Back to Journals » Journal of Pain Research » Volume 12
Neck pain in episodic migraine: a cross-sectional study
Authors Yu Z, Wang R, Ao R, Yu S
Received 6 January 2019
Accepted for publication 2 April 2019
Published 20 May 2019 Volume 2019:12 Pages 1605—1613
DOI https://doi.org/10.2147/JPR.S200606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Zhe Yu, Rongfei Wang, Ran Ao, Shengyuan Yu
Department of Neurology, Chinese PLA General Hospital, Beijing 100853, People’s Republic of China
Purpose: It has been reported that neck pain is more prevalent in episodic migraineurs (EM) than in the general population. Subjects with episodic migraine exhibited widespread hypersensitivity in cranio-cervical region. Our objectives were to explore the potential factors associated with the presence of neck pain for EM, and whether there were differences in pericranial muscle tenderness between EM with and without neck pain.
Patients and methods: Fifty EM with neck pain (34.76±8.04) and 50 age- and sex-matched EM without neck pain (34.26±9.39) were enrolled. The characteristics of headaches and some lifestyle factors were assessed in two groups. The migraine disability score and neck disability index were also recorded. During migraine-free period, cranio-cervical muscle tenderness scores and mechanical pain threshold were assessed for all patients.
Results: There were no significant differences in pain intensity (p=0.44), migraine disability (p=0.71), duration (p=0.44) or frequency (p=0.85) of headache between EM with and without neck pain. The lifestyle factors including smoking, alcohol, coffee, body mass index≧23kg/m2, poor sleeping (<8 h/day) and time spent on TV and computers (>2 h/day) were not associated with the presence of neck pain in this study. Compared with EM without neck pain, those with neck pain had higher neck tenderness (p<0.01) and higher cephalic tenderness scores (p<0.01). Neck Disability Index scores were positively correlated with neck and total muscle tenderness scores.
Conclusion: There was a significant difference in cranio-cervical muscle tenderness scores between EM with and without neck pain. For EM, the factors studied in the current research seemed not associated with the onset of neck pain, and further studies including other factors are needed.
Keywords: episodic migraine, neck pain, muscle tenderness, neck disability
Introduction
Migraine is a common health problem and in the Global Burden of Disease Survey 2010, it was ranked as the third most prevalent disorder worldwide.1 In China, the one-year prevalence of migraine is 9.3%.2 It was reported that neck pain affects 14–71% of the general population.3 Both migraine and neck pain have a significant impact on individuals, their families, communities and businesses.2–6
Migraine can be classified into two categories: episodic migraine and chronic migraine. The episodic form is the most common manifestation, and it is estimated that each year, approximately 2.5% of episodic migraineurs (EM) develop new-onset chronic migraine.7 Epidemiological studies have reported that EM are more likely to experience neck pain than healthy individuals, but the underlying mechanism remains unclear.8,9 A potential mechanism may explain the clinical finding was the convergence of trigeminal and cervical afferents in the trigeminocervical nucleus,10–12 but based on the trigeminocervical system, whether it attributed to the central or peripheral sensitization has been widely debated. At one hand, for migraineurs, the occurrence of headache is due to the activation of the trigeminal afferents innervating the pain-sensitive structures, including cranial vessels, dura matter, etc.13,14 Some researchers suggested that increased attacks and prolonged inputs from the intracranial process could lead to a sensitization of the second-order neurons in the trigeminocervical nucleus receiving cervical input, which may contribute to the clinical phenomena of cervical hypersensitivity.15,16 However, considering that EM experience a low frequency of headaches, whether this mechanism plays an important role in the presence of neck pain remains unclear. In addition, to date, whether there are differences in characteristics of migraine between EM with neck pain and without neck pain has not been studied. On the other hand, it was also indicated that the cervical input could increase the central excitability of dural afferent input.10,12 Someone suggested that the neck pain was a kind of preexisting dysfunction of the musculoskeletal system, which could be the potential trigger for migraine attacks.12,17
In the clinic, neck pain could increase the disability and affect the migraineurs’ response to treatment.18,19 Given the high prevalence of neck pain in EM and its negative effect on migraine treatment, it is important to protect these patients from neck pain. To our knowledge, the factors associated with the onset of neck pain have not been studied in EM. According to previous studies, some lifestyle factors, such as body mass index (BMI), smoking, alcohol, coffee intake, etc., were considered as potential risk factors of migraine.8,9,20,21 Simultaneously, conflicting results were reported that some of them increased the risk of neck pain in general people, eg, BMI, smoking, etc.5,22 In this study, we want to explore that whether these lifestyle factors were associated with the presence of neck pain in EM, because it seemed that the common factors could also account for the high prevalence of neck pain in EM.
