Back to Journals » Drug Design, Development and Therapy » Volume 17
Network Pharmacology and Experimental Validation to Explore the Molecular Mechanisms of Compound Huangbai Liquid for the Treatment of Acne
Authors Di H, Liu H, Xu S, Yi N , Wei G
Received 7 September 2022
Accepted for publication 30 November 2022
Published 11 January 2023 Volume 2023:17 Pages 39—53
DOI https://doi.org/10.2147/DDDT.S385208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Huifeng Di, Hui Liu, Shuna Xu, Na Yi, Guangchen Wei
Jinan City People’s Hospital, Jinan, 271100, People’s Republic of China
Correspondence: Guangchen Wei, Department of Pharmacy, Jinan City People’s Hospital, 001 Xuehu Street, Jinan, 271100, People’s Republic of China, Email [email protected]
Background: Acne is a highly prevalent skin disease, and inflammation plays an important role. Compound Huangbai Liquid (CHL) is a classical traditional Chinese medicine (TCM) with remarkable clinical therapeutic effects on acne. However, a holistic network pharmacological approach to explain the mechanism of CHL in the treatment of acne has not been explored.
Methods: In this study, active components and action targets of Compound Huangbai Liquid were assessed via BATMAN-TCM. The target genes related to acne were extracted from GeneCards, DisGeNet and OMIM databases. Venn diagrams to predict potential targets for the treatment of acne. Protein–Protein interaction (PPI) analysis was proceeded through String database to obtain the core protein, and the protein interaction network was constructed by Cytoscape 3.9.1. Gene Ontology (Go) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed on Metascape platforms and bioinformatics.com.cn. TCM-compound-target-disease network and disease-target pathway network were constructed using Cytoscape to give the visual result. Finally, the results were further verified by establishing the mouse acne animal model.
Results: This approach identified 165 active compounds, 1117 gene targets, 156 acne-related targets, and 34 potential target proteins for the treatment of acne with CHL. The biological processes were primarily related to cellular response to lipid, response to lipopolysaccharide, and regulation of secretion. The CHL was significantly associated with ten pathways including the Chagas disease and pathways in cancer. Animal experiments showed that CHL could significantly alleviate the levels of inflammatory factors and TLR4/NF-κB/p38 MAPK signaling pathway in acne.
Conclusion: This study revealed the multiple active components, multiple targets, and multiple pathways of CHL in the treatment of acne, which provided a new perspective for the study of the mechanism of traditional Chinese medicine in the treatment of acne.
Keywords: Compound Huangbai Liquid, acne, network pharmacology, inflammation
Introduction
Acne is a highly prevalent chronic inflammatory skin disease,1 which is a common problem affecting more than 85% of adolescents and usually lasts into adulthood, causing scarring and hyperpigmentation.2,3 The occurrence of acne is closely related to many factors, including sebum secretion, hormone levels, bacterial infection and so on. Among them, the change of sebum secretion is considered to be one of the important factors of acne.4 The pathogenesis of acne is complex, and inflammation is one of the important clinical characteristics.1,5 Research has confirmed that inflammation plays a pivotal role in the onset, development and resolution of acne. The IL-1 family of cytokines is the initiator and key factor of acne.6 Recent studies have found that sebaceous cells are affected by P. acnes significantly enhanced IL-1β release.7 However, the up-regulated expression of IL-1 can stimulate local endothelial cells and blood vessels around follicles, leading to an inflammatory cascade.8 IL-1β was found to induce the expression of proinflammatory cytokines IL-6 and IL-8 in sebocytes, suggesting that IL-1β plays an important role in acne.9 Keratinocytes are also closely related to the development of acne. It was found that P. acnes is a powerful inducer of IL-1β release from keratinocytes in a dose-dependent manner.10 In addition to IL-1, cytokines such as IL-6, tumor necrosis factor (TNF), and IL-10 are also involved in the inflammatory response of acne.11 In addition, Cutibacterium acnes is an important factor in the activation of toll-like receptors (TLR-2 and TLR-4), which leads to the activation of mitogen-activated protein kinase (MAPK) and transcription factor-nuclear factor-Kappa B (NF-κB) pathways.12 Anti-acne medications usually include retinoids, benzoyl peroxide, antibiotics and hormonal preparations.13 However, in recent years, traditional Chinese medicine (TCM) has also been very effective in anti-acne treatment.
