Back to Journals » Drug Design, Development and Therapy » Volume 16
Network Pharmacology-Based Combined with Experimental Validation Study to Explore the Underlying Mechanism of Agrimonia pilosa Ledeb. Extract in Treating Acute Myocardial Infarction
Authors Zhang M, Chen J, Wang Y, Kang G, Zhang Y , Han X
Received 12 April 2022
Accepted for publication 30 July 2022
Published 15 September 2022 Volume 2022:16 Pages 3117—3132
DOI https://doi.org/10.2147/DDDT.S370473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Muqing Zhang,1,2,* Jian Chen,3,4,* Yanwei Wang,2 Guobin Kang,2 Yixin Zhang,3,4 Xue Han3,4
1College of Integrative Medicine, Hebei University of Chinese Medicine, Shijiazhuang, People’s Republic of China; 2Affiliated Hospital, Hebei University of Chinese Medicine, Shijiazhuang, People’s Republic of China; 3School of Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang, People’s Republic of China; 4International Joint Research Center on Resource Utilization and Quality Evaluation of Traditional Chinese Medicine, Shijiazhuang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yixin Zhang; Xue Han, Tel +86 311 89926316, Fax +86 311 89926316, Email [email protected]; [email protected]
Purpose: The network pharmacology approach and validation experiment were performed to investigate the potential mechanisms of Agrimonia pilosa Ledeb. (APL) extract against acute myocardial infarction (AMI).
Methods: The primary compounds of APL extract were identified by High-Performance Liquid Chromatography (HPLC) analysis. The intersecting targets of active compounds and AMI were determined via network pharmacology analysis. A mouse model of AMI was established by subcutaneous injection of isoproterenol (Iso). Mice were treated with APL extract by intragastric administration. We assessed the effects of APL extract on the electrocardiography (ECG), cardiac representative markers, representative indicators of oxidative stress, pathological changes, and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, as well as apoptosis-related indicators in the mice.
Results: Five candidate compounds were identified in APL extract. Enrichment analyses indicated that APL extract could exert myocardial protective effects via the PI3K/Akt pathway. ST segment elevation and increased heart rate were obviously reversed in APL extract groups compared to Iso group. We also detected significant decreases in lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase MB (CK-MB), malondialdehyde (MDA), and reactive oxygen species (ROS), as well as a significant increase in superoxide dismutase activities (SOD) after APL extract treatment. In addition, APL extract markedly decreased the number of apoptotic cardiomyocytes after AMI. In the APL extract groups of AMI mice, there were increased expression levels of p-PI3K, p-Akt, and B-cell lymphoma-2 (Bcl-2) protein, and there were decreases in Bcl-2-associated X (Bax), cysteinyl aspartate-specific proteases-3 (caspase-3), and cleaved-caspase-3 protein expression levels, as well as the Bax/Bcl-2 ratio.
Conclusion: APL extract had a protective effect against Iso-induced AMI. APL extract could ameliorate AMI through antioxidant and anti-apoptosis actions which may be associated with the activation of the PI3K/Akt signaling pathway.
Keywords: acute myocardial infarction, APL extract, isoproterenol, oxidative stress, apoptosis, PI3K/Akt
Introduction
Acute myocardial infarction (AMI) involves myocardial necrosis due to persistent and acute ischemia and hypoxia in the coronary arteries, which can cause heart failure in severe cases.1 AMI is one of the main factors leading to death in patients with cardiovascular diseases.2 One effective treatment involves timely opening of occluded coronary arteries to restore blood flow.3 The principles of therapy are to remedy the dying myocardium, minimize the infarct size, protect heart function, and promptly manage all types of complications. Ischemic myocardium can be restored to normal perfusion, but reperfusion can aggravate injury if performed late after ischemia.4 When the blood supply from the coronary artery cannot meet the myocardial demand, a series of damaging changes occurs, such as myocardial ultrastructural changes, myocardial cell metabolic imbalance, excessive oxidative stress, and myocardial cell necrosis.5 Serious cases may be life-threatening due to arrhythmia or shock.
Isoproterenol (Iso)-induced AMI has become one of the most characteristic models commonly used to evaluate cardioprotective drugs.6 Iso is a β-receptor agonist which can increase myocardial oxygen consumption by enhancing myocardial contraction and heart rate, causing cardiac overload, impairment of myocardial microcirculation, and myocardial infarction. The pathogenesis of Iso-induced AMI mice is related to oxidative stress and apoptosis.7 Apoptosis is a process of autonomous and orderly cell death that is controlled by genes and influenced by changes in the internal environment.8 Many physiological responses are associated with apoptosis, such as normal development of an embryo, renewal of cells, and drug-induced cell death.9 And apoptosis disorders are directly or indirectly associated with cardiovascular disease.10–13
Oxidative stress can induce both oxidative damage and apoptosis, and moderate apoptosis can protect cardiomyocytes from damage in stressful conditions.14–16 Excessive oxidative stress can promote apoptosis and even lead to pathological damage.17 The phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is a significant intracellular signal transduction route that has a protective function in apoptosis.18 This pathway has been shown to modulate the apoptosis by regulating the expression of apoptosis-related factors.13
Agrimonia pilosa Ledeb. (APL) extract is obtained from the aerial parts of APL. APL is a medicinal plant that contains a variety of chemical components that are known to have antithrombotic, antioxidant, and anticancer effects, as well as the ability to scavenge reactive oxygen species and regulate nitric oxide production.19 The flavonoids from APL have been reported to have significant free-radical-scavenging activity, protective effects against oxidative DNA damage, antioxidant activity, and aldose-reductase-inhibiting ability.20,21 Furthermore, APL could help to improve glucose metabolism and prevent apoptosis in brain tissue in an ischemic area after middle cerebral artery occlusion.22 However, the effects of aqueous extracts of APL on mice with AMI have not yet been examined.