Trigeminal sensory fibers that innervate the meninges also project branches that supply the pericranial muscles.14 The increased muscle tenderness was considered to be associated with muscle hyperesthesia.23,24 During acute migraine, migraineurs do have increased pericranial muscle tenderness.25 Previous studies revealed that subjects with episodic migraine exhibited widespread hypersensitivity in cranio-cervical region.26 Given the fact that neck pain may be a disorder concerning pericranial muscles and upper cervical nerve,5,22 for EM, whether the muscle tenderness and the mechanical pain threshold in the cranio-cervical region are related with the presence of neck pain remained unknown.
The present study aimed to explore the potential factors associated with the presence of neck pain for EM, and whether there were differences in muscle tenderness of the cranio-cervical region between EM with and without neck pain.
Material and methods
Participants
All the participants were consecutively recruited from among a group of patients who visited the International Headache Center of the Chinese PLA General Hospital between January 2015 and June 2015. The study was approved by the Ethical Committee of the Chinese PLA General Hospital and conducted following the principles of the Declaration of Helsinki, all of whom signed the informed consents of the Ethics Committee of PLA General Hospital. The migraine diagnosis was made by experienced neurologists in the headache center according to the International Classification of Headache Disorders, 3rd Edition (beta version) (ICHD-3 beta).27 Episodic migraine was defined as migraine with <15 days of headache per month.13 Patients between 18 and 60 years of age, who were diagnosed with episodic migraine, were included in the present study. Individuals with systemic diseases, chronic migraine, combined with other types of headache, a history of cranio-cervical trauma or any musculoskeletal dysfunction were excluded. Neck pain is defined as a subjective pain in the neck and shoulder region. Neck pain can refer to the upper limb.6,28 For migraineurs with neck pain, those who reported neck pain between the migraine attacks were included. Patients with systemic diseases, such as rheumatoid arthritis, ankylosing spondylitis or lupus erythematous; history of neck or head trauma (eg, whiplash) or surgery; musculoskeletal dysfunction (eg, fibromyalgia); neck pain only occurring during the migraine phase; or combined with medication overuse, were excluded.
Characteristics of headache and lifestyle factors
A staff member of the headache center recorded the demographic information including age, sex, the frequency and duration of headaches, occupation, height, weight, etc. Every patient who was diagnosed with episodic migraine was asked to complete the migraine Disability Assessment questionnaire (MIDAS) to assess headache-related disability.29 Migraineurs answered five questions, scoring the number of days with activity limitations due to migraine in the past 3 months. MIDAS is considered to be a scientifically reliable and valid measure of migraine disability and can improve communication between patients and physicians. Scores are as follows: Grade I: 0–5 little or no disability, Grade II: 6–10 mild disability, Grade III: 11–20 moderate disability and Grade IV: ≥21 severe disability.
The lifestyle factors were estimated by a semi-structured interview, which was similar to the previous studies on assessing the potential factors associated with migraine.8,9,28 Smoking was assessed by the question “Do you smoke?”. Those who answered “yes” would be considered as smokers. The alcohol consumption was measured with the question “have you consumed any alcoholic drink in the last 2 weeks?”. The coffee consumption was assessed through the question “have you consumed any coffee in the last 2 weeks?”. Sleep condition was assessed with the question “how long do you sleep every day in the last six months?”. The time spent on TV and computer was assessed by the question “how much time do you spend in the TV and computer every day in the last six months?”.