Through thousands of years of development, TCM has been widely used to treat various diseases.14 TCM has accumulated a lot of experience in the treatment of skin diseases, including psoriasis, vitiligo, eczema, acne and so on.15–18 In addition, TCM is easy to obtain, economical, and has a good clinical effect on acne with few side effects.15,19 Qing-Shang-Fang-Feng-Tang, Zhen-Ren-Huo-Ming-Yin, Jia-Wei-Xiao-Yao-San, Wu-Wei-Xiao-Du-Yin and Huang-Lian-Jie-Du-Tang were used to treat acne.15 Different decoctions according to its own characteristics have played an effective role in acne treatment. Compound Huangbai Liquid (CHL), which is composed of five traditional Chinese herbs, Forsythiae Fructus (Lianqiao), Phellodendri chinensis Cortex (Huangbai), Honeysuckle (Jinyin Hua), dandelion (Pugong Ying) and centipede (Wugong), has shown superior efficacy in acne treatment in China. However, the mechanism is still unclear and needs further study.
Network pharmacology is a modern research method based on network to elucidate the relationship between drug, target and disease and the mechanism of action of drug.20 It can predict the mechanism of a drug’s action on a disease.21 In this study, pharmacological components of CHL were used to identify its target, and then fused with acne target to obtain a potential target for acne treatment. The signal pathways related to the treatment of acne were obtained through the enrichment analysis of functions and pathways and verified by experiments, providing new ideas and directions for comprehensively and systematically explaining the mechanism of action of CHL in the treatment of acne. The flowchart of the study is shown in Figure 1.
 |
Figure 1 Flow diagram of network pharmacological analysis of Compound Huangbai Liquid for acne treatment. |
Materials and Methods
Drug and Disease Targets Identification
The traditional Chinese medicine (TCM) in the Bioinformatics Analysis Tool for molecular mechanism of Traditional Chinese Medicine (BATMAN-TCM) (http://bionet.ncpsb.org.cn/batman-tcm/) was searched with keywords “LIAN QIAO”, “HUANG BAI”, “JIN YIN HUA”, “PU GONG YING”, “WU GONG”. In BATMAN-TCM platform, active compounds and target genes were screened according to a score cutoff ≥20 and P < 0.05.
Potential target genes of acne were collected from GeneCards (https://www.genecards.org), DisgeNet (https://www.disgenet.org/) and Online Mendelian Inheritance in Man (OMIM) (https://omim.org/) databases with the keyword of “acne”.
Prediction of Compound Huangbai Liquid Targets for Acne Treatment
The Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) was drawn to predict the potential targets for the treatment of acne, and the results were derived.
Construction and Analysis of Protein–Protein Interaction (PPI) Network
The common targets of drugs and diseases are potential therapeutic targets. Protein-protein interaction (PPI) network was constructed by String 11.0 (https://string-db.org) database and visualized by Cytoscape 3.9.1 software. The core target protein was identified through the construction of PPI network.
Gene Function and Pathway Enrichment Analysis
The Gene Ontology (GO) analysis commonly describes three distinct aspects gene functions, including biological pathways (BP), cellular components (CC), and molecular function (MF). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis (http://www.genome.jp/kegg/) can help us to find the core pathway. GO biological function analysis and KEGG enrichment analysis (http://www.genome.jp/kegg/) were performed by Metascape platforms and bioinformatics.com.cn. The cut-off criteria for significant enrichment of KEGG pathway were P <0.05.
Network Construction
To understand complex relational herbs, compounds, targets, pathway and diseases, we used Cytoscape 3.9.1 to build “TCM-compound-target-disease network and Disease-target pathway network”.
Culture of Propionibacterium acnes (P. acnes) Strains
The Propionibacterium acnes (P. acnes, ATCC6919) were purchased from BeNa Culture Collection. P. acnes were cultured on blood agar plates, and isolated colonies were anaerobic cultured in BHI medium at 37°C. The P. acnes bacterial suspension was diluted with fresh BHI medium 1:100 until the bacterial suspension was re-cultured to OD 600 of 0.1–0.3. The cultures were centrifuged (3220g, 5 minutes) and washed by BHI for 3 times. The suspension concentration of the culture was about 1 × 107 CFU on BHI medium.
