Back to Journals » Drug Design, Development and Therapy » Volume 16
Network Pharmacology Analysis of Hewei Jiangni Granule for Gastroesophageal Reflux Disease and Experimental Verification of Its Anti-Neurogenic Inflammation Mechanism
Authors Cheng Y , Kou F , Zhang X, Dai Y, Shi L, Xie C, Li X, Li J
Received 25 November 2021
Accepted for publication 27 April 2022
Published 5 May 2022 Volume 2022:16 Pages 1349—1363
DOI https://doi.org/10.2147/DDDT.S348985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Yuan Cheng,1,2,* Fushun Kou,1,2,* Xiaosi Zhang,1,2 Yi Dai,3 Lei Shi,2 Chune Xie,2 Xiaohong Li,2 Junxiang Li2
1Second Clinical Medical College, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Gastroenterology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Department of Pharmacotherapy and Oriental Medicine, School of Pharmacy, Hyogo University of Health Sciences, Hyogo, Japan
*These authors contributed equally to this work
Correspondence: Junxiang Li; Xiaohong Li, Department of Gastroenterology, Dongfang Hospital, Beijing University of Chinese Medicine, No. 6, 1st Section Fangxingyuan, Fangzhuang, Fengtai District, Beijing, 100078, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Proton pump inhibitors, as the first-line drugs for treating gastroesophageal reflux disease (GERD), are unable to completely relieve patients’ symptoms and patients are prone to recurrence after prolonged drug withdrawal. Thus, it is crucial to find herbal medicines as a complementary and alternative treatment. Hewei Jiangni granule (HWJNG) is a classical Chinese medicinal formula with clinical therapeutic effects on GERD, but its pharmacological mechanism of action remains unclear. This study aimed to explore and then verify the pharmacological mechanisms of HWJNG in GERD therapy.
Methods: A network pharmacology approach was applied to explore and then verify the pharmacological mechanisms of HWJNG in GERD therapy. The active ingredients of HWJNG, as well as therapeutic targets of GERD were acquired from specialized databases. The “herb-ingredient-gene-target” network for HWJNG in GERD treatment was built. The protein–protein interaction (PPI) network was constructed to screen the core coincident targets. Then, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed. The core targets and signaling pathways associated with the anti-neurogenic inflammatory effect were partially verified via experiments in vivo at molecular level.
Results: In total, 179 chemical ingredients in HWJNG and 298 intersection targets between GERD and HWJNG were selected from databases. A large proportion of core targets and top signaling pathways were involved in neurogenic inflammation. HWJNG significantly alleviated pathological injuries of esophagus and reversed dilated intracellular spaces. Additionally, HWJNG markedly inhibited the excessive release of inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor receptor (TNF-a), as well as regulated stimulation sensors including transient receptor potential vanilloid type 1 (TRPV1) and its related neuroinflammatory mediators in GERD mice.
Conclusion: HWJNG is a promising therapeutic strategy for GERD treatment via regulation of multiple targets and pathways, its effects in alleviating neurogenic inflammation are especially acknowledged.
Keywords: Hewei Jiangni granule, gastroesophageal reflux disease, network pharmacology, neurogenic inflammation
Plain Language Summary
Gastroesophageal reflux disease (GERD) is one of the most common upper gastrointestinal disorders worldwide with a high burden of morbidity and high cost of management. Proton pump inhibitors, as the first-line drugs, cannot completely relieve symptoms and patients are prone to recurrence after prolonged drug withdrawal. Considering the relative safety and multiple beneficial effects, it is crucial to find herbal medicines as complementary and alternative treatment. Hewei Jiangni granule (HWJNG) is a classical Chinese medicinal formula with clinical therapeutic effects on GERD, but its pharmacological mechanism of action remains unclear. In the study, the “herb-ingredient-gene-target” network for HWJNG in GERD treatment was built by network pharmacology. A large proportion of core targets and top signaling pathways were involved in neurogenic inflammation. With in vivo experimental validation, HWJNG may ameliorate pathological damage of GERD and improve structural integrity of the mucosa via regulation of two aspects, which mainly involve inflammatory cytokines and neurotransmitters mediating pain. HWJNG is a promising therapeutic strategy for GERD treatment via regulation of multiple targets and pathways, especially through alleviating neurogenic inflammation.
Introduction
Gastroesophageal reflux disease (GERD) is a series of syndromes with a complex matrix of contributing pathophysiology, which is defined as the effortless movement of stomach contents into the esophagus or mouth causing troublesome symptoms or complications. The prevalence of GERD ranges from 8–33% at present and is expected to increase over time.1 Although proton pump inhibitors (PPIs) can effectively reduce acid reflux, and are thought to be the first-line treatment for GERD, the complex underlying pathobiology of GERD is poorly defined.2,3 PPI only relieves about 50% of the reflux symptoms, and the symptoms have the possibility of recurrence after prolonged drug withdrawal.4–6 Considering the relative safety and multiple beneficial effects, it is crucial to find medicinal herbs as a complementary and alternative treatment.
The notable features of traditional Chinese medicine (TCM) with a long history, are characterized by syndrome differentiation and focus on recovering overall stable function.