Network pharmacology has unique advantages in the process of systematically discovering potential active ingredients and targets in traditional Chinese medicine. It can enable comprehensive network analysis of drug action; reveal the mechanisms of drugs in depth; verify the multi-component, multi-target, and multi-pathway characteristics of traditional Chinese medicine; and provide new strategies and directions for modernization research on traditional Chinese medicine.23 As one such medicine, APL has a wide variety of compounds with significant medicinal effects on the cardiovascular system, which could be highly valuable for exploitation.24 However, its benefits in AMI, including its specific effects and potential mechanisms, remain largely unexplored.
Motivated by previous High-Performance Liquid Chromatography (HPLC) results, this study utilizes network pharmacology and experimental validation to further elucidate the potential mechanisms of APL extract against AMI. We constructed a drug-component-target interaction network to screen potential targets and pathways of APL extract. Furthermore, we validated the protective effects and mechanisms using a mouse model of AMI.
Materials and Methods
Materials
APL extract (Cat.#: HL-210302) was obtained from Xi’an Huilin Biotechnology Co., Ltd. (Shanxi, China), Iso was acquired from Amylet Scientific Inc. (Michigan, USA), and verapamil (Ver) was obtained from HeFeng Pharmaceutical Co., Ltd. (Shanghai, China). Pentobarbital sodium was acquired from Shanghai Rongbai biological technology Co., Ltd. (Shanghai, China), 4% paraformaldehyde solution was acquired from Labgic technology Co., Ltd. (Beijing, China), and sodium chloride injection was acquired from Shijiazhuang No.4 pharmaceutical Co., Ltd. (Hebei, China). Shimadzu LC-20AD was purchased from Shimadzu (Tokyo, Japan). Acetonitrile, methanol, and formic acid (chromatographic pure) were obtained from Fisher Chemical (Pittsburgh, PA, USA), while ellagic acid (≥98%), (+)-catechin (≥98%), quercetin (≥98%), quercitrin (≥98%), and taxifolin (≥98%) standard substances were obtained from Alfa Biotechnology Co., Ltd (Chengdu, China).
HPLC Detection
APL extract was dissolved with methanol (2 mg/mL) for qualitative analysis. The chromatographic conditions were as follows: ZORBAX StableBond C18 chromatographic column (4.6 mm × 250 mm, 5 μm) (Agilent, USA), 0.2% formic acid aqueous solution as mobile phase A, acetonitrile solution as mobile phase B, flow rate of 1 mL/min, column temperature of 30°C, PDA detector, detection wavelength of 254 nm, injection volume of 10 μL, and 80 min run time. The elution program was as follows: 0–50 min, 5–30% B; 50–70 min, 30–55% B; 70–80 min, 55–95% B.
Target Prediction and Protein-Protein Interaction (PPI) Network Construction
The active ingredients were confirmed by HPLC in combination with literature to provide a reference for subsequent network pharmacological studies. The target proteins of the active ingredients in APL extract were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://old.tcmsp-e.com/), Swiss Target Prediction (http://swisstargetprediction.ch/), and DrugBank (https://www.drugbank.ca/) databases.25–27 The Genecards database (https://www.genecards.org/) was used to search for targets related to AMI.28 Homo sapiens was selected for analysis, and the standard names of all protein targets were matched through UniProt (https://www.uniprot.org/).29 Component and disease targets were searched for intersections and introduced into Cytoscape 3.7.2 to construct network maps. Then, the intersecting targets were imported into String (https://string-db.org/) to build a PPI network.30 Homo sapiens was used as a filtering criterion, and the minimum interaction score was stipulated as high confidence (0.700).
Enrichment Analysis
Cross-targets were imported into Metascape (http://metascape.org/) for Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analysis.31 The core targets and related pathways were screened according to the p value for visualization and analysis. These methods were used in combination with the literature to select suitable indicators for animal experimental validation.
Experimental Animals
Sixty Kunming mice (25 ± 5 g) were acquired from Hebei Medical University (Experimental animal license number: SCXK (Ji) 2018–004) and housed in a standard environment with freely obtainable water and food. All mice were acclimatized for at least 1 week and fasted overnight before experiments. All experimental processes were carried out according to the ethical guidelines authorized by the Ethics Committee of Hebei University of Chinese Medicine (Certificate number: DWLL2020073).
Experimental Design
The animals were randomly divided into six groups: control (Con) group, Iso group (85 mg/kg), Ver group (2 mg/kg), APL extract low-dose (Low) group (75 mg/kg), APL extract middle-dose (Middle) group (150 mg/kg), and APL extract high-dose (High) group (300 mg/kg). The maximum clinical dose (12 g) was used as the middle-dose, the extraction rate of APL extract was 10:1, and the doses were calculated according to the body surface area normalization method. After intragastric administration of APL extract for 7 d, mice were injected subcutaneously with Iso for two consecutive days. After the experiment, the protective effect of APL extract on myocardial injury was observed.