Preparation of Synthetic Sebum
Synthetic sebum was composed of squalene, triolein, oleic acid, jojoba oil and Vitamin E (±a-tocopherol). We were all purchased from Macklin (St Huatuo, Shanghai, China). The purities of the squalene were 98% and 99% for oleic acid and triolein. The 90% pure jojoba oil and 95% pure Vitamin E (±a-tocopherol) were used as synthetic sebum. Each drug was dissolved in dichloromethane: methanol (2:1). On the day of the experiment, 17% oleic acid, 44.7% triolein, 25% jojoba oil 12.4% squalene and 0.9% Vitamin E were used to prepare sebum.
Ethics Statement and Animal Experiments
The specific pathogen-free (SPF) male BALB/c mice (5–6 weeks; 20–22g) were purchased from the Peng Yue experimental animal center (Jinan, China) with the permission number SCXK 2022-0009. Approval for all animals used in this study in the experiment was received from Jinan city people’s hospital. Animals were housed in the Laboratory Animal Center of Jinan city people’s hospital. Strictly abide by the national laboratory animal-related laws, regulations and standards, including but not limited to《Guidelines for ethical review of the welfare of laboratory animals》 (GB/T35892-2018), IGP 2012 and IAVE Guidelines 2010. The mice were housed at a temperature between 20°C and 25°C and humidity between 50% and 70% for one week. Food and drinking water were freely available.
The mice were randomly divided into 4 groups of eight each: (1) saline group (saline), (2) P. acnes group (P. acnes), (3) P. acnes+ Compound Huangbai Liquid group (P. acnes+CHL), (4) P. acnes+ SB203580 group (P. acnes+ SB203580). Animal models of acne were established by injecting intradermally with approximately 1 × 107 CFU of P. acnes in 50 μL BHI media. Immediately after injection, 20 μL of fresh synthetic sebum was applied to the skin once a day. The saline group was injected intradermally with equal volume of saline, and the same volume of saline was applied to the skin. The establishment of animal models of acne lasted for 7 days. From day 8 to 21, apply 1.5 mL of Compound Huangbai Liquid to the P. acne+Huangbai group mice skin twice a day for 15–20 minutes. The drug was administered continuously for 14 days. 5 mg/kg/day SB203580 is injected intraperitoneally on days 11, 14, and 17. The particular grouping and protocol is shown in Figure 2.
 |
Figure 2 Experimental protocol. |
Histopathological and Immunohistochemistry Analysis of Skin Tissue
The skin of mice in each group was fixed with 4% paraformaldehyde, embedded in paraffin and stained with hematoxylin and eosin (H&E). The protein expression levels of TLR4, NF-κB p65, P38 MAPK, phospho-NF-κB p65 and phospho-p38 MAPK were detected by immunohistochemistry. In this experiment, anti-TLR4 (Abcam, MA, USA), anti-NF-κB p65 (Abcam, MA, USA), anti-p38 MAPK (Abcam, MA, USA), phospho-NF-κB p65 (Abcam, MA, USA) and phospho-p38 MAPK (Abcam, MA, USA) were used in the immunohistochemical staining. The experimental methods refer to Chen’s research.22 Histopathological changes and immunohistochemistry protein expression were observed under microscope. Image-pro Plus 6.0 (Media Cybernetics) was used to analyze the experimental results, and the average optical density was used to represent the protein expression level “Mean density=integrated optical density/area of interest”.
Preparation of Tissue Homogenate
The skin on the back of the mice was collected, the tissue was washed with ice PBS buffer, and the excess water was absorbed by filter paper and weighed. The tissue is placed in a glass homogenizer and ground on ice. A certain volume of pre-cooled PBS was added into the homogenizer to make its mass fraction 10% of tissue homogenate. After centrifugation (9400 g, 4°C, 10 min), the supernatant was collected and stored at −80°C until tested.
Enzyme-Linked Immunosorbent Assay
The ELISA kits for IL-6, IL-1β and TNFα were purchased from eBioscience (San Diego, CA, USA). IL-6, IL-1β and TNFα were tested using ELISA kits according to the manufacturer’s instructions. The sensitivities of the ELISA kits were 3 pg/mL for IL-6, 1.2 pg/mL for IL-1β, 3.7 pg/mL for TNFα.
Statistical Analysis
All data of each group were expressed with mean ± SEM. In this experiment, all analyses were carried out using SPSS 19.0, while statistical graphs were generated using GraphPad Prism 8.0 software. Differences were considered significant at *P < 0.05 and **P < 0.01 according to one-way ANOVA analysis.