Hewei Jiangni granule (HWJNG) is a traditional Chinese herbal compound developed by Dongfang Hospital Affiliated to Beijing University of Chinese Medicine and comes from “Banxia Xiexin decoction”. It mainly consists of ten herbs: Radix scutellariae (Huang Qin: HQ), Rhizoma coptidis (Huang Lian: HL), Rhizoma pinelliae (Ban Xia: BX), Rhizoma zingiberis (Gan Jiang: GJ), Gentianae radix et rhizama (Long Dan Cao: LDC), Taraxaci herba (Pu Gong Ying: PGY), Bulbus fritillariae thunbergia (Zhe Bei Mu: ZBM), Fructus aurantii immaturus (Zhi Shi: ZS), Radix trichosanthis fructusycyrrhizae (Quan Gua Lou: QGL), and Radix glycyrrhizae (Gan Cao: GC). Previous studies have demonstrated that HWJNG is effective and safe in treating pathological injuries of esophagus and symptoms of acid reflux in GERD. Compared with PPI, the recurrence rate after HWJNG treatment was lower after long-term drug withdrawal.7–9 However, its mechanism of action remains unclear. Thus, the purpose of this study was to systematically explore the critical pharmacologic mechanism of HWJNG on GERD.
Network pharmacology is a new discipline based on the theory of systems biology and computer technology, which analyzes the network of “herb ingredient-disease-gene-target” and reveals the complex relationship between active ingredients and multi-target mechanisms of TCM. It has attracted new research interests and become a powerful strategy for TCM.10 This paper aimed to explore core active compounds, critical targets and top pathways for HWJNG treatment of GERD via network pharmacology.
Materials and Methods
Bioactive Compounds Screening and Collection of Compound-Related Targets
The bioactive components of HWJNG were selected from literature and Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://old.tcmsp-e.com/tcmsp.php) and confirmed by conditions with optimal toxicokinetic absorption, distribution, metabolism, and excretion (ADME) rules, that is oral bioavailability (OB) ≥30%, drug-likeness (DL) ≥0.18.10–12 Compound name and molecular structure were collected from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and the corresponding component Smiles and SDF structure files were downloaded. In addition, those main relevant ingredients which were mentioned in the literature but out of compliance are still retained. Three public databases including TCMSP, Swiss Target Prediction (http://www.swisstargetprediction.ch/) and Similarity Ensemble Approach (SEA, http://sea.bkslab.org/) were employed to identify the compound-related targets depending on chemical similarities and pharmacophore models. The standard names of target proteins’ ID were obtained from Universal Protein Resource (UniProt, https://www.UniProt.org/) database by limiting the species with “Homo sapiens”.
Disease-Associated Gene Mining
The GERD-related target proteins were screened from two sources: (1) the Human Gene Database (Gene Cards, https://www.genecards.org/), (2) database of gene-disease associations (DisGeNET, https://www.disgenet.org/home/). The keyword “gastroesophageal reflux disease” was used to obtain the disease-associated targets by mapping the putative targets of the ingredients in HWJNG and the known therapeutic targets in GERD.
Construction of Network and Enrichment Analyses
The Network Analysis of Cytoscape software (version 3.8.0, http://www.cytoscape.org/) analyzed the network interactions among herbs, ingredients, targets and diseases. Closeness, degree and betweenness were regarded as the main analysis indicators to collect the topological parameter value of each point and visually find out the core genes in the interaction network via the MCC algorithm of the cytoHubba plug-in.13 Nodes represented herbs, ingredients, and targets while edges indicated interactions among them. The online tool STRING14 is designed for analyzing interactions of protein-protein interactions (PPI) and proteintopology analysis is executed by selecting genes with a score of ≥0.7. To explore the gene ontology (GO) and the underlying biological process of selected genes, the Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/) was used.15 GO annotation contains three parts, which include biological process (BP), cell component (CC) and molecular function (MF), and these can identify the biological properties of genes and genomes of all organisms. The Kyoto Encyclopedia Gene and Genome Database (KEGG) was applied for pathway enrichment analysis, and significant (P-value <0.05) genes were selected.
Experimental Validation
Animals
All experimental procedures involving mice in this study were approved by the Animal Ethics Committee of Beijing University of Chinese Medicine (NO.BUCM-4-2020122103-4168), and were in strict accordance with the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of China. Fifty female C57BL/6 mice (weight 20±2 g) were purchased from Weitonglihua Laboratory Animal Research Center in Beijing (Number: SCXK-2019-0013). During the 7-day adaptation period before modeling, the animals were housed under standard light (12 h light/dark cycle), temperature (24 ± 1℃), and relative humidity (55 ± 5%) conditions and fed with a pelletized commercial chow diet.
Regents and Materials
The HWJNG consists of the following 10 herbs: Radix scutellariae (9 g), Radix glycyrrhizae (3 g), Fructus aurantii immaturus (9 g), Rhizoma pinelliae (9 g), Rhizoma coptidis (6 g), Taraxaci herba (9 g), Rhizoma zingiberis (9 g), Gentianae radix et rhizama (9 g), Bulbus fritillariae thunbergia (9 g) and Radix trichosanthis fructusycyrrhizae (9 g) and was purchased from Beijing Kangrentang (Beijing, China). The ratio of conversion of the drug dosage between mice and humans was 9.1.16 So the amount of the granules needed was a dose of 0.24 g/kg.day. The required dose of omeprazole sodium enteric-coated tablets, produced by Shandong New Times Pharmaceutical (national medicine standard: H20044871), was 0.06 mg/kg.day according to the same ratio of conversion.