Measurements of Electrocardiography (ECG) Changes
Mice were anaesthetized with pentobarbital sodium (40 mg/kg) 24 h after the last Iso injection and then fixed on a fixation plate. ECG was recorded by the RM6240BD Biological Signal Collection System (Chengdu Instrument Factory, Chengdu, China).
Myocardial Enzyme Testing
Serum levels of lactate dehydrogenase (LDH, Cat.#: 210,101), creatine kinase (CK, Cat.#: 201,101), and creatine kinase MB (CK-MB, Cat.#: 201,101) were used as indicators of evaluation of AMI. Blood was removed from eyeballs and centrifuged at 3500 rpm for 20 min at 4°C. The supernatant was gathered. LDH, CK, and CK-MB levels were determined using commercial kits according to the manufacturer’s instructions (Shenzhen Icubio Biomedical Technology Co. Ltd, Shenzhen, China).
Histopathological Varieties of Myocardial Tissues
The experimental mice were sacrificed after performing ECG. The heart tissues were taken from all experimental groups, immediately rinsed with saline, and fixed in a 4% paraformaldehyde solution. After fixation, heart tissues were embedded in paraffin, sliced, and stained with hematoxylin and eosin (H&E). Histopathological evaluation was then performed under a biological microscope.
Oxidative Stress-Related Indicators
The heart samples were cut into 5 μm thick cryosections which were stained with DHE (Cat.#: D7008) dye solution and incubated for 30 min at 37°C in the dark. After rinsing the dye solution with PBS three times, ROS levels were observed and photographed under a fluorescence microscope. The serums were assayed for the levels of malondialdehyde (MDA, Cat.#: 200,425; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and superoxide dismutase (SOD, Cat.#: 200,425; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) activities to evaluate oxidative stress in mice with AMI.
TUNEL Assay
Myocardial apoptosis was assayed using an in situ apoptosis detection kit (Servicebio, Wuhan, China). Myocardial tissue sections were rinsed with phosphate-buffered saline, immobilized in 4% paraformaldehyde solution, and incubated in proteinase K for 10 min. Apoptotic nuclei were identified by green TUNEL staining. Then each myocardial sample was photographed with a fluorescence microscope at 200 × (Nikon, Japan).
Western Blot Assay
Heart tissues were lysed with RIPA Lysis Buffer (Servicebio, Wuhan, China), and total proteins were extracted and denatured. The protein concentration was determined using an Ultra-micro spectrophotometer (METTLER TOLEDO, Shanghai, China). Protein (50 µg) was sampled from each group, separated by 10% SDS‑PAGE at constant pressure, and transferred onto PVDF membranes with constant flow (Transfer Membranes, Shanghai, China). Blots were sealed with 5% skim milk on a shaker at room temperature for 1.5 h and then incubated with specific primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Cat.#: AF7021), PI3K (Cat.#: AF6241), p-PI3K (Cat.#: AF3241), Akt (Cat.#: AF6261), p-Akt (Cat.#: AF0016), B-cell lymphoma-2 (Bcl-2, Cat.#: AF6139), Bcl-2-associated X (Bax, Cat.#: AF0120), cysteinyl aspartate-specific proteases-3 (caspase-3, Cat.#: AF6311), and cleaved-caspase-3 (Cat.#: AF7022) (Affinity Biosciences, Jiangsu, China) at 1:2000 and 4°C overnight. The specific protein was incubated with a secondary antibody (Cat.#: S0001, Affinity Biosciences, Jiangsu, China) for 50 min at room temperature after washing the PVDF membranes three times with TBST. Proteins were detected using an Intelligent Image Workstation (6000Plus, Guangzhou Biolight Biotechnology Co., Ltd, Guangzhou, China).
Statistical Analysis
The acquired data were expressed as the mean ± standard error of the mean (SEM) and analyzed by measuring the statistical significance between groups using a one-way analysis of variance test, followed by a Tukey’s test. SPSS version 26 was used to analyze the data, and p-values < 0.05 were considered statistically significant.
Results
Active Chemical Constituents of APL Extract
The results of HPLC analysis (Figure 1A) showed that the most critical active ingredients of APL extract were (+)-catechin, ellagic acid, taxifolin, quercitrin, and quercetin (Figure 1B) with contents of 24.24 mg/g, 9.76 mg/g, 11.03 mg/g, 9.84 mg/g, and 0.78 mg/g, respectively. A linear regression analysis was performed by plotting the standard curve of peak area (Y) and concentration (X) for the standard substance, and the results showed a good linear relationship between the peak area and concentration (Table 1).
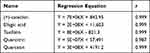 |
Table 1 Regression Equation |
 |
Figure 1 APL extract. (A) Standard chromatograms. (B) Chemically active ingredients. 1. (+)-catechin, 2. ellagic acid, 3. taxifolin, 4. quercitrin, 5. quercetin. |
Network Analysis
Based on the TCMSP, Swiss Target Prediction, and DrugBank databases, 152 targets were obtained for 5 active ingredients (Figure 2A). A total of 899 AMI targets were collected and screened from the Genecards database. Combining targets of APL extract with AMI-related targets, 93 common targets were identified (Figure 2B). Based on these, a PPI network of intersecting targets was constructed for active ingredients of APL extract and AMI (Figure 2C). Moreover, APL extract mainly influenced AMI through 20 common targets, including AKT1, TNF, and JUN.