Result
Compounds and Target Genes in Compound Huangbai Liquid
We searched Forsythiae Fructus (Lianqiao), Phellodendri chinensis Cortex (Huangbai), Honeysuckle (Jinyin Hua), dandelion (Pugong Ying) and centipede (Wugong) in BATMAN-TCM database and collected the ingredients and potential targets. Then, the parameters score cutoff ≥20 and P < 0.05 were used as conditions to screen the active components of Compound Huangbai Liquid, and the number of compounds and target genes were obtained as shown in Table 1. Using the BATMAN-TCM database, 165 compounds were retrieved: 47 in Forsythiae Fructus, 37 in Phellodendri chinensis Cortex, 72 in Honeysuckle, 8 in Dandelion, and 7 in Centipede (5 herbs shared 6 compounds). Of the 47 compounds in Forsythiae Fructus, 32 satisfied the criterion of cutoff ≥20 and P < 0.05. Of the 37 compounds in Phellodendri chinensis Cortex, 28 satisfied the criterion of cutoff ≥20 and P < 0.05. Of the 72 compounds in Honeysuckle, 46 satisfied the criterion of cutoff ≥20 and P < 0.05. Of the 8 compounds in Dandelion, 8 satisfied the criterion of cutoff ≥20 and P < 0.05. Of the 7 compounds in Centipede, 5 satisfied the criterion of cutoff ≥20 and P < 0.05. Among the 32 compounds obtained in Forsythiae Fructus, 543 target genes were obtained. Among the 28 compounds obtained in Phellodendri chinensis Cortex, 325 target genes were obtained. Among the 46 compounds obtained in Honeysuckle, 853 target genes were obtained. Among the 8 compounds obtained in Dandelion, 123 target genes were obtained. Among the 5 compounds obtained in Centipede, 212 target genes were obtained. More detailed drug compounds and target information were available in the Supplementary Data (Tables S1–S5).
 |
Table 1 The Number of Compounds and Target Genes in Compound Huangbai Liquid |
Prediction of Potential Targets
In Table 2, a total of 156 acne-related targets were screened from GeneCards, DisgeNet and OMIM databases. The related targets of active components of Compound Huangbai Liquid and those of acne were plotted by Venn diagram (Figure 3), and 34 overlapping targets were obtained, which were potential targets for the treatment of acne by Compound Huangbai Liquid (Table 3), including IL1RN, TNF, UROD, SOX9, etc.
 |
Table 2 Acne-Related Target Genes |
 |
Table 3 Potential Targets of Acne Treatment |
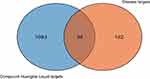 |
Figure 3 A Venn diagram of the Compound Huangbai Liquid related potential targets and acne related targets. |
Protein–Protein Interaction (PPI) Network Construction
Thirty-four potential targets were imported into the multiple proteins of the String database to obtain the protein interaction network data, which was then imported into the Cytoscape 3.9.1 software. Then, the network was further optimized to obtain PPI network (Figure 4A and Figure 4B). In Figure 4A, there are 31 nodes, 221 edges, and the average degree value is 14.258. The redder the color and the larger the circle, the more core the protein plays, such as IL-6, TNF and IL-1B, which are the core nodes of the network.
GO and Pathway Analysis
Thirty-four overlapping targets were performed for enrichment analysis using bioinformatics.com.cn. The top 20 items of biological process (BP), cell component (CC) and molecular function (MF) were screened by P < 0.05, as shown in Figure 5A. The results showed that BP targets were mainly enriched in cellular response to lipid, response to lipopolysaccharide, regulation of secretion, response to hormone, multi-multicellular organism process, etc. The targets of CC were mainly enriched in basal part of cell, receptor complex, raft and side of membrane, transport vesicle, etc. The targets of MF were mainly enriched in receptor ligand activity, nuclear receptor activity, hormone activity, heme binding, G protein-coupled receptor binding, etc. Based on the results of enriched biological process ontologies, the anti-acne effect of the CHL may result from a complex multibiological process synergetic effect.
 |
Figure 5 GO and KEGG pathway enrichment analysis. (A) Biological process categories. (B) Cellular component categories. (C) Molecular function categories. (D) KEGG pathway analysis. |
In terms of pathway analysis, 34 targets are involved in 10 KEGG Pathways with significant P values (P < 0. 05), including Chagas disease and pathways in cancer, etc. As can be seen from the bubble diagram (Figure 5D), the larger the bubble in the diagram, the more genes are enriched in the pathway, and the redder the color, the smaller the P value.