IL-6, TNF-a, IL-1β, protease activated receptors-2 (PAR2), substance P (SP) and calcitonin gene-related peptide (CGRP) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Cusabio Biotech (Newark, USA). The primary antibody against rabbit TRPV1 (ab6166, 1:2000 dilution), antibody against mouse tryptase (ab2378,1:10000 dilution), antibody against rabbit PAR2 (ab180953, 1:100 dilution), antibody against mouse SP (ab14184,1:2000 dilution) as well as secondary immunoglobulin G H&L (HRP) (ab205718, 1:2000 dilution) were obtained from Abcam (Cambridge, UK). The primary antibody against rabbit CGRP (#14959, 1:400 dilution) and anti-mouse secondary immunoglobulin G antibody (#8890s, 1:1000 dilution) were obtained from CST (Danvers, MA, USA).
Animal Model and Treatments
After acclimating to the environment for 1 week, 50 female C57BL/6 mice were randomly divided into 5 groups with 10 mice per group: the control group, model group, low dose of HWJNG (0.24 g/kg) group, high dose of HWJNG (0.48 g/kg) group, and omeprazole group (0.06 mg/kg) group. HWJNG and omeprazole were dissolved in deionized water and gavage feeding is performed once daily from the 15th to the 28th day of the experiment, 0.2 mL per 20 g in weight each time. Mice in control and model group received equivalent volume of deionized water over the same period of time. Meanwhile mice had access to food and water ad libitum. The treatment duration was 2 weeks.
The method of mice with restraint stress was adopted to perform the pre-experiment based on GERD model of WulaMu et al.,17 and a protocol was decided: The model mice were placed in restraint cages and immersed vertically to the level of the xiphoid process in a water bath of 22 ± 2°C for 2 hours per day for 28 consecutive days. In the morning following the last restraint stress, all mice underwent a 20–22 h fasting period. All mice were sacrificed due to cervical dislocation by the end of 4th week, and the esophageal samples was collected for the study.
Histopathological Staining of Esophageal Mucosa
The esophagus tissues were fixed in 10% formalin for 24 h. Then, the tissues were embedded in paraffin and sectioned (at 3 μm thickness), deparaffinized, and stained with hematoxylin and eosin (H&E). Stained sections were dehydrated by pure alcohol. The sections were mounted on slides, observed under light microscope and evaluated to grade injury indexes.18
Transmission Electron Microscopy (TEM) of Tissue Sections
The 1 mm3 tissue pieces were cut into 50 nm thick ultra-thin tissue sections. The collected sections were stained with uranyl acetate-lead citrate (Electron Microscopy China, Beijing, China) and then observed under TEM (HT7800, Hitachi, Tokyo, Japan). The Image-Pro Plus 6.0 Image Analysis System (IPP6.0, Media Cybernetics, Rockville, MD) was used for measurement. For each specimen, at least 20 perpendicular cross-sections in different TEM images were randomly selected for intercellular space width measurement (2500 magnification). Each photomicrograph was used to count amounts of desmosomes (15,000 magnification), and then the morphology of mitochondria (8000 magnification) was observed. The sample size is 5 per group.
Test of pH Value in the Esophagus
All mice were fasted for 12 h prior to this experiment. When the mice were under the depth of anesthesia with 10% chloral hydrate (0.03 mL per 10 g in weight), and the electrode of the pH value recorder was placed at 0.5 cm above the gastroesophageal junction, after 1 min, the instantaneous pH values of the lower third of esophagus of the mice were recorded.
Enzyme-Linked Immunosorbent Assay (ELISA)
The extracted esophageal mucosa was weighed and homogenized with tissue extraction reagent on ice for 3 min using a homogenizer. IL6, TNF-α and IL-1β were measured using mouse ELISA kits (Cusabio Biotech, Newark, USA). Five samples were randomly chosen from each group for analysis.
Immunohistochemical (IHC) Analysis
Sections (3μm) of paraffin-embedded esophagus tissue were deparaffinized and rehydrated and were then incubated with 3% H2O2 for 10 min to quench endogenous peroxidase activity. The sections were incubated overnight at 4℃ with primary antibodies against TRPV1, SP, PAR2, CGRP and mast cell tryptase (MCT), respectively and then washed with PBS three times. Different sections were then incubated respectively with suitable secondary immunoglobulin G antibodies at 37℃ for 90 min. Images were analyzed by Image-Pro Plus 6.0 software, and positive staining rates were calculated.
RT-qPCR Analysis
The total RNA was isolated from esophagus tissues using TRIzol reagent (TIANGEN, DP424, Beijing, China). Then mRNA was reverse transcribed to cDNA using the reverse transcription reagent kit (Invitrogen, Carlsbad, USA). Real-time PCR was performed in a Step One Software Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The thermal cycling program used for PCR was as follows: 95℃ for 30 s, followed by 40 cycles at 95℃ for 4 s, 60℃ for 40 s and 95℃ for 5 min. The amplification curve and melting curve for real-time fluorescence quantitative PCR were performed by the end of the reaction. The Ct value of β-actin in the same sample was taken as the reference gene, and relative quantitative expression levels were calculated by the 2−ΔΔCt method. Each group was analyzed in triplicate. The primer sequences used in the study are listed in Table 1.
 |
Table 1 Primers Sequences of RT-qPCR |
Statistical Analysis
We used QQ plots and Shapiro–Wilk test to assess data distribution. Statistical analysis was performed by the SPSS 20.0 statistical software. The data of normal distribution were shown as the mean ± standard deviation (SD). Analysis of variance (ANOVA) and Dunnett’s T3 test were carried out to determine statistical significance for multiple comparisons. The least significant difference (LSD) test was performed under the assumption of equal variances. When the data were not normally distributed, the median and interquartile range (IQR) were commonly used. Kruskal–Wallis H (K) test and Nemenyi test (R, PMCMRplus) were chosen to determine statistical significance for multiple comparisons. Statistical significance was established at P <0.05, P <0.01 or P <0.001.