Enrichment Analysis
KEGG pathway and GO enrichment analyses were done to identify the potential mechanisms of APL extract for the treatment of AMI. The 93 intersecting targets of APL extract and AMI were enriched in 179 KEGG pathways, and the top 20 pathways are shown in Figure 3A. The results showed that the mechanisms of APL extract for AMI mainly involves pathways in cancer, lipids and atherosclerosis, fluid shear stress and atherosclerosis, AGE-RAGE, and the PI3K-Akt signaling pathway.
 |
Figure 3 KEGG pathway and GO enrichment analyses. (A) KEGG pathway analysis of candidate targets of APL extract against AMI. (B) GO terms of candidate targets of APL extract against AMI. |
GO analysis showed that the top 10 terms were markedly enriched in BP, CC, and MF (Figure 3B). The results demonstrated that these 93 targets were tightly correlated with the following terms. The biological processes were mainly related to the response to inorganic substances, positive regulation of cell migration, and positive regulation of cell motility. The cellular components were primarily involved the membrane raft, membrane microdomain, and caveola. The molecular functions principally focused on cytokine receptor binding, protein domain specific binding, and DNA-binding transcription factor binding. Furthermore, it has been observed that the PI3K/Akt signaling pathway plays a pivotal role in the pathogenesis of AMI and mainly participates in the apoptotic process.32 Therefore, animal experiments were performed to investigate the mechanism of the PI3K/Akt signaling pathway of AMI.
Effect of APL Extract on ECG of AMI Model Mice
The ECG results showed that Iso caused an increase in heart rate and elevation in the ST segment in comparison to the Con group (P < 0.01). Conversely, APL extract pre-treatment notably reduced the increase in heart rate and the elevated ST segment in comparison to the Iso group (P < 0.05 or P < 0.01). Moreover, in Iso-induced AMI mice, high doses of APL extract resulted in near-normal heart rate and ST segment (Figure 4). The data suggested that APL extract could reverse the changes of ECG.
Effect of APL Extract on Serum LDH, CK, and CK-MB Enzyme Activities
In the Iso group, the results of the serum biochemical analysis suggested that LDH, CK, and CK-MB levels rose prominently in comparison to the Con group (P < 0.01). The activities of the marker enzymes such as LDH, CK, and CK-MB in the serum presented a significant reduction after administration of APL extract (P < 0.05 or P < 0.01). The data revealed that APL extract could abate cardiac enzyme activity in AMI mice (Figure 5).
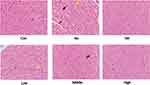
Effect of APL Extract on Histopathology of AMI in Mice
In the Con group, myocardial cells were morphologically normal, well structured, and tightly dense. There was no obvious edema, injury, necrosis, inflammatory cell infiltration, or other manifestations. The myocardial cells were morphologically altered, and the arrangement was disordered in the Iso group. Edema, cell necrosis, and infiltration of myocardial fibers by inflammatory cellular factors were observed. These pathological changes confirmed the successful induction of AMI by Iso.
After administration of the various doses of APL extract, the myocardial tissue had better morphology, and there were fewer necrotic and inflammatory infiltrated cells. The effect in the High group was the most pronounced, and the pathological features were substantially improved and nearly normal (Figure 6). These changes suggested that the treatment of mice with APL extract could improve pathological patterns.
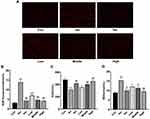
Effect of APL Extract on ROS, SOD, and MDA Levels
The data showed that the SOD activity in mice treated with Iso was significantly lower, and the levels of ROS and MDA were significantly higher than normal (P < 0.01), demonstrating that mice were in a state of excessive stress in the Iso group. The treatment of AMI mice with APL extract substantially increased the SOD activities and reduced the number of ROS and the MDA level (P < 0.05 or P < 0.01). We found that the doses used in the Middle and High group had favorable influences on oxidative stress injury in mice after the occurrence of infarction (Figure 7).
Effect of APL Extract on the Apoptosis of Myocardial Cells in Mice
The TUNEL assay was applied to inspect apoptotic cells in myocardial tissues after AMI and the protective effect of APL extract. The results showed that apoptosis was not detectable in the Con group. However, the rate of apoptosis in myocardial tissues in the Iso group was dramatically increased compared to the Con group. In contrast, the number of apoptotic cells was substantially decreased by APL extract treatment, demonstrating that APL extract was capable of inhibiting the apoptosis of myocardial tissue cells in mice (Figure 8).
 |
Figure 8 APL extract decreased cardiomyocyte apoptosis in AMI mice. Typical pictures of immunofluorescent TUNEL staining (magnification 200 ×). |
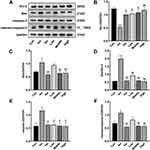
Effect of APL Extract on Apoptosis Proteins in Myocardial Tissue of Mice with AMI
The results indicated that Iso reduced the expression of Bcl-2 (P < 0.05) and increased the expressions of Bax, Bax/Bcl-2, caspase-3, and cleaved-caspase-3 (P < 0.05 or P < 0.01). Additionally, APL extract treatment was found to significantly reverse these changes, downregulating Bax, Bax/Bcl-2, caspase-3, and cleaved-caspase-3 while upregulating Bcl-2 (P < 0.05 or P < 0.01). These data indicated that APL extract could inhibit apoptosis by upregulating the expression levels of anti-apoptotic protein and downregulating those of anti-apoptotic protein (Figure 9). Therefore, APL extract may exert a significant inhibitory effect on Iso-induced myocardial tissue apoptosis in mice.