Cytoscape 3.9.1 software was employed to draw the TCM-compound-target-disease and disease-target-pathway networks (Figure 6A and Figure 6B). The TCM-compound-target-disease network comprised a total of 106 nodes (1 disease node, 5 herb nodes, 68 compound nodes, 32 target nodes) and 308 edges. According to the node degree parameters of the nodes in the network (shown in Table 2), the top five compounds in the network were 3-Methyl-2-(2-Pentenyl)-2-Cyclopenten-1-One, beta-Pinene, carvacrol, ursolic acid, D-Limonene, and could interact with 13, 12, 9, 7, 7 targets, respectively. From the perspective of the target, the top 5 were AR, VDR, PPARD, PTGS2 and IL1B, which could interact with 35, 20, 15, 14, 13 compounds, respectively.
Based on targets and top 10 KEGG pathway analyses, disease-target-pathway network was constructed using Cytoscape 3.9.1. As shown in Figure 6B, the interaction network has 43 nodes and 86 edges. The pathway with the most enriched genes was Chagas disease, with 19 genes, followed by the pathways in cancer and Hypertrophic cardiomyopathy with 10 genes and 8 genes, respectively. KEGG Pathway Maps for Chagas Disease as is shown in Figure 7. Therefore, based on the outcomes of PPI analysis, GO-BP analysis, KEGG analysis and disease-target-pathway network, we determined that the CHL might reverse the pathological changes associated with acne via six essential targets, namely, the TLR4, NF-κB, p38 MAPK, TNFα, IL-6 and IL-1β.
 |
Figure 7 The anti-acne pathways of the Compound Huangbai Liquid (CHL). The red squares represent the core therapeutic targets in the pathway. |
Compound Huangbai Liquid Alleviated Acne-Like Skin Lesions Induced by P. acnes
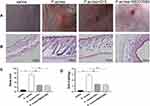
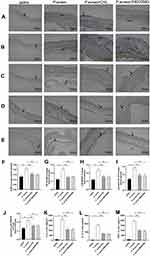
As shown in Figure 8A, status of back skin in each group on day 22. The saline group presented a healthy skin condition. Mice in the P. acnes group skin is red and swollen, with raised papules and hemorrhagic spots. After CHL treatment, the raised papules have almost disappeared. Similarly, after SB203580 treatment, the raised papules almost disappeared and the skin was scabbed. In addition, the skin was stained with H&E to investigate any histopathological changes (Figure 8B). The acne-like skin lesions were found in the P. acnes group, but not in the saline group. As seen in Figure 9B, the sebaceous glands of the epidermis and subcutaneous hair follicles were clear in the saline group. In the P. acnes group, the epidermis was thickened, the granular layer and spinous layer of hair follicle epithelium were obviously thickened, and the keratinization was excessive. A large number of keratinized material accumulation can be seen at the mouth of the hair follicle and infundibular part, and the infundibular part is expanded into a pot. The number of dermal hair follicles increases, and the adjacent hair follicles fuse with each other. After CHL treatment, the degree of hair follicle orifice dilation and epidermis thickening were alleviated, the number of hair follicles was significantly reduced, and keratinization was obviously loose. SB203580 and CHL have the same therapeutic effect on acne lesions. We did a disease score (Figure 8C) and a pathology score (Figure 8D) based on the scoring criteria of previous studies.23 CHL can significantly relieve acne-like lesions in mice by disease score and pathology score.
Compound Huangbai Liquid Alleviated the Expression of TLR4/NF-κB/p38 MAPK and Cytokines
To clarify that CHL may reverse acne-related pathological changes via TLR4, NF-κB and p38 MAPK, mice were treated with CHL. Immunohistochemistry for TLR4 (Figure 9A), NF-κB p65 (Figure 9B), p38 MAPK (Figure 9C), phospho-NF-κB p65 (Figure 9D) and phospho-p38 MAPK (Figure 9E) in the skin tissue showed that the expression of TLR4 (Figure 9F), NF-κB p65 (Figure 9G), p38 MAPK (Figure 9H), phospho-NF-κB p65 (Figure 9I) and phospho-p38 MAPK (Figure 9J) in the P. acnes group increased significantly (p < 0.01), compared to the saline group. The expression of TLR4, NF-κB p65, p38 MAPK, phospho-NF-κB p65 and phospho-p38 MAPK in the P. acnes+CHL group and P. acnes+SB203580 group significantly reduced after treatment with CHL and SB203580 (p < 0.05 or 0.01), compared to the P. acnes group.