Results
HWJNG-Compound-Target Network Analysis
The putative targets of the candidate ingredients in HWJNG were identified in the TCMSP database (OB ≥30% and DL ≥0.18): 54 ingredients in Radix scutellariae, 37 ingredients in Radix glycyrrhizae, 13 ingredients in Fructus aurantii immaturus, 15 ingredients in Rhizoma pinelliae, 12 ingredients in Rhizoma coptidis, 10 ingredients in Taraxaci herba, 9 ingredients in Rhizoma zingiberis, 9 ingredients in Gentianae radix et rhizama, 7 ingredients in Bulbus fritillariae thunbergia and 14 ingredients in Radix trichosanthis fructusycyrrhizae. These results indicated there are 179 chemical compounds in total in the ten herbs that are HWJNG ingredients. According to the Smiles and SDF structure files of the above components, the targets were mainly obtained from TCMSP, and the two databases (Swiss Target Prediction and Similarity Ensemble Approach) were used to supplement the targets, and a total of 706 potential targets of HWJNG were obtained by deduplication. By screening the GeneCards database and DisGeNET database, 3884 GERD-related targets were selected. A total of 298 genes were obtained by observing the intersection of the ingredients of herbs and the known targets of disease (Figure 1A). These 298 genes were selected as potential targets for further analysis, with PPI maps generated by confidence scores ≥0.4 in the STRING database, among which only 282 targets had high interactions (Figure 1B). Then, to elucidate the relationship between the herbs, active compounds and potential targets, the herb-compound-target network of HWJNG was built and demonstrated in Figure 1C. This network was composed of 445 nodes and 4045 edges in total. Specifically, quercetin, genistein, ursolic acid, kaempferol, 10,13-eicosadienoic, luteolin, (3S,6S)-3-(benzyl)-6-(4-hydroxybenzyl) piperazine-2,5-quinone, beta-D-ribofuranoside xanthine-9, gondoic acid, and wogonin were predicted as the important active compounds according to degree and betweenness centrality by topological analysis, indicating their critical roles in HWJNG (Table 2).
 |
Table 2 General Information of the Top 20 Ingredients in Network of HWJNG in Treating GERD |
HWJNG-GERD Target Network and Enrichment Analysis
The network of 282 genes is shown in Figure 2A, including 145 nodes and 2493 edges with an average 17.68 node degree. TP53, AKT1, IL6, STAT3, VEGFA, MAPK1, INS, EGFR, TNF and JUN represented the crucial targets of HWJNG based on degree. To establish the underlying mechanism of HWJNG in the treatment of GERD, we used Cytoscape ClueGO plugin to conduct GO and KEGG functional enrichment analysis based on the 282 co-target genes. GO analysis is comprised of three parts: BP, CC and MF. GO enrichment results were obtained, of which were BP 77, CC 12, and MF 49. The top 10 enrichment results in BP, CC and MF are, respectively, shown in Figures 2B. The key targets involved in BP were protein phosphorylation signaling pathway, transcription from RNA polymerase II promoter, G1/S transition of mitotic cell cycle, lipopolysaccharide-mediated signaling pathway, and cell division. GO CC enrichment analysis demonstrated that the targets were primarily related to the cell junction, postsynaptic membrane, proteinaceous extracellular matrix, and receptor complex. MF was mostly involved in ATP binding, cytokine activity and transcription factor activity.
KEGG pathway analysis of HWJNG for GERD treatment was performed with DAVID Bioinformatics Resources 6.8 tool, a total of 155 pathways were selected, and the 29 significant KEGG pathways (P <0.05) are shown in Figure 2C by a bubble chart. The pathways included inflammatory reaction, neurotrophin signaling pathway, cell cycle, gap junction, nerve tissue conduction and so on. A large number of pathways related to inflammatory response such as PI3K-Akt signaling pathway, TNF signaling pathway, T cell receptor signaling pathway, Toll-like receptor signaling pathway, and Cytokine-cytokine receptor interaction existed in key pathways. The potential pathways were primarily involved in the categories of the inflammatory response, neurosensitivity, and immunologic regulation.
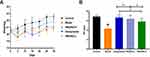
HWJNG Gained Body Weight, and Improved PH Value of Mice
The weights of mice between the control and model groups were statistically significantly different by the end of the fourth week, and the former was heavier (P<0.05). Our study indicated that weights of HWJNG group, whether low or high dose, were heavier in the fourth week than those of model group (P <0.01). The weights of mice between omeprazole and model were not significantly different (P >0.05). These results are shown in Figure 3A. As shown in Figure 3B, the pH value of esophagus in model group was lower than that in the control group (P <0.01), indicating that there was pathological acid reflux in the model group. The pH values of both the HWJNG group and omeprazole group were higher than that of the model group (P <0.01), acid-suppressant effect between omeprazole and low dose of HWJNG group were not statistically significant. The low-dose of HWJNG had more significant acid-suppressant effect than the high-dose group (P <0.05).
