 2015;173:459–467. doi:10.1016/j.jep.2015.04.017
14. Xu L, Qi Y, Lv L, et al. In vitro anti-proliferative effects of Zuojinwan on eight kinds of human cancer cell lines. Cytotechnology. 2014;66(1):37–50. doi:10.1007/s10616-013-9534-x
15. Sun MY, Sun J, Tao J, Yuan YX, Ni ZH, Tang QF. Zuo Jin Wan reverses DDP resistance in gastric cancer through ROCK/PTEN/PI3K signaling pathway. Evid Based Compl Alter Med. 2018;2018:4278568.
16. Sui H, Pan SF, Feng Y, et al. Zuo Jin Wan reverses P-gp-mediated drug-resistance by inhibiting activation of the PI3K/Akt/NF-kappaB pathway. BMC Complement Altern Med. 2014;14:279.
17. Yan R, Wang Y, Shen W, Liu Y, Di X. Comparative pharmacokinetics of dehydroevodiamine and coptisine in rat plasma after oral administration of single herbs and Zuojinwan prescription. Fitoterapia. 2011;82(8):1152–1159. doi:10.1016/j.fitote.2011.07.012
18. Chou ST, Hsiang CY, Lo HY, et al. Exploration of anti-cancer effects and mechanisms of Zuo-Jin-Wan and its alkaloid components in vitro and in orthotopic HepG2 xenograft immunocompetent mice. BMC Complement Altern Med. 2017;17(1):121. doi:10.1186/s12906-017-1586-6
19. Du J, Sun Y, Wang XF, Lu YY, Zhou QM, Su SB. Establishment of an experimental breast cancer ZHENG model and curative effect evaluation of zuo-Jin Wan. Evid Based Compl Alter Med. 2013;2013:324732.
20. Pan J, Xu Y, Song H, Zhou X, Yao Z, Ji G. Extracts of Zuo Jin Wan, a traditional Chinese medicine, phenocopies 5-HTR1D antagonist in attenuating Wnt/beta-catenin signaling in colorectal cancer cells. BMC Complement Altern Med. 2017;17(1):506. doi:10.1186/s12906-017-2006-7
21. Sun M-Y, Wang -D-D, Sun J, et al. The Zuo Jin Wan Formula increases chemosensitivity of human primary gastric cancer cells by AKT mediated mitochondrial translocation of cofilin-1. Chin J Nat Med. 2019;17(3):198–208. doi:10.1016/S1875-5364(19)30022-6
22. Sui H, Liu X, Jin BH, et al. Zuo Jin Wan, a traditional Chinese herbal formula, reverses P-gp-mediated MDR in vitro and in vivo. Evid Based Compl Alter Med. 2013;2013:957078.
23. Zuo H, Zhang Q, Su S, Chen Q, Yang F, Hu Y. A network pharmacology-based approach to analyse potential targets of traditional herbal formulas: an example of Yu Ping Feng decoction. Sci Rep. 2018;8(1):11418. doi:10.1038/s41598-018-29764-1
24. Zeng L, Yang K. Exploring the pharmacological mechanism of Yanghe Decoction on HER2-positive breast cancer by a network pharmacology approach. J Ethnopharmacol. 2017;199:
25. Jing C, Sun Z, Xie X, et al. Network pharmacology-based identification of the key mechanism of Qinghuo Rougan Formula acting on uveitis. Biomed Pharmacother. 2019;120:109381.
26. Xie G, Peng W, Li P, et al. A network pharmacology analysis to explore the effect of astragali radix-radix angelica sinensis on traumatic brain injury. Biomed Res Int. 2018;2018:3951783.
27. Kim SK, Lee S, Lee MK, Lee S. A systems pharmacology approach to investigate the mechanism of Oryeong-san formula for the treatment of hypertension. J Ethnopharmacol. 2019;244:112129.
28. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13.
29. Maier JK, Labute P. Assessment of fully automated antibody homology modeling protocols in molecular operating environment. Proteins. 2014;82(8):1599–1610. doi:10.1002/prot.24576
30. Sadhukhan P, Kundu M, Chatterjee S, et al. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater Sci Eng C Mater Biol Appl. 2019;100:
31. Ruan H, Zhan YY, Hou J, et al. Berberine binds RXRalpha to suppress beta-catenin signaling in colon cancer cells. Oncogene. 2017;36(50):6906–6918. doi:10.1038/onc.2017.296
32. Pan Y, Shao D, Zhao Y, et al. Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK- HIF-1alpha. Int J Biol Sci. 2017;13(6):794–803. doi:10.7150/ijbs.18969
33. Zheng X, Zhang F, Shao D, et al. Gram-scale production of carrier-free fluorescent berberine microrods for selective liver cancer therapy. BioFactors (Oxford, England). 2018;44(5):496–502. doi:10.1002/biof.1450
34. Guo XX, Li XP, Zhou P, et al. Evodiamine induces apoptosis in SMMC-7721 and HepG2 cells by suppressing NOD1 signal pathway. Int J Mol Sci. 2018;19(11).