The ELISA test showed that the expression of IL-6 (Figure 9K), IL-1β (Figure 9L) and TNFɑ (Figure 9M) in the P. acnes group increased significantly (p < 0.01), compared to the saline group. The production of IL-6, IL-1β and TNFα in the P. acnes+CHL group and P. acnes+SB203580 significantly reduced after treatment with CHL and SB203580 (p < 0.01), compared to the P. acnes group.
Dicussion
Acne is a cutaneous chronic inflammatory disorder with complex pathogenesis. Hyperseborrhea and dysseborrhea, altered keratinization of the pilosebaceous duct, Cutibacterium acnes and inflammation play crucial roles in the pathophysiology of acne.13 The doctors and patients in China are showing an increasing interest in the Compound Huangbai Liquid, which is a more natural and generally safer therapeutic option for acne. In the past, because Traditional Chinese medicine (TCM) cannot be understood at the molecular level and systemically, it is not well accepted.24,25 In recent years, especially after the COVID-19 epidemic, TCM has attracted worldwide attention. “Network pharmacology” was proposed in 2007 and 2008 and has since quickly become the frontier of current drug research and new drug research model.26,27 Network pharmacology can discover the active ingredients and target genes of traditional Chinese medicine, and reveal the underlying mechanism of action, which may increase the global acceptance of Chinese medicine. The mechanism of Compound Huangbai Liquid in the treatment of acne is still unclear which inhibits the development of Compound Huangbai Liquid. Therefore, network pharmacology provides a new way to explore the pharmacological mechanism of Compound Huangbai Liquid.
In this study, 165 effective active ingredients of Compound Huangbai Liquid were obtained through BATMAN-TCM database, and the core anti-inflammatory ingredients of Compound Huangbai Liquid were screened out as 3-Methyl-2-(2-Pentenyl)-2-Cyclopenten-1-One, beta-Pinene, carvacrol, ursolic acid, D-Limonene, etc. For example, 3-Methyl-2-(2-Pentenyl)-2-Cyclopenten-1-One mediated the downregulation of Vitamin D receptor (VDR) expression and exhibited an inhibitory effect on excessive sebaceous gland activity.28 At the same time, we found a compound that modulates multiple targets. For instance, ursolic acid suppressed the expression of the TNFα in vitro and inhibited the production of inflammatory cytokine IL-1β and IL-6.29 In conclusion, the 165 active compounds in the Compound Huangbai Liquid showed synergistic interaction in the treatment of acne. Therefore, based on the outcomes of PPI analysis, GO analysis, KEGG analysis and network analysis, TLR4/NF-κB/p38 MAPK pathway, and inflammatory cytokines IL-6, IL-1β and TNFα played a key role, which were closely related to inflammation response of acne.
Immunohistochemical and ELISA results showed that the expression of TLR4, NF-κB, p38 MAPK, IL-6, IL-1β and TNFα in P. acnes groups was significantly higher than that in saline group. In vivo studies have shown that P. acnes affects the activation of TLR4 in keratinocytes and increases TLR4 expression in acne-like lesions.30 Overexpression of TLR4 leads to the activation of NF-κB and MAPK signaling pathways most are activated by TLR4.31 This causes keratinocytes to produce IL-1β, IL-6 and TNFα.12 This is consistent with the results of this study. It was reported that proinflammatory cytokine IL-1β is not only involved in the immune response but also played a potential role in the disease of pilosebaceous unit.32,33 Clinical studies have found that IL-1β expression levels are upregulated around the hair follicles of patients with inflammatory acne lesions.31 In addition, IL-1β may be responsible for skin inflammation and the resulting keratinocyte proliferation, and may play an important role in plugging of the follicular ostia and microcomedone formation.33 IL-6, a key cytokine in a variety of inflammatory and immune responses. Il-6 overexpression is associated not only with NF-κB and p38 MAPK but also with the induction of IL-1β. IL-1β has been shown to be a strong IL-6 inducer in sebocytes.31,34 IL-6 is an agonist and catalyst of inflammatory response and high expression of IL-6 mediates and intensifies inflammatory response of acne-like lesions. TNF is produced mainly by monocytes and lymphocytes and has been shown to be involved in a variety of inflammatory diseases.35 There is evidence that TNFα is involved not only in sebaceous lipogenesis but also in the formation of lipid droplets in cells in an in vitro model of inflammatory acne.36,37 Therefore, acne-related pathological changes were related to the expression of the IL-6, IL-1β and TNFα.