The BMI was classified as BMI< 23kg/m2 and BMI≥23 kg/m2 because obesity was defined as BMI≧23.0 kg/m2 in China.30 Poor sleeping was defined as sleeping less than 8 hrs per day in the previous study, and it was reported that individuals who sleep less than 8 hrs per day showed a higher prevalence of migraine.9 The sleep condition was divided into patients who sleep less than 8 hrs per day and those who sleep more than 8 hrs per day. The time spent on TV and computer would be divided according to the median of the data in all the patients.31
Pain intensity of migraine
The visual analog scale (VAS) which is a well-established measurement was used to collect the pain intensity data.32 It consists of a 100 mm line, on which 0 indicates no pain and 100 indicates the worst pain imaginable by the patient. Migraine is a type of paroxysmal disease and usually lasts several hours. It can be aggravated by routine physical activity (eg, walking or climbing stairs). Coupled with distance and social factors, it is very difficult to schedule appointments with EM during an acute migraine attack. In the present study, the patients were asked to assess the pain intensity of migraine in the latest attack using a VAS during a migraine-free period. A headache diary with VAS was then given to individuals to record the features of headache experiencing migraine attacks. The final VAS scores could be corrected according to the headache diary, which ensured the validity of results.
Neck disability index
The Neck Disability Index (NDI) questionnaire has been considered as a reliable way to assess individuals’ neck disability.33,34 It consists of 10 items regarding the effect of neck pain on activities of daily life and has been reported to be correlated with the cervical range of motion. The total score of individuals can range from 0 to 50. The NDI is scored as follows: 0 to 4 (absence of disability), 5 to 14 (mild disability), 15 to 24 (moderate disability), 25 to 34 (severe disability) and ≥35 (complete disability). In this study, the Chinese version of the NDI was applied to the patients.35
Mechanical pain threshold
Von Frey hairs (VFH; North Coast, Touch-test TM Sensory Evaluator, USA; force 0.16–300 g) were used for assessing the mechanical pain threshold.36 The test was performed during a migraine-free period. The lowest force (0.16 g) was applied at the beginning and was followed by the next highest force until the subject signaled that he or she was experiencing a pricking pain. The areas tested were the forehead (V1) and the posterior neck (C2, C3). Pain intensity, NDI and mechanical pain threshold were assessed by a second staff member of the headache center who was blinded to the group allocation.
Muscle tenderness scores
The pericranial muscle tenderness scoring system was a reliable way to reflect the sensitivity of the muscle.24,25,37,38 To ensure the reliability and validity of the muscle TS, the use of a palpometer was recommended, and the palpometer has been previously described in detail.24,37 In this study, a clinician who was blinded to the groups was trained using a palpometer to exert a palpation pressure of moderate intensity (140 U). The muscle tenderness scores and von Frey were assessed in the same day. The palpometer has previously been described in detail.25,37 Eight pairs of cranio-cervical muscles were assessed by palpation. The muscles were divided into two groups: a cephalic muscle group (frontal, temporal, lateral pterygoid and masseter muscles) and a neck muscle group (insertions at the mastoid processes, sternocleidomastoid and trapezius muscles and neck muscle insertions).38 The tenderness score (TS) of each muscle ranged from 0 to 3, according to the patients’ reflection: 0= no discomfort; 1= visible mild discomfort in face without verbal report of pain; 2= visible discomfort with verbal report of pain and 3=marked visible discomfort or withdraw and report of considerable pain.25,26,37,38 The cephalic tenderness score (cephalic TS) was calculated by summation of the scores from the cephalic muscle group. The neck tenderness score (neck TS) was represented by the sum of the scores of the neck muscle group. The total tenderness score (TTS) was the sum of the cephalic TS and neck TS scores.25,38
Sample size