35. Su T, Yang X, Deng JH, et al. Evodiamine, a novel NOTCH3 methylation stimulator, significantly suppresses lung carcinogenesis in vitro and in vivo. Front Pharmacol. 2018;9:434.
36. Massi A, Bortolini O, Ragno D, et al. Research progress in the modification of quercetin leading to anticancer agents. Molecules (Basel, Switzerland). 2017;22(8).
37. Zhang Z, Li B, Xu P, Yang B. Integrated whole transcriptome profiling and bioinformatics analysis for revealing regulatory pathways associated with quercetin-induced apoptosis in HCT-116 cells. Front Pharmacol. 2019;10:798.
38. Ji Y, Li L, Ma YX, et al. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J Nutr Biochem. 2019;69:
39. Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed Pharmacother. 2019;114:108839.
40. Cheng S, Gao N, Zhang Z, et al. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of Bax. Clin Cancer Res. 2010;16(23):5679–5691. doi:10.1158/1078-0432.CCR-10-1565
41. Maurya AK, Vinayak M. PI-103 and quercetin attenuate PI3K-AKT signaling pathway in T-cell lymphoma exposed to hydrogen peroxide. PLoS One. 2016;11(8):e0160686. doi:10.1371/journal.pone.0160686
42. Chuang CH, Chan ST, Chen CH, Yeh SL. Quercetin enhances the antitumor activity of trichostatin A through up-regulation of p300 protein expression in p53 null cancer cells. Chem Biol Interact. 2019;306:
43. Lu J, Wang Z, Li S, et al. Quercetin Inhibits the Migration and Invasion of HCCLM3 Cells by Suppressing the Expression of p-Akt1, Matrix Metalloproteinase (MMP) MMP-2, and MMP-9. Med Sci Monit. 2018;24:
44. Refolo MG, D’Alessandro R, Malerba N, et al. Anti proliferative and pro apoptotic effects of flavonoid quercetin are mediated by CB1 receptor in human colon cancer cell lines. J Cell Physiol. 2015;230(12):2973–2980. doi:10.1002/jcp.25026
45. Kim HS, Wannatung T, Lee S, et al. Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer. Apoptosis. 2012;17(9):938–949. doi:10.1007/s10495-012-0719-0
46. Kim GT, Lee SH, Kim YM. Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 colon cancer cells. J Cancer Prev. 2013;18(3):264–270. doi:10.15430/JCP.2013.18.3.264
47. Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. 2011;68(6):1449–1457. doi:10.1007/s00280-011-1641-9
48. Alwhaibi A, Verma A, Adil MS, Somanath PR. The unconventional role of Akt1 in the advanced cancers and in diabetes-promoted carcinogenesis. Pharmacol Res. 2019;145:104270. doi:10.1016/j.phrs.2019.104270
49. Xu H, Lin F, Wang Z, et al. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018;412:69–80. doi:10.1016/j.canlet.2017.09.030
50. Xue S, Zhou Y, Zhang J, et al. Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibition of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am J Transl Res. 2019;11(4):2580–2589.
51. Yang HL, Thiyagarajan V, Shen PC, et al. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J Exp Clin Cancer Res. 2019;38(1):186. doi:10.1186/s13046-019-1196-x
52. Elzaiat M, Herman L, Legois B, Leger T, Todeschini AL, Veitia RA. High-throughput exploration of the network dependent on AKT1 in mouse ovarian granulosa cells. Mol Cell Proteomics. 2019;18(7):1307–1319. doi:10.1074/mcp.RA119.001461
53. Zhang M, Liu LP, Chen Y, et al. Wogonin induces apoptosis in RPMI 8226, a human myeloma cell line, by downregulating phospho-Akt and overexpressing Bax. Life Sci. 2013;92(1):55–62. doi:10.1016/j.lfs.2012.10.023
54. Liu JS, Huo CY, Cao HH, et al. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine. 2019;61:152843.
55. Hossan MS, Chan ZY, Collins HM, et al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/mTOR signal transduction inhibition. Cancer Lett. 2019;453:57–73. doi:10.1016/j.canlet.2019.03.034
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.