In this study, the expression of TLR4, NF-κB, p38 MAPK, IL-6, IL-1β and TNFα in the P. acnes+CHL group decreased significantly compared with the P. acnes group. PPI results suggest that TLR4, IL-6, IL-1β and TNF were the potential target of compound CHL in the treatment of acne. KEGG results suggest that CHL treats acne by Chagas Disease signaling pathway. However, NF-κB and p38 MAPK have been involved in the Chagas Disease signaling pathway. There is evidence that inhibition of TLR4, NF-κB, p38 MAPK pathways and inhibiting secretion of IL-6, IL-1β and TNFα can relieve acne-like lesions.29 Therefore, in this study, CHL may act as a potent anti-inflammatory by inhibiting P. acnes-mediated IL-6, IL-1β and TNFα expression via suppressing the TLR4/NF-κB/p38 MAPK activation pathways.
In summary, in this study, the molecular mechanism of Compound Huangbai Liquid in the treatment of acne was predicted by network pharmacology. In addition, the experimental results suggest that Compound Huangbai Liquid plays a good role in the treatment of acne by regulating TLR4/NF-κB/p38 MAPK inflammatory signaling pathway. This study not only provides clinical theoretical basis for the treatment of acne with Compound Huangbai Liquid but also suggests that the production and secretion of pro-inflammatory cytokines may be an effective choice for the treatment of acne.
Data Sharing Statement
The datasets generated or analyzed in the current study can be found in the databases mentioned in this study. All data generated or analyzed during this study are included in the article published herein or in the Supplementary Data file.
Acknowledgment
There is no fund support for this study, and all expenses are self-funded.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Harper JC, Thiboutot DM. Pathogenesis of acne: recent research advances. Adv Dermatol. 2003;19:1–10.
2. Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27.
3. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi:10.1016/S0140-6736(11)60321-8
4. Li X, He C, Chen Z, Zhou C, Gan Y, Jia Y. A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol. 2017;16(2):168–173. doi:10.1111/jocd.12345
5. Tan JK, Gold LS, Alexis AF, Harper JC. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37:S60–S62.
6. Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1î±-like bioactivity are present in the majority of open comedones in acne vulgaris. J Investig Dermatol. 1992;98(6):895–901. doi:10.1111/1523-1747.ep12460324
7. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Investig Dermatol. 2014;134(11):2747–2756. doi:10.1038/jid.2014.221
8. Kistowska M, Gehrke S, Jankovic D, et al. IL-1β drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Investig Dermatol. 2014;134(3):677–685. doi:10.1038/jid.2013.438
9. Mastrofrancesco A, Kokot A, Eberle A, et al. KdPT, a tripeptide derivative of α-melanocyte–stimulating hormone, suppresses IL-1β–mediated cytokine expression and signaling in human sebocytes. J Immunol. 2010;185(3):1903–1911. doi:10.4049/jimmunol.0902298
10. Wang D, Duncan B, Li X, Shi J. The role of NLRP3 inflammasome in infection-related, immune-mediated and autoimmune skin diseases. J Dermatol Sci. 2020;98(3):146–151. doi:10.1016/j.jdermsci.2020.03.001
11. Dreno B, Gollnick H, Kang S, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29:3–11. doi:10.1111/jdv.13190
12. Lee YB, Byun EJ, Kim HS. Potential role of the microbiome in acne: a comprehensive review. J Clin Med. 2019;8(7):987. doi:10.3390/jcm8070987
13. Cong T-X, Hao D, Wen X, Li X-H, He G, Jiang X. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311(5):337–349. doi:10.1007/s00403-019-01908-x
14. Wu P, Liang S, He Y, et al. Network pharmacology analysis to explore mechanism of three flower tea against nonalcoholic fatty liver disease with experimental support using high-fat diet-induced rats. Chin Herb Med. 2022;14:273–282. doi:10.1016/j.chmed.2022.03.002
15. Chen H-Y, Lin Y-H, Chen Y-C. Identifying Chinese herbal medicine network for treating acne: implications from a nationwide database. J Ethnopharmacol. 2016;179:1–8. doi:10.1016/j.jep.2015.12.032
16. Chiang -C-C, Cheng W-J, Lin C-Y, et al. Kan-Lu-Hsiao-Tu-Tan, a traditional Chinese medicine formula, inhibits human neutrophil activation and ameliorates imiquimod-induced psoriasis-like skin inflammation. J Ethnopharmacol. 2020;246:112246. doi:10.1016/j.jep.2019.112246
17. Ghafourian E, Ghafourian S, Sadeghifard N, et al. Vitiligo: symptoms, pathogenesis and treatment. Int J Immunopathol Pharmacol. 2014;27(4):485–489. doi:10.1177/039463201402700403
18. Uzun S, Wang Z, McKnight TA, et al. Improvement of skin lesions in corticosteroid withdrawal-associated severe eczema by multicomponent traditional Chinese medicine therapy. Allergy Asthma Clin Immunol. 2021;17(1):1–4. doi:10.1186/s13223-021-00555-0