The PASS software 15.0 was used to calculate the sample size of this study. The independent t test was conducted to calculate the difference between two independent means (EM with neck pain and EM without neck pain). We set an alpha probability of 0.05 and a power of 90% to detect changes in a bilateral comparison. The means and the standard deviation in two groups (EM with neck pain: 7.29±4.26; EM without neck pain: 3.29±3.33) were obtained from our previous pilot study with 14 patients per group, considering total muscle tenderness as the main variable. The total sample size was 42 patients.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0. The data of patients were analyzed by descriptive statistics, such as means ± SD. For continuous variables, the Shapiro–Wilk test was used to determine whether the data were normally distributed (p>0.05). For the results that were normally distributed, the independent t test was used. For the results that were not normally distributed, the Mann–Whitney test was used. For categorical data, chi-square test was used. The multinomial regression model was performed to analyze whether the lifestyle factors were associated with the presence of neck pain. OR with their corresponding 95% confidence intervals were calculated. Spearman’s correlation (r) tests were used to determine the association between muscle tenderness scores and NDI (r) in two groups, respectively. For the association, values<0.3 represented weak correlation, from 0.3 to 0.7 moderate correlation and >0.7 strong correlation. Statistical significance was set at p<0.05.39
Results
The characteristics of migraine and lifestyle factors
Fifty EM with neck pain (F:39 M:11 34.76±8.04 years old) and fifty age- and sex-matched EM without neck pain (F:38 M:12 34.26±9.22 years old) were finally recruited (Figure 1). There were no significant differences in pain intensity (p=0.44), MIDAS (p=0.71), duration (p=0.44) or frequency (p=0.85) of headache between those with and without neck pain (Table 1). According to the multinomial regression (Table 2), the lifestyle factors including smoking, alcohol, coffee, BMI≥23 kg/m2, poor sleeping (<8 h/day) and time spent on TV and computers (>2 h/day) were not associated with the presence of neck pain in this study.
 | Table 1 Demographic and characteristics of headache in EM with and without neck pain |
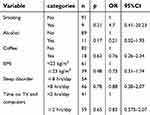 | Table 2 The associations between lifestyle factors and the presence of neck pain according to the multinomial regression model |
 | Figure 1 Patients' recruitment. Abbreviations: TTH, tension-type headache; NP, neck pain; EM, episodic migraineurs. |
Muscle tenderness and NDI
Compared with the EM without neck pain (Figure 2), EM with neck pain had higher neck TS (p<0.01) and higher cephalic TS (p<0.01). The TTS was equally higher (p<0.01) in the groups of patients who had neck pain. Neck TS was positively correlated with cephalic TS in the present study (r: 0.65 p<0.01).
 | Figure 2 Cephalic tenderness scores, neck tenderness scores, total tenderness scores were assessed (mean ± SE). ***p<0.01. Abbreviations: TS, tenderness scores; EM, episodic migraineurs. |
The correlations between NDI scores and muscle TS were calculated in two groups, respectively. There were moderate positive correlations between the neck muscle TS and NDI score in both two groups (Table 3). In the group of EM with neck pain, NDI scores were correlated with neck TS (r:0.37 p<0.01) and total TS (r:0.30 p=0.03). In the group of EM without neck pain, NDI scores were correlated with neck TS (0.30 p=0.01) and total TS (0.20 p=0.04).
 | Table 3 Correlations between tenderness and neck disability index (NDI) scores |
Mechanical pain threshold
There was no statistical difference in mechanical pain threshold of the forehead regions (p=0.06) and neck regions (p=0.12) between EM with neck pain and those without neck pain (Figure 3). However, both cephalic and neck pain threshold of EM with neck pain were lower than those EM without neck pain. Neck TS was negatively correlated with the pain threshold of the neck region (r=−0.28 p<0.01) and cephalic TS negatively correlated with the pain threshold of the trigeminal region (r=−0.22 p=0.03).
 | Figure 3 Cephalic pain threshold and neck pain threshold were assessed using von Frey hair (mean ± SE).Abbreviation: EM, episodic migraineurs. |
Discussion
Our main findings indicated that there was a significant difference in cranio-cervical muscle tenderness scores between EM with and without neck pain, and the total muscle TS were positively correlated with NDI scores. No differences in the characteristics of migraine were found between two groups, and the lifestyle factors studied in the current research were not associated with the presence of neck pain.