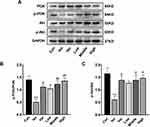
Effect of APL Extract on the PI3K/Akt Signaling Pathway
To determine whether APL extract activates the PI3K/Akt signaling pathway, the expressions of PI3K, p-PI3K, Akt, and p-Akt were inspected. There were no prominent changes in PI3K and Akt protein levels among the different groups according to the Western blot analysis. The results indicated that the expression of p-PI3K and p-Akt decreased after Iso treatment compared to the Con group (P < 0.01). Conversely, in comparison with the Iso group, the expressions of p-PI3K and p-Akt were increased with APL extract therapy (P < 0.05 or P < 0.01) (Figure 10). These data implied that APL extract probably occurred regulating the PI3K/Akt signaling pathway.
Discussion
Since AMI often causes irreversible myocardial damage, it has high morbidity and mortality rates.33 Currently, direct percutaneous coronary intervention and thrombolytic therapy are widely used to treat AMI.34 However, these treatments produce side effects such as reperfusion injury, so it is necessary to develop newer and safer natural products. Oxidative stress and apoptosis are the key factors in AMI. Hence, compounds exhibiting both antioxidant and anti-apoptosis activities are indispensable for developing treatment strategies for AMI. Clinical studies have been confirmed that certain natural compounds can provide significant cardioprotection against I/R injury of patients with AMI, potentially through the suppression of oxidative stress and apoptosis.35,36 Previous studies have shown that APL extract against cerebral ischemic-reperfusion injury might be relevant with its antioxidant potential.22 Here, the active components of APL extract were first identified, and then network pharmacology was performed to predict the targets and pathways of APL extract for the treatment of AMI in this study. Finally, we investigated the therapeutic effect of APL extract on AMI and confirmed that it has a myocardial protective effect against AMI caused by Iso (Figure 11).
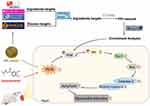 |
Figure 11 Diagram of the likely protective mechanism of APL extract against Iso-induced AMI. |
The KEGG and GO enrichment analyses suggested that the mechanisms of APL extract’s action against AMI could involve multiple factors. Based on these results, the PI3K/Akt pathway was examined through additional screening. The pathway is tightly related to oxidative stress and apoptosis to a large degree, which suggests that the effect of APL extract on AMI is related to apoptosis, as confirmed by subsequent experiments.37 The results of animal experiments showed that APL extract could protect against AMI caused by Iso.
AMI is a severe consequence of coronary atherosclerotic heart disease that is defined and described based on ECG, pathology, and biochemical findings. Subcutaneous injection of Iso for two consecutive days in Kunming mice caused dramatic changes in ECG. This could be due to the continuous loss of cell membrane potential in the injured myocardium leading to ECG changes.38,39 Serum levels of cardiac enzymes LDH and CK are sensitive markers and CK-MB is sensitive and specific markers for the diagnosis of AMI.40 When myocardial infarction occurs, a large amount of intracellular myocardial enzymes are released into the blood. Therefore, the serum levels of myocardial enzymes LDH, CK, and CK-MB can reflect the degree of myocardial damage. Previous research studies have revealed that Iso caused marked increases in the serum levels of LDH, CK, and CK-MB activities, reflecting the extreme damage to myocardium cells.41 The results in this study suggested that the treatment with APL extract reduced the serum cardiac myocyte enzyme activity in mice. This confirmed the alleviating effect of APL extract on post-infarction myocardial injury, thus reducing the leakage of cardiac enzymes.
Moreover, H&E staining can visualize the extent of myocardial injury. The results suggested that the myocardial cells had altered morphology, disordered arrangement, and cell necrosis in the Iso group. In the APL extract groups, cell morphology and arrangement were restored, and cell necrosis was significantly reduced. These suggested that APL extract might promote cardiac neural remodeling and improve ECG characteristics in AMI mice.
The administration of Iso has been reported to induce oxidative stress and myocardial apoptosis in mice.42 Many reports have suggested that oxidative stress is associated with the pathogenesis of AMI and the levels of oxidative markers in myocardial tissue, such as ROS, SOD, and MDA.43 ROS has a vital role in oxidative damage to biological macromolecules such as DNA and proteins. SOD plays a critical role in defending against cellular damage caused by oxygen free radicals and can inhibit apoptosis in some cells. MDA is a final oxidative product of lipid peroxidation, and increased production of free radicals can cause damage to cardiac cell membranes.44 In the present study, treatment with APL extract markedly increased SOD level and decreased the number of ROS and MDA level, proving that APL extract can exert myocardial protection through antioxidant. Furthermore, TUNEL staining revealed significant myocardial apoptosis. Administration of Iso has been reported to increase the activity of caspase-3 and DNA damage, which shows a process of apoptosis in cardiomyocytes.45 The TUNEL assay demonstrated that treatment with APL extract significantly decreased myocardial apoptosis in mice with AMI. Thus, the results showed that APL extract could protect myocardial cells from injury by inhibiting apoptosis.