HWJNG Ameliorated Pathological Damage
HE staining was conducted to reveal histological changes in the esophagus of the different groups, which can directly demonstrate the therapeutic effects. The percentage of basal layer thickness increasing and papillary hyperplasia were remarkably higher in model mice than those in control mice. However, these injuries were attenuated to varying extents by HWJNG and omeprazole treatment, including all pathological quantitative indicators as shown in Figure 4.
 |
Figure 4 Hematoxylin and eosin staining of esophageal mucosa in lower third of esophagus of mice. Abbreviation: HWJNG-L, Hewei Jiangni granule low dose; HWJNG-H, Hewei Jiangni granule high dose. |
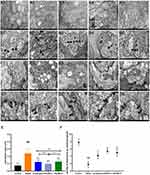
The effects of HWJNG on intercellular space width measurement (Figure 5A and B), morphology of mitochondria (Figure 5C) and amounts of desmosomes (Figure 5D) were further explored, which were observed by TEM after staining with uranyl acetate-lead citrate. As a marker of GERD, the width of epithelial intercellular space was obviously wider in the esophagus tissue of GERD mice than that in control mice (P <0.001). This widening in intercellular space was reversed by HWJNG and omeprazole group (P <0.001) (Figure 5E). The level of mitochondrial damage was higher in model mice, and injury of mitochondrial could be inhibited by HWJNG and omeprazole treatment. The decreased amounts of desmosomes in model mice indicated gap junctions problems in epithelial cells of esophagus tissues (P <0.001), and the number of desmosomes was increased by HWJNG (P <0.001) and omeprazole treatment (P <0.01) (Figure 5F). Considering the treatment of different doses of HWJNG on the pathological damage and general conditions of GERD, the low dose of HWJNG group had the better efficacy. Follow-up mechanism verification was also carried out targeting the low-dose group.
HWJNG Inhibited the Expression of Inflammatory Cytokines
As shown in Figure 6A, inflammatory cytokines including IL-1β, IL-6 and TNF-α were chosen for experimental validation based on the “top-20 Hub Target” screened by PPI analysis. The protein levels of inflammatory cytokines, including IL-1β, IL-6 and TNF-α were significantly increased in the GERD group compared with those in the control group (P <0.001). The protein levels of the above-mentioned inflammatory cytokines were significantly reduced in the group treated with HWJNG compared with those of model group (P <0.01) in Figure 6A. Meanwhile, the levels of IL-6 and STAT3 mRNA were significantly increased in model mice compared with those of control mice (P <0.05). In addition, compared with those in model group, the levels of IL-6 and STAT3 were significantly decreased by 2 weeks with HWJNG treatment (P <0.05) in Figure 6B. The results illustrated the potential inhibition of multiple inflammatory factors by HWJNG.
HWJNG Regulated the Expression of Stimulation Sensor and Relational Neuroinflammatory Mediators
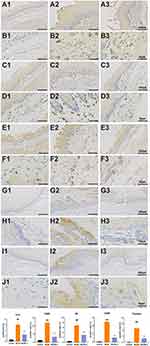
Considering the importance in the result of GO and KEGG functional enrichment analysis in the present study, TRPV1 and related neuroinflammation factors, including SP, PAR-2, CGRP and MCT were selected for experimental validation as major inflammation-related signaling and nerve tissue conduction pathways. As shown in Figure 6C, the mRNA level of TRPV1 was remarkably higher in the esophagus tissue of model than those of control mice. The excessive increases in TRPV1 expression was reversed by HWJNG treatment. These findings were determined by RT-qPCR (P <0.001). Significant increases were observed in the protein expression levels of SP, PAR-2 and CGRP in the esophageal tissues of the model group compared with those in the control group, indicating that neuroinflammatory mediators related TRPV1 were secreted at high levels in the esophageal mucosa of GERD (P<0.001). Compared with the model group, the protein level of HWJNG group was lower (P<0.001) (Figure 6D). The protein levels of TRPV1 and its related neuroinflammatory mediators, including SP, CGRP, PAR2 and MCT, were subsequently detected by IHC (P <0.001) as shown in Figure 7. The findings indicated that HWJNG could regulate neuroinflammatory mediators to ameliorated pathological damage of GERD.
Discussion
The etiology and pathogenesis of GERD are complicated. The interaction of multiple factors may lead to its pathogenesis, including esophageal hypersensitivity, inflammation of esophageal mucosa, abnormal esophageal contractility and acid reflux, as well as decreased esophageal mucosal resistance. Different symptom manifestations have their own dominant mechanisms.19 Stress could aggravate heartburn in patients suffering from GERD, and evoke esophageal inflammation. Stress and anxiety also may also intensify the central response to esophageal irritation and inflammation. Meanwhile, females have greater esophageal afferent sensitivity than males. Therefore, we performed a pre-experiment and chose female C57BL/6 to build the model finally.20–22 As a hospital-made preparation, HWJNG was widely used in GERD treatment. Previous clinical study found that HWJNG is effective and safe in treating GERD. Currently, the main active ingredients of HWJNG were not explored to reflect the multi-target and multi-pathway activities, therapeutic characteristics and related mechanisms as a TCM compound. In the present study, the bioactive compounds and the molecular mechanisms of HWJNG for GERD treatment were first performed by the network pharmacology approach, which screened core targets and signaling pathways by PPI network, GO and KEGG analysis. The evaluation on pharmacodynamics of HWJNG showed a low dose is more effective than high dose regarding the acid suppressant effect. Low dose of HWJNG is equivalent to the standard dose for human, and high dose of HWJNG is approximately twice the recommended dose for human. Therefore, we consider the overuse of medications may not have good results.