In this study, only EM were included and there were no differences in the migraine disability, intensity, frequency and disease duration of headaches between those with neck pain and without neck pain. It was suggested that the increased migraine attacks may lead to the central sensitization, and neck pain could emerge from the sustained central sensitization.15,16 However, EM experienced a low frequency of headaches, and the mean frequency of migraine in these patients was less than four attacks per month. Moreover, given the fact that two groups had similar profiles regarding headache, the central sensitization may not account for the presence of neck pain in these EM. The current results were partly in accordance with the previous study.17 Some researchers found that compared with healthy controls, EM had a higher prevalence of some dysfunctions in cervical musculoskeletal system, which could not be explained by the central sensitization.17 They suggested that the neck pain may be a kind of preexisting cervical dysfunctions and could serve as one of the triggers for migraine attacks.12,17 Combined with the previous and current studies, we speculated that the peripheral mechanism may play an important role in the presence of neck pain in the EM.
Our findings confirmed that local muscle TS were negatively correlated with mechanical pain threshold (assessed by VFH) in these areas, which was consistent with the results of previous studies.23,24,37 In these studies, the negative correlations between pericranial muscle TS and pressure pain threshold have been demonstrated.23,24,37 Although many studies have been performed to study the muscle tenderness in migraineurs, the conflicting results were reported. Some studies revealed increased total TS in patients with migraineurs,23,25 whereas some researchers found that there was no difference between migraineurs and people without migraine.40 To our knowledge, this was the first study to estimate differences in the muscle tenderness between EM with and without neck pain. We found that cranio-cervical muscle TS were significantly increased in EM with neck pain. Considering the high prevalence of neck pain in migraine,8,9 the current study may be a good supplement to the previous studies regarding pericranial muscle tenderness in migraineurs. Although no statistical difference in the mechanical pain threshold between two groups was found in this study, there was a trend that the EM with neck pain had a lower mechanical pain threshold in cranio-cervical region. So, the results of these two manners were partly consistent.
We also found that both neck and cephalic TS were associated with the presence of neck pain, and neck TS was positively correlated with cephalic TS. These results could be explained by the involvement of the trigeminocervical systems. The cervical and trigeminal afferents converge in the trigeminocervical nucleus.10–12 It was suggested that peripheral nociceptor sensitization could sensitize second-order neurons in the trigeminocervical nucleus, which resulted in the increased sensitivity to the stimuli from both the trigeminal and cervical regions.10,12
In the current study, the lifestyle factors including obesity, smoking, alcohol, coffee, sleep conditions and computer use were studied in EM. We did not find any associations between these factors and the presence of neck pain, and it seemed that some other factors may contribute to the onset of neck pain in EM. In the general population, a large number of studies have identified associations between age and neck pain, and a higher prevalence of neck pain among women was also indicated.6,22 However, these factors were well controlled in this study. In order to keep EM informed how to keep away from neck pain, a study including other factors is needed in the future.
This study may have potential clinical implications. First of all, although it has been reported that neck pain could increase the disability and affect the migraineurs’ response to treatment, how to treat neck pain in migraine have not been studied. Our findings suggested that the presence of neck pain in EM may not be explained by the central sensitization, and the peripheral mechanism may play an important role. Thus, for these patients, apart from the anti-migraine drugs aiming to suppress sensitization, the treatment focused on the cervical musculoskeletal systems may be a good supplement to lift the burden of the diseases. In addition, the positive association between neck muscle TS and NDI scores was found in our research, which was consistent with a cross-sectional study based on thirty-two migraines.41 The pressure pain thresholds were measured in that study, and the negative correlations between NDI scores and pressure pain thresholds were obtained. To date, the NDI questionnaire has not been a commonly used measure to assess the disability of migraineurs in the clinic. Muscle tenderness is easily detected and recorded by manual palpation, and it could be an alternative indirect index to reflect neck disability in migraineurs.