19. Bedout VD, Nichols AJ. Traditional Chinese medicine approaches. In: Integrative Dermatology. Springer; 2021:235–248.
20. Shao L, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi:10.1016/S1875-5364(13)60037-0
21. Li H, Zhao L, Zhang B, et al. A network pharmacology approach to determine active compounds and action mechanisms of ge-gen-qin-lian decoction for treatment of type 2 diabetes. Evid Based Complement Altern Med. 2014;2014. doi:10.1155/2014/495840
22. Chen W, He L, Zhong L, et al. Identification of active compounds and mechanism of Huangtu decoction for the treatment of ulcerative colitis by network pharmacology combined with experimental verification. Drug Des Devel Ther. 2021;15:4125. doi:10.2147/DDDT.S328333
23. Kolar SL, Tsai C-M, Torres J, Fan X, Li H, Liu GY. Propionibacterium acnes–induced immunopathology correlates with health and disease association. JCI Insight. 2019;4(5). doi:10.1172/jci.insight.124687
24. Qiu J. Traditional medicine: a culture in the balance. Nature. 2007;448(7150):126–129. doi:10.1038/448126a
25. Stone R. Lifting the veil on traditional Chinese medicine. Am Assoc Adv Sci. 2008;2008:709–710.
26. Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1111. doi:10.1038/nbt1007-1110
27. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi:10.1038/nchembio.118
28. Makrantonaki E, Ganceviciene R, Zouboulis CC. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato-Endocrinology. 2011;3(1):41–49. doi:10.4161/derm.3.1.13900
29. Soleymani S, Farzaei MH, Zargaran A, Niknam S, Rahimi R. Promising plant-derived secondary metabolites for treatment of acne vulgaris: a mechanistic review. Arch Dermatol Res. 2020;312(1):5–23. doi:10.1007/s00403-019-01968-z
30. Jugeau S, Tenaud I, Knol A, et al. Induction of toll‐like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153(6):1105–1113. doi:10.1111/j.1365-2133.2005.06933.x
31. Chen Y, Ji N, Pan S, et al. Roburic acid suppresses NO and IL-6 production via targeting NF-κB and MAPK pathway in RAW264. 7 cells. Inflammation. 2017;40(6):1959–1966. doi:10.1007/s10753-017-0636-z
32. Guy R, Green MR, Kealey T. Modeling acne in vitro. J Investig Dermatol. 1996;106(1):176–182. doi:10.1111/1523-1747.ep12329907
33. Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38(3):177–188. doi:10.1016/j.jdermsci.2005.01.007
34. Liu X, Ye F, Xiong H, et al. IL-1β induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-κB signaling pathway. Exp Cell Res. 2015;331(1):223–231. doi:10.1016/j.yexcr.2014.08.040
35. Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13(2):5094–5107. doi:10.2741/3066
36. Choi JJ, Park MY, Lee HJ, et al. TNF-α increases lipogenesis via JNK and PI3K/Akt pathways in SZ95 human sebocytes. J Dermatol Sci. 2012;65(3):179–188. doi:10.1016/j.jdermsci.2011.11.005
37. Rico MJ. The role of inflammation in acne vulgaris. Pract Dermatol. 2013;8:22–33.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.