In HWJNG, Rhizoma coptidis and Radix scutellariae are the “monarch herbs”, Rhizoma pinelliae and Rhizoma zingiberis are the “minister herbs”, Fructus aurantii immaturus, Rhizoma pinelliae, Taraxaci herba, Gentianae radix et rhizama, Bulbus fritillariae thunbergia and Trichosanthis fructus are the “adjuvant herbs”, Radix glycyrrhizae is the “guide herb”. Our team supposes that Rhizoma coptidis, Radix scutellariae, Rhizoma pinelliae and Rhizoma zingiberis have the effect of regulating cold and heat, reducing inversion and stopping vomiting, which is in line with the theoretical guidance of TCM of “acrid opening and bitter downbearing”. In total, 298 targets affected by 179 active compounds in the HWJNG were selected in the active component-target network of HWJNG. In terms of pharmaceutical ingredients, quercetin, genistein, ursolic acid, kaempferol, luteolin, gondoic acid and wogonin were found to be important active compounds in HWJNG, playing a major role in the treatment of GERD. Quercetin, belonging to the sub-class flavonoids family, is one of the most prominent dietary antioxidants. Venkateswara Rao et al. reported quercetin could obviously decrease the elevated plasma histamine content and prevent the esophageal mucosal damage.23–25 Genistein is the component with the highest content of soy isoflavones, which has a two-way estrogen-regulating effect. Estrogen may play a role in the gender differences of GERD symptoms. Furthermore, genistein inhibited the phosphorylation of STAT3 to downregulate the expression of cytokines and exhibited strong anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6.21,26 Ursolic acid, a triterpenoid compound found in natural plants, demonstrated potent antagonistic activity towards TRPV1 tested at 100 μM.27,28
Esophageal mucosal inflammation and impaired mucosal integrity are related to patients with GERD.29–32 In the previous study, patients with non-erosive reflux disease (NERD) have absence of gross mucosal changes in the esophagus, while patients with erosive reflux disease (ERD) develop mucosal injury in the esophagus; both subtypes of GERD have the same distinctive sign of diagnosis, that is the presence of dilated intracellular spaces (DISs) to assess the histopathological samples.33–35 This study demonstrated that HWJNG is able to reverse DIS. Chronic inflammation exists widely in patients with GERD and is essential for the progression of GERD. Cytokines such as IL-1β, IL-6 and TNF-α and signal transducer and activator of transcription 3 (STAT3) play key roles. IL-1β and TNF are primary activators of IL6 expression. Moreover, abnormal acid exposure (AAE) exhibited obviously increased IL-1β and TNF-α expression compared with normal acid exposure.36 Although PI3K-Akt and MAPK signaling pathway had higher P value in GO and KEGG enrichment analysis, progress of inflammatory reaction and nerve tissue conduction, which could be regulated by the HWJNG, were selected for experimental validation. This is more specific and in line with current research hotspots.
TRPV‑1 is a key receptor responding to mechanical or acid irritation, as well as thermal stimulation. Increased epithelial permeability and DIS are induced by activation of TRPV-1 due to physiologic and/or pathologic gastroesophageal reflux in mice. TRPV1 is overexpressed in the esophageal mucosa of ERD and NERD compared with health controls, which explains, in large part, the similar severity of reflux symptoms in both groups, regardless of the presence or absence of erosion. In addition to inflammatory response, physiological reflux could contribute to GERD symptoms in the condition with esophageal hypersensitivity. Therefore, TRPV1 is at the intersection of inflammation and nerve tissue conduction, which is worth exploring.37–42 Considerable evidence confirmed the important production of inflammatory mediators and neurotransmitters in the pathogenesis of GERD. MCs degranulate and release tryptase, which can up-regulate the release of neurotransmitter SP and CGRP via PAR-2. TRPV1 activation in primary afferent neurons evokes the sensation of burning pain and may induce neurogenic inflammation following the release of SP and CGRP.43,44 Meanwhile, neuroinflammatory mediators could increase the stimulation of esophageal mucosa. All these findings indicated that HWJNG ameliorated pathological damage of GERD and structural integrity of the mucosa via regulating two aspects, which mainly involved inflammatory cytokines and neurotransmitters mediating pain.
Many studies have reported the reaction cascade of a series of cellular infiltrations and cytokine release that results in an inflammatory response and damage in the esophageal tissue.45,46 It is worth noting that HWJNG exerts protective effects via targets and pathways related to inflammatory response, which have been confirmed by network pharmacology analysis in the current study. It is also preliminarily confirmed HWJNG inhibits neuroinflammatory mediators and a key receptor of stimulation to reduce the stimulation of the esophageal mucosa. Growing evidence shows that GERD patients, especially NERD, are characterized by enhanced esophageal sensitivity to chemical and mechanical stimuli, which is caused by enhanced excitability of visceral sensory neurons or overexpression of acid-sensing receptors in the epithelial layer and afferent fibers in the lamina propria.47 In the next step, new experimental framework verifications are required to further explore other crucial mechanisms of HWJNG. Meanwhile, we also realize that our current research is still insufficient, and we will use Ultra-high Performance Liquid Chromatography/Quadrupole Time-of-flight Mass Spectrometry (UHPLC/Q-TOF-MS) to analyze the composition of HWJNG extracts and the active components that can be absorbed into the blood and esophagus, and use Surface Plasmon Resonance (SPR)-MS to explore the target of its main active constituent ingredients.