There are some limitations in the present study. First, given that all the participants were recruited from the headache center, selections bias could not be ruled out. Second, because only EM were enrolled in this study, all of our findings applied only to EM. Neck pain in patients with chronic migraine still requires further study in the future. Third, no-headache individuals with neck pain and without neck pain should be included to help us make a better understanding the role of the migraine in the neck disability, muscle tenderness and pain threshold in these patients. Therefore, further study regarding neck pain in EM is still needed in the future.
Conclusion
There was a significant difference in cranio-cervical muscle tenderness scores between EM with and without neck pain. For EM, the factors studied in the current research seemed not associated with the onset of neck pain, and further studies including other factors are needed.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (grant 2017YFC1307701).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLD) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
2. Yu S, Liu R, Zhao G, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52(4):582–591.
3. Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15(6):834–848. doi:10.1007/s00586-004-0864-4
4. Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210. doi:10.1111/j.1468-2982.2007.01288.x
5. Hogg-Johnson S, van der Velde G, Carroll LJ. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S39–S51. doi:10.1097/BRS.0b013e31816454c8
6. Hoy DG, Protani M, De R, et al. The epidemiology of neck pain. Best Pract Res Clin Rheumatol. 2010;24(6):783–792. doi:10.1016/j.berh.2011.01.019
7. Manack A, Buse D, Lipton R. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep. 2011;15(1):70–78. doi:10.1007/s11916-010-0157-z
8. Landgraf MN, von Kries R, Heinen F, Langhagen T, Straube A, Albers L. Self-reported neck and shoulder pain in adolescents is associated with episodic and chronic migraine. Cephalalgia. 2016;36(8):807–811. doi:10.1177/0333102415610875
9. Ferna´Ndez-de-Las-Pen˜as C, Herna´ndez-Barrera V, Carrasco-Garrido P, et al. Population based study of migraine in Spanish adults: relation to socio-demographic factors, lifestyle and co-morbidity with other conditions. J Headache Pain. 2010;11(2):97–104. doi:10.1007/s10194-009-0176-5
10. Bartsch T, Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002;125(Pt 7):1496–1509.
11. Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126(Pt 8):1801–1813. doi:10.1093/brain/awg190
12. Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8(7):679–690. doi:10.1016/S1474-4422(09)70090-0
13. Goadsby PJ. Migraine pathophysiology. Headache. 2005;45(Suppl 1):S14–S24. doi:10.1111/j.1526-4610.2005.4501003.x
14. Dodick DW. Migraine. Lancet. 2018;391(10127):1315–1330. doi:10.1016/S0140-6736(18)30478-1
15. Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Curr Pain Headache Rep. 2003;7(5):371–376.
16. Hagen K, Einarsen C, Zwart JA, Svebak S, Bovim G. The co-occurrence of headache and musculoskeletal symptoms amongst 51 050 adults in Norway. Eur J Neurol. 2002;9(5):527–533.