The PI3K/Akt pathway mediates many cellular biological activities, such as cell proliferation, cell differentiation, and cell apoptosis.46 Earlier researches have demonstrated that the PI3K/Akt signaling pathway might participate in the regulation of cardiomyocyte apoptosis.47,48 Activation of PI3K promotes phosphorylation of Akt and affects its conformational changes, initiating the apoptotic process.13 After PI3K activation, it phosphorylates downstream Akt, and the PI3K/Akt signaling pathway regulates Bcl-2-family gene expression.49–51 Bcl-2 inhibits Bax activity and prevents it from embedding in the outer mitochondrial membrane, which in turn inhibits caspase-3 activity and exerts an anti-apoptotic effect.52–54
As expected, APL extract markedly increased the expression levels of p-PI3K and p-Akt proteins and activated the PI3K/Akt signaling pathway. Additionally, transcriptional regulation of the PI3K/Akt signaling pathway could regulate the expression of anti-apoptotic protein Bcl-2 and pro-apoptotic proteins Bax, caspase-3, cleaved-caspase-3, thereby reducing cardiomyocyte apoptosis.55,56 Other studies have indicated that the PI3K/Akt pathway also reduces AMI by inhibiting cardiomyocyte apoptosis.57 APL extract could improve AMI through antioxidant and anti-apoptosis effects which may be related to the activation of the PI3K/Akt signaling pathway.
The present study indicates that the protective effect of APL extract against Iso-induced myocardial infarction in mice may be related to its antioxidant and anti-apoptotic activity. However, one limitation of this study is that despite the current results exhibited a mechanism by which APL extract affects AMI is related to the PI3K/Akt pathway, further addition of inhibitors is needed to verify the specific mechanisms by which APL extract affects AMI.
Conclusion
We have demonstrated the protective effects of APL extract in mice with AMI. APL extract could ameliorate AMI through antioxidant and anti-apoptosis actions which may be associated with the activation of the PI3K/Akt signaling pathway. The study has emphasized the potential development value of APL extract for the treatment of AMI-related diseases.
Data Sharing Statement
The original data in this study are included in this article, and the authors have affirmed the accuracy of the data.
Acknowledgments
This work was supported by the Foundation of Administration of Traditional Chinese Medicine of Hebei Province (No. 2021051).
Disclosure
The authors declare no conflict of interest.
References
1. Li KS, Bai Y, Li J, et al. LncRNA HCP5 in hBMSC-derived exosomes alleviates myocardial ischemia reperfusion injury by sponging miR-497 to activate IGF1/PI3K/AKT pathway. Int J Cardiol. 2021;342:72–81. doi:10.1016/j.ijcard.2021.07.042
2. Wang L, Wang L, Wang Q. Constitutive activation of the NEAT1/miR-22-3p/Ltb4r1 signaling pathway in mice with myocardial injury following acute myocardial infarction. Aging. 2021;13(11):15307–15319. doi:10.18632/aging.203089
3. Kong Q, Dai L, Wang Y, et al. HSPA12B attenuated acute myocardial ischemia/reperfusion injury via maintaining endothelial integrity in a PI3K/Akt/mTOR-dependent mechanism. Rep. 2016;6:33636. doi:10.1038/srep33636
4. Jong RD, Pluijmert NJ, Vries MD, et al. Annexin A5 reduces infarct size and improves cardiac function after myocardial ischemia-reperfusion injury by suppression of the cardiac inflammatory response. Rep. 2018;8(1):6753. doi:10.1038/s41598-018-25143-y
5. Zhai M, Li B, Duan W, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63(2):e12419. doi:10.1111/jpi.12419
6. Nguelefack-Mbuyo EP, Nokam F, Tchinda NL, et al. Vasorelaxant and antioxidant effects of aframomum pruinosum gagnep. (Zingiberaceae) seed extracts may mediate their cardioprotective activity against isoproterenol-induced myocardial infarction. J Evid Based Complement Altern Med. 2022;2022:7257448. doi:10.1155/2022/7257448
7. Zhao Z, Liu M, Zhang Y, et al. Cardioprotective effect of monoammonium glycyrrhizinate injection against myocardial ischemic injury in vivo and in vitro: involvement of Inhibiting oxidative stress and regulating Ca2+ homeostasis by L-type calcium channels. Drug Des Devel Ther. 2020;14:331–346. doi:10.2147/dddt.S232130
8. Chang X, Tian M, Zhang Q, et al. Grape seed proanthocyanidin extract ameliorates cisplatin-induced testicular apoptosis via PI3K/Akt/mTOR and endoplasmic reticulum stress pathways in rats. J Food Biochem. 2021:e13825. doi:10.1111/jfbc.13825
9. Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1):BSR20180992. doi:10.1042/bsr20180992
10. Xing X, Guo S, Zhang G, et al. miR-26a-5p protects against myocardial ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT signaling pathway. Braz J Med Biol Res. 2020;53(2):e9106. doi:10.1590/1414-431X20199106