Conclusion
In this study, the “herbs-targets-disease” network pharmacology research is used to predict the core targets and pathways at multiple levels and partially verified via experiments in vivo. The results indicate that HWJNG plays a key role in GERD treatment associated with anti-neurogenic inflammation. HWJNG provides a promising therapeutic strategy for further experimental research and clinical treatment of GERD.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
This work is supported by the National Natural Science Foundation of China, Key Projects of Beijing University of Traditional Chinese Medicine, and Beijing Science and Technology Program Project-Capital characteristics Project. We deeply appreciate all the members of our team.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (81803907); Key Projects of Beijing University of Traditional Chinese Medicine (No. 2020-JYB-ZDGG-128) and Beijing Science and Technology Program Project-Capital characteristics Project (Z181100001718067).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):267–276. doi:10.1053/j.gastro.2017.07.045
2. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. 2018;67(7):1351–1362. doi:10.1136/gutjnl-2017-314722
3. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880. doi:10.1136/gutjnl-2012-304269
4. Savarino E, De BN, De CC, et al. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus. 2017;30(2):1–9. doi:10.1111/dote.12511
5. Talley NJ, Zand Irani M. Optimal management of severe symptomatic gastroesophageal reflux disease. J Intern Med. 2021;289(2):162–178. doi:10.1111/joim.13148
6. Cheng Y, Liu J, Tan X, et al. Direct comparison of the efficacy and safety of vonoprazan versus proton-pump inhibitors for gastroesophageal reflux disease: a systematic review and meta-analysis. Dig Dis Sci. 2021;66(1):19–28. doi:10.1007/s10620-020-06141-5
7. Li JX. Clinical observation on 50 cases of reflux esophagitis treated with Hewei Jiangni granule. J Tradit Chin Med. 2002;1(09):162–170..
8. Qin Y, Li JX, Li XH. Effects of modified banxia xiexin decoction on symptoms and quality of life in non-erosive reflux disease. J Beijing Univ Tradit Chin Med. 2013;36(04):9591319.
9. Liu JL, Kou FS, Tan X, et al. Hewei Jiangni granule alleviates visceral hypersensitivity in a rat model of non-erosive reflux disease via transient receptor potential channel signaling[J]. J Tradit Chin Med Sci. 2020;7(2):162–170. doi:10.1016/j.jtcms.2020.05.002
10. Zhang GB, Li QY, Chen QL, Su SB. Network pharmacology: a new approach for Chinese herbal medicine research. Evid Based Complement Alternat Med. 2013;2013:621423. doi:10.1155/2013/621423
11. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi:10.1186/1758-2946-6-13
12. Xia QD, Xun Y, Lu JL, et al. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Prolif. 2020;53(12):e12949. doi:10.1111/cpr.12949
13. Otasek D, Morris JH, Bouças J, Pico AR, Demchak B. Cytoscape automation: empowering workflow-based network analysis. Genome Biol. 2019;20(1):185. doi:10.1186/s13059-019-1758-4
14. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):607–613. doi:10.1093/nar/gky1131
15. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.211
16. Hang JH, Huang XH, Chen ZY, et al. Dose conversion among different animals and healthy volunteers in pharmacological study. Chin J Pharmacol Toxicol. 2004;9(9):1069–1072.
17. Wulamu W, Yisireyili M, Aili A, et al. Chronic stress augments esophageal inflammation, and alters the expression of transient receptor potential vanilloid 1 and protease-activated receptor 2 in a murine model. Mol Med Rep. 2019;19(6):5386–5396. doi:10.3892/mmr.2019.10192
18. Yerian L, Fiocca R, Mastracci L, et al. Refinement and reproducibility of histologic criteria for the assessment of microscopic lesions in patients with gastroesophageal reflux disease: the Esohisto project. Dig Dis Sci. 2011;56(9):2656–2665. doi:10.1007/s10620-011-1624-z
19. Jung HK, Tae CH, Song KH, et al. Korean society of neurogastroenterology and motility. 2020 Seoul consensus on the diagnosis and management of gastroesophageal reflux disease. J Neurogastroenterol Motil. 2021;27(4):453–481. doi:10.5056/jnm21077
20. Katzka DA, Pandolfino JE, Kahrilas PJ. Phenotypes of gastroesophageal reflux disease: where Rome, Lyon, and Montreal meet. Clin Gastroenterol Hepatol. 2020;18(4):767–776. doi:10.1016/j.cgh.2019.07.015
21. Kim YS, Kim N, Kim GH. Sex and gender differences in gastroesophageal reflux disease. J Neurogastroenterol Motil. 2016;22(4):575–588. doi:10.5056/jnm16138
22. Zhong C, Liu K, Wang K, et al. Developing a diagnostic understanding of GERD phenotypes through the analysis of levels of mucosal injury, immune activation, and psychological comorbidity. Dis Esophagus. 2018;31(10):doy039. doi:10.1093/dote/doy039
23. Rao CV, Vijayakumar M. Effect of quercetin, flavonoids and alpha-tocopherol, an antioxidant vitamin, on experimental reflux oesophagitis in rats. Eur J Pharmacol. 2008;589(1–3):233–238. doi:10.1016/j.ejphar.2008.04.062