17. Luedtke K, Starke W, May A. Musculoskeletal dysfunction in migraine patients. Cephalalgia. 2018;38(5):865–875. doi:10.1177/0333102417716934
18. Ford S, Calhoun A, Kahn K, et al. Predictors of disability in migraineurs referred to a tertiary clinic: neck pain, headache characteristics, and coping behaviors. Headache. 2008;48(4):523–528. doi:10.1111/j.1526-4610.2008.00859.x
19. Okuma H, Kitagawa Y, Takagi S. Clinical efficacy of rizatriptan for patients with migraine: efficacy of drug therapy for migraine accompanied by tension headache-like symptoms, focusing on neck stiffness. J Headache Pain. 2005;6(6):455–458. doi:10.1007/s10194-005-0249-z
20. Tai MS, Yap JF, Goh CB. Dietary trigger factors of migraine and tension-type headache in a South East Asian country. J Pain Res. 2018;11:1255–1261. doi:10.2147/JPR.S158151
21. Yu S, Liu R, Yang X, et al. Body mass index and migraine: a survey of the Chinese adult population. J Headache Pain. 2012;13(7):531–536. doi:10.1007/s10194-012-0470-5
22. Cohen SP. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin Proc. 2015;90(2):284–299. doi:10.1016/j.mayocp.2014.09.008
23. Fernández-de-Las-Peñas C, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Side-to-side differences in pressure pain thresholds and pericranial muscle tenderness in strictly unilateral migraine. Eur J Neurol. 2008;15(2):162–168. doi:10.1111/j.1468-1331.2007.02020.x
24. Bendtsen L. Central sensitization in tension-type headache—possible pathophysiological mechanisms. Cephalalgia. 2000;20(5):486–508. doi:10.1046/j.1468-2982.2000.00070.x
25. Jensen K, Tuxen C, Olesen J. Pericranial muscle tenderness and pressure-pain threshold in the temporal region during common migraine. Pain. 1988;35(1):65–70.
26. Palacios-Ceña M, Ferracini GN, Florencio LL, et al. The number of active but not latent trigger points associated with widespread pressure pain hypersensitivity in women with episodic migraines. Pain Med. 2017;18(12):2485–2491. doi:10.1093/pm/pnx130
27.
28. Blaschek A, Decke S, Albers L, et al. Self-reported neck pain is associated with migraine but not with tension-type headache in adolescents. Cephalalgia. 2014;34(11):895–903. doi:10.1177/0333102414523338
29. Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–S28.
30. He YH, Jiang GX. Obesity and its associations with hypertension and type 2 diabetes among Chinese adults age 40 years and over. Nutrition. 2009;25:1143–1149. doi:10.1016/j.nut.2009.04.003
31. MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19–40.
32. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1–2):95–97. doi:10.1016/S0304-3959(97)00005-5
33. Schellingerhout JM, Verhagen AP, Heymans MW. Measurement properties of disease-specific questionnaires in patients with neck pain: asystematic review. Qual Life Res. 2012;21(4):659–670. doi:10.1007/s11136-011-9965-9
34. Bobos P, MacDermid JC, Walton DM, Gross A, Santaguida PL. Patient-reported outcome measures used for neck disorders: an overview of systematic reviews. J Orthop Sports Phys Ther. 2018;48(10):775–788. doi:10.2519/jospt.2018.8131
35. Wu S, Ma C, Mai M, Li G. Translation and validation study of Chinese versions of the neck disability index and the neck pain and disability scale. Spine (Phila Pa 1976). 2010;35(16):1575–1579. doi:10.1097/BRS.0b013e3181c6ea1b
36. LoPinto C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2006;26(7):852–856. doi:10.1111/j.1468-2982.2006.01121.x
37. Bendtsen L, Jensen R, Jensen NK, et al. Pressure-controlled palpation: a new technique which increases the reliability of manual palpation. Cephalalgia. 1995;15(3):205–210. doi:10.1046/j.1468-2982.1995.015003205.x
38. Aaseth K, Grande RB, Lundqvist C, et al. Pericranial tenderness in chronic tension-type headache: the Akershus population-based study of chronic headache. J Headache Pain. 2014;15:58.
39. Sani F, Todman J. Experimental Design and Statistics for Psychology: A First Course. Oxford, UK: Blackwell Publising; 2006.
40. Jensen R, Rasmussen BK, Pedersen B, et al. Muscle tenderness and pressure pain thresholds in headache. A population study. Pain. 1993;52(2):193–199.
41. Gonçalves MC, Chaves TC, Florencio LL, et al. Is pressure pain sensitivity over the cervical musculature associated with neck disability in individuals with migraine? Journal of Bodywork and Movement Therapies. 2015;19(1):67–71. doi:10.1016/j.jbmt.2014.02.007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