11. Xing X, Guo S, Zhang G, et al. Ferulic acid protects cardiomyocytes from TNF-α/cycloheximide-induced apoptosis by regulating autophagy. Arch Pharm Res. 2020;43(8):863–874. doi:10.1007/s12272-020-01252-z
12. Zhang H, Song Y, Feng C. Improvement of cerebral ischemia/reperfusion injury by daucosterol palmitate-induced neuronal apoptosis inhibition via PI3K/Akt/mTOR signaling pathway. Metab Brain Dis. 2020;35(6):1035–1044. doi:10.1007/s11011-020-00575-6
13. Jian J, Xuan F, Qin F, et al. Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. Drug Des Devel Ther. 2015;9:5933–5945. doi:10.2147/dddt.S92549
14. Mao S, Luo X, Li Y, et al. Role of PI3K/AKT/mTOR pathway associated oxidative stress and cardiac dysfunction in takotsubo syndrome. Curr Neurovasc Res. 2020;17(1):35–43. doi:10.2174/1567202617666191223144715
15. Tang L, Wang F, Xiao L, et al. Yi-Qi-Jian-Pi formula modulates the PI3K/AKT signaling pathway to attenuate acute-on-chronic liver failure by suppressing hypoxic injury and apoptosis in vivo and in vitro. J Ethnopharmacol. 2021;280:114411. doi:10.1016/j.jep.2021.114411
16. Tong HY, Dong Y, Huang XJ, et al. Anshen buxin liuwei pill, a mongolian medicinal formula, could protect H2O2-induced H9c2 myocardial cell injury by suppressing apoptosis, calcium channel activation, and oxidative stress. J Evid Based Complementary Altern Med. 2022;2022:5023654. doi:10.1155/2022/5023654
17. Chen Y, Feng X, Hu X, et al. Dexmedetomidine ameliorates acute stress-induced kidney injury by attenuating oxidative stress and apoptosis through inhibition of the ROS/JNK signaling pathway. Oxid Med Cell Longevity. 2018;2018:4035310. doi:10.1155/2018/4035310
18. Kumar R, Sharma A, Kumari A, et al. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology. 2019;20(2):171–189. doi:10.1007/s10522-018-9785-1
19. Fei X, Yuan W, Jiang L, et al. Opposite effects of Agrimonia pilosa Ledeb aqueous extracts on blood coagulation function. Ann Transl Med. 2017;5(7):157. doi:10.21037/atm.2017.03.17
20. Zhu L, Chen J, Tan J, et al. Flavonoids from Agrimonia pilosa Ledeb: free radical scavenging and DNA oxidative damage protection activities and analysis of bioactivity-structure relationship based on molecular and electronic structures. Molecules. 2017;22(3):195. doi:10.3390/molecules22030195
21. Set K, Seung H, Hong-Won S, et al. Phytochemical analysis of Agrimonia pilosa Ledeb, its antioxidant activity and aldose reductase inhibitory potential. Int J Mol Sci. 2017;18(2):379. doi:10.3390/ijms18020379
22. Zhu HY, Bie YL, Wang J, et al. Experimental study on the protection of agrimony extracts from different extracting methods against cerebral ischemia-reperfusion injury. Chin Med Sci J. 2017;32(4):239–247. doi:10.24920/j1001-9294.2017.048
23. Li L, Dai W, Li W, et al. Integrated network pharmacology and metabonomics to reveal the myocardial protection effect of Huang-Lian-Jie-Du-Tang on Myocardial Ischemia. Front Pharmacol. 2020;11:589175. doi:10.3389/fphar.2020.589175
24. Jang HH, Bae JH, Kim MJ, et al. Agrimonia pilosa Ledeb. Ameliorates hyperglycemia and hepatic steatosis in ovariectomized rats fed a high-fat diet. Nutrients. 2020;12(6):1631. doi:10.3390/nu12061631
25. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi:10.1186/1758-2946-6-13
26. Gfeller D, Grosdidier A, Wirth M, et al. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014;42(W1):W32–38. doi:10.1093/nar/gku293
27. Wishart DS, Wu A. Using drug bank for in silico drug exploration and discovery. Curr Protoc Bioinf. 2016;54:
28. Stelzer G, Rosen N, Plaschkes I, et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinf. 2016;54:
29. Abdelmonem M, Ibrahim SM, Essam RM, et al. Lutein exerts its cardioprotective effect against the experimental model of isoprenaline-induced myocardial infarction via MIAT/miR-200a/Nrf2/TXINP pathway. J Biochem Mol Toxicol. 2021;35(11):e22899. doi:10.1002/jbt.22899
30. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
31. Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi:10.1038/s41467-019-09234-6
32. Liu XY, Zhang W, Zhao M, et al. Anti-apoptotic effect of MiR-223-3p suppressing pik3c2a in cardiomyocytes from myocardial infarction rat through regulating PI3K/Akt signaling pathway. Cardiovasc Toxicol. 2021;21(8):669–682. doi:10.1007/s12012-021-09658-x
33. Sun JH, Yang HX, Yao TT, et al. Gentianella acuta prevents acute myocardial infarction induced by isoproterenol in rats via inhibition of galectin-3/TLR4/MyD88/NF-кB inflammatory signalling. Inflammopharmacology. 2021;29(1):205–219. doi:10.1007/s10787-020-00708-4