24. Sohn US, Lee SE, Lee SH, et al. The protective mechanism of quercetin-3-O-β-D-glucuronopyranoside (QGC) in H2O2-induced injury of feline esophageal epithelial cells. Arch Pharm Res. 2016;39(9):1324–1334. doi:10.1007/s12272-016-0808-7
25. Wu P, Zhou L, Li YJ, et al. Protective effects of quercetin against chronic mixed reflux esophagitis in rats by inhibiting the nuclear factor-κB p65 and interleukin-8 signaling pathways. J Dig Dis. 2015;16(6):319–326. doi:10.1111/1751-2980.12249
26. Xu J, Xiong H, Zhao Z, et al. Genistein suppresses allergic contact dermatitis through regulating the MAP2K2/ERK pathway. Food Funct. 2021;12(10):4556–4569. doi:10.1039/D0FO03238G
27. Di Y, Xu T, Tian Y, et al. Ursolic acid protects against cisplatin-induced ototoxicity by inhibiting oxidative stress and TRPV1-mediated Ca2+-signaling. Int J Mol Med. 2020;46(2):806–816. doi:10.3892/ijmm.2020.4633
28. Zhang Y, Sreekrishna K, Lin Y, et al. Modulation of transient receptor potential (TRP) channels by Chinese herbal extracts. Phytother Res. 2011;25(11):1666–1670. doi:10.1002/ptr.3427
29. Ribolsi M, Savarino E. Exploring the association between esophageal mucosal inflammation, impaired motility, and GERD severity. Neurogastroenterol Motil. 2021;33(10):e14211. doi:10.1111/nmo.14211
30. Ustaoglu A, Nguyen A, Spechler S, et al. Mucosal pathogenesis in gastro-esophageal reflux disease. Neurogastroenterol Motil. 2020;32(12):e14022. doi:10.1111/nmo.14022
31. Ustaoglu A, Woodland P. Esophageal afferent innervation and its role in gastro-esophageal reflux disease symptoms. Curr Opin Gastroenterol. 2021;37(4):372–377. doi:10.1097/MOG.0000000000000749
32. Yu M, Chang C, Undem BJ, Yu S. Capsaicin-sensitive vagal afferent nerve-mediated interoceptive signals in the esophagus. Molecules. 2021;26(13):3929. doi:10.3390/molecules26133929
33. Cui R, Zhang H, Zhou L, et al. Diagnostic value of dilated intercellular space and histopathologic scores in gastroesophageal reflux disease. Dis Esophagus. 2015;28(6):530–537. doi:10.1111/dote.12256
34. Xie C, Sifrim D, Li Y, Chen M, Xiao Y. Esophageal baseline impedance reflects mucosal integrity and predicts symptomatic outcome with proton pump inhibitor treatment. J Neurogastroenterol Motil. 2018;24(1):43–50. doi:10.5056/jnm17032
35. Kahrilas PJ. Dilated intercellular spaces: extending the reach of the endoscope. Am J Gastroenterol. 2005r;100(3):549–550. doi:10.1111/j.1572-0241.2005.41918.x
36. Zavala-Solares MR, Fonseca-Camarillo G, Valdovinos M, et al. Gene expression profiling of inflammatory cytokines in esophageal biopsies of different phenotypes of gastroesophageal reflux disease: a cross-sectional study. BMC Gastroenterol. 2021;21(1):201. doi:10.1186/s12876-021-01707-7
37. Taft TH, Guadagnoli L, Carlson DA, et al. Validation of the short-form esophageal hypervigilance and anxiety scale. Clin Gastroenterol Hepatol. 2021;20:e64–e73. doi:10.1016/j.cgh.2020.12.021
38. Ustaoglu A, Sawada A, Lee C, et al. Heartburn sensation in nonerosive reflux disease: pattern of superficial sensory nerves expressing TRPV1 and epithelial cells expressing ASIC3 receptors. Am J Physiol Gastrointest Liver Physiol. 2021;320(5):G804–G815. doi:10.1152/ajpgi.00013.2021
39. Bamps D, Vriens J, de Hoon J, Voets T. TRP channel cooperation for nociception: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2021;61:655–677. doi:10.1146/annurev-pharmtox-010919-023238
40. Silva RO, Bingana RD, Sales TMAL, et al. Role of TRPV1 receptor in inflammation and impairment of esophageal mucosal integrity in a murine model of nonerosive reflux disease. Neurogastroenterol Motil. 2018;30:e13340.
41. Delvalle NM, Dharshika C, Morales-Soto W, et al. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell Mol Gastroenterol Hepatol. 2018;6(3):321–344. doi:10.1016/j.jcmgh.2018.05.009
42. Patcharatrakul T, Kriengkirakul C, Chaiwatanarat T, Gonlachanvit S. Acute effects of red chili, a natural capsaicin receptor agonist, on gastric accommodation and upper gastrointestinal symptoms in healthy volunteers and gastroesophageal reflux disease patients. Nutrients. 2020;12(12):3740. doi:10.3390/nu12123740
43. Voisin T, Bouvier A, Chiu IM. Neuro-immune interactions in allergic diseases: novel targets for therapeutics. J Int Immunol. 2017;29:247–261. doi:10.1093/intimm/dxx040
44. Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev. 2018;282(1):168–187. doi:10.1111/imr.12622
45. Nan L, Nam HH, Park BY, Kim BT, Choo BK. Ameliorative effects of Magnolia sieboldii buds hexane extract on experimental reflux esophagitis. Phytother Res. 2020;34(9):2385–2396. doi:10.1002/ptr.6689
46. Nam HH, Nan L, Choo BK. Dichloromethane extracts of geranium koreanum kom. alleviates esophagus damage in acute reflux esophagitis-induced rats by anti-inflammatory activities. Int J Mol Sci. 2018;19(11):3622. doi:10.3390/ijms19113622
47. Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):277–288. doi:10.1053/j.gastro.2017.09.047
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.