34. Shi H, Zhou P, Gao G, et al. Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J Food Biochem. 2021;45(7):e13757. doi:10.1111/jfbc.13757
35. Rodrigo R, Hasson D, Prieto JC, et al. The effectiveness of antioxidant vitamins C and E in reducing myocardial infarct size in patients subjected to percutaneous coronary angioplasty (PREVEC Trial): study protocol for a pilot randomized double-blind controlled trial. Trials. 2014;15:192. doi:10.1186/1745-6215-15-192
36. Xing K, Fu X, Jiang L, et al. Cardioprotective effect of anisodamine against myocardial ischemia injury and its influence on cardiomyocytes apoptosis. Cell Biochem Biophys. 2015;73(3):707–716. doi:10.1007/s12013-015-0642-4
37. Deng H, Yu B, Li Y. Tanshinone IIA alleviates acute ethanol-induced myocardial apoptosis mainly through inhibiting the expression of PDCD4 and activating the PI3K/Akt pathway. Phytother Res. 2021;35(8):4309–4323. doi:10.1002/ptr.7102
38. Evran B, Karpuzoğlu H, Develi S, et al. Effects of carnosine on prooxidant-antioxidant status in heart tissue, plasma and erythrocytes of rats with isoproterenol-induced myocardial infarction. Pharmacol Rep. 2014;66(1):81–86. doi:10.1016/j.pharep.2013.08.008
39. Lu Y, Yang M, Peng M, et al. Kuanxiong aerosol inhibits apoptosis and attenuates isoproterenol-induced myocardial injury through the mitogen-activated protein kinase pathway. J Ethnopharmacol. 2021;269:113757. doi:10.1016/j.jep.2020.113757
40. Chen Y, Tao Y, Zhang L, et al. Diagnostic and prognostic value of biomarkers in acute myocardial infarction. Postgrad Med J. 2019;95(1122):210–216. doi:10.1136/postgradmedj-2019-136409
41. Wu J, Cai W, Du R, et al. Sevoflurane alleviates myocardial ischemia reperfusion injury by inhibiting P2X7-NLRP3 mediated pyroptosis. Front Mol Biosci. 2021;8:768594. doi:10.3389/fmolb.2021.768594
42. Gao L, Ruan Z, Chen G. MicroRNA-383-5p regulates oxidative stress in mice with acute myocardial infarction through the AMPK signaling pathway via PFKM. Dis Markers. 2021;2021:8587535. doi:10.1155/2021/8587535
43. Jeong EM, Liu M, Sturdy M, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52(2):454–463. doi:10.1016/j.yjmcc.2011.09.018
44. Zhou R, Xu Q, Zheng P, et al. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol. 2008;586(1–3):244–250. doi:10.1016/j.ejphar.2008.02.057
45. Li H, Xie YH, Yang Q, et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One. 2012;7(11):e48872. doi:10.1371/journal.pone.0048872
46. Jafari M, Ghadami E, Dadkhah T, et al. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol. 2019;234(3):2373–2385. doi:10.1002/jcp.27262
47. Wang B, Shravah J, Luo H, et al. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun. 2009;389(1):105–111. doi:10.1016/j.bbrc.2009.08.097
48. Liao Y, Li H, Pi Y, et al. Cardioprotective effect of IGF-1 against myocardial ischemia/reperfusion injury through activation of PI3K/Akt pathway in rats in vivo. J Int Med Res. 2019;47(8):3886–3897. doi:10.1177/0300060519857839
49. Li X, Hu X, Wang J, et al. Short-term hesperidin pretreatment attenuates rat myocardial ischemia/reperfusion injury by inhibiting high mobility group box 1 protein expression via the PI3K/Akt pathway. Cell Physiol Biochem. 2016;39(5):1850–1862. doi:10.1159/000447884
50. Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10(3):177. doi:10.1038/s41419-019-1407-6
51. Lee HJ, Lee EK, Seo YE, et al. Roles of Bcl-2 and caspase-9 and −3 in CD30-induced human eosinophil apoptosis. J Microbiol Immunol Infect. 2017;50(2):145–152. doi:10.1016/j.jmii.2015.05.017
52. Rahmani M, Nkwocha J, Hawkins E, et al. Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML cells. Cancer Res. 2018;78(11):3075–3086. doi:10.1158/0008-5472.Can-17-3024
53. Aamazadeh F, Ostadrahimi A, Rahbar Saadat Y, et al. Bitter apricot ethanolic extract induces apoptosis through increasing expression of Bax/Bcl-2 ratio and caspase-3 in PANC-1 pancreatic cancer cells. Mol Biol Rep. 2020;47(3):1895–1904. doi:10.1007/s11033-020-05286-w
54. Tang Z, Yang C, Zuo B, et al. Taxifolin protects rat against myocardial ischemia/reperfusion injury by modulating the mitochondrial apoptosis pathway. PeerJ. 2019;7:e6383. doi:10.7717/peerj.6383
55. Chen Z, Chua CC, Ho YS, et al. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280(5):H2313–2320. doi:10.1152/ajpheart.2001.280.5.H2313
56. Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285(3):416–431. doi:10.1111/febs.14186
57. Shen P, Chen J, Pan M. The protective effects of total paeony glycoside on ischemia/reperfusion injury in H9C2 cells via inhibition of the PI3K/Akt signaling pathway. Mol Med Rep. 2018;18(3):3332–3340. doi:10.3892/mmr.2018.9335
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.