Back to Journals » Journal of Inflammation Research » Volume 16
NEAT1/microRNA 339-5p/SPI1 Axis Feedback Loop Contributes to Osteogenic Differentiation in Acute Suppurative Osteomyelitis in Children
Received 27 March 2023
Accepted for publication 24 June 2023
Published 29 June 2023 Volume 2023:16 Pages 2675—2687
DOI https://doi.org/10.2147/JIR.S410339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Dongsheng Zhu,1,* Zhitao Zhu,2,* Han Qi3
1Department of Pediatric Surgery, the First People’s Hospital of Lianyungang, Affiliated to Xuzhou Medical University, Lianyungang, Jiangsu, 222000, People’s Republic of China; 2Department of Radiology, the Second People’s Hospital of Lianyungang, Lianyungang, Jiangsu, 222000, People’s Republic of China; 3Department of Emergency Surgery, the Second People’s Hospital of Lianyungang, Lianyungang, Jiangsu, 222000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongsheng Zhu, Email [email protected]
Objective: Long non-coding RNA plays an important role in osteogenic differentiation. Nuclear enriched abundant transcript 1 (NEAT1) has been revealed to promote osteogenic differentiation in human bone marrow mesenchymal stem cells (hBMSCs), but the underlying regulatory mechanism remains unknown in acute suppurative osteomyelitis of children.
Methods: Osteogenic medium (OM) was used to induce osteogenic differentiation. Quantitative real-time PCR and Western blotting were used to evaluate gene expression. The effects of NEAT1, microRNA 339– 5p (miR-339-5p), and salmonella pathogenicity island 1 (SPI1) on osteogenic differentiation were assessed in vitro using alizarin red S staining assays and alkaline phosphatase activity. Interactions between NEAT1, miR-339-5p, and SPI1 were identified using immunoprecipitation, luciferase reporter assays, and chromatin immunoprecipitation.
Results: During osteogenic differentiation, expression of NEAT1 was up-regulated in hBMSCs, and miR-339-5p level was down during osteogenic differentiation. Knockdown of NEAT1 reduced the osteogenic differentiation of hBMSCs, and down-regulation of miR-339-5p may counteract the effect of NEAT1 silencing. SPI1 was a target of miR-339-5p by luciferase reporter assay and was also a transcription factor of NEAT1 by chromatin immunoprecipitation. A positive NEAT1-miR-339-5p-SPI1 feedback loop was found to be present during osteogenic differentiation in hBMSCs.
Conclusion: It was the first study to reveal that the NEAT1-miR-339-5p-SPI1 feedback loop can promote osteogenic differentiation in hBMSCs and shed a new light on the role of NEAT1 during osteogenic differentiation.
Keywords: NEAT1, miR-339-5p, SPI1, osteogenic differentiation
Introduction
Osteomyelitis is a very common orthopedic disease, often in children, that can cause severe bone loss and destruction due to a bacterial infection, resulting in progressive bone destruction.1 Staphylococcus aureus (SA) is believed to be the most common causative bacterium of osteomyelitis in pediatric patients.2 Staphylococcal protein A (SPA) is a protein present in the cell wall of SA and is the main virulence component of SA. SPA plays an important role in promoting the occurrence of osteomyelitis.3 Inflammation, new formation, and bone remodeling are the three main phases in the process of bone regeneration process for the repair of osteomyelitis.4 Pathological changes in osteomyelitis are mainly characterized by bone destruction and bone defects, and are always associated with an imbalance between osteoblasts and osteoclasts during bone remodeling. Human bone mesenchymal stem cells (hBMSCs) have a strong ability to differentiate in vitro and can be induced to differentiate into chondrocytes, osteoblasts, nerve cells or myocardial cells, and are considered ideal grafts for bone tissue engineering.5 However, in order to maintain bone homeostasis, osteogenic differentiation plays a critical role.6 Therefore, it is important to explore the mechanisms of osteogenic differentiation in hBMSCs. Nowdays, hBMMSCs are treated with SPA to construct the cell models of osteomyelitis.7,8
Long non-coding RNA (lncRNA), is a type of RNA that has a length of more than 200 nucleotides and does not have the ability to encode proteins; Mounting evidence has demonstrated that osteogenic differentiation in hBMSCs involves multiple factors, including lncRNA.9 Nuclear enriched abundant transcript 1 (NEAT1) has been suggested to play a crucial role in biological differentiation and proliferation.10,11 Previous studies have found that NEAT1 modulates BMP2 in renal interstitial fibroblasts and promotes osteogenic differentiation in humans.12 In addition, another study in hBMSCs revealed that NEAT1 expression is up-regulated and promotes osteogenic differentiation by modulating the miR-29b-3p/BMP1 pathway in hBMSCs.13 However, it remains to be further explored how NEAT1 regulates osteogenic differentiation in hBMSCs in childhood osteomyelitis. MicroRNA 339–5p (miR-339-5p) has been found to potentially affect osteogenic differentiation, down-regulation of miR-339 promotes differentiation of hBMSCs, and alleviates osteoporosis in human by targeting DLX.14 Salmonella pathogenicity island 1 (SPI1) has also been found to act as a hub gene in osteoporosis patients, which promotes osteogenic differentiation.15 However, the role of NEAT1, miR-339-5p and SPI1 in osteomyelitis is unclear.
In this study of osteomyelitis in children, gene expression analysis was performed. Our findings suggest that the axial feedback loop of NEAT1/microRNA 339–5p/SPI1 contributes to osteogenic differentiation in acute suppurative osteomyelitis in children.
Materials and Methods
Statement of Ethical
This research was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of The Lianyungang No. 1 People’s Hospital, affiliated to Xuzhou Medical University (December 2021; approval no. 20210303), and all legal guardians of the children provided written informed consent.
Patients and Specimens
Blood samples were taken from children with bacterial osteomyelitis. All osteomyelitis patients with positive bacteremia results were confirmed by at least one of percutaneous puncture, surgical sample, and blood culture. Blood samples were collected from 12 children with bacterial osteomyelitis prior to surgery or treatment; at the same time, blood samples were also taken from 12 healthy volunteers.
Cell Culture
The hBMSCs were obtained from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc) and also with 100 U/mL of penicillin in a humidified incubator containing 5% CO2 at 37°C. The hBMSCs were grown in an osteogenic medium (OM), supplemented with 0.05 mmol/L ascorbate, 100 mmol/L dexamethasone, and 10 mmol/L b-glycerophosphate for osteogenic induction, and not for osteogenic induction, only in growth medium (GM). hBMMSCs were treated with SPA (1 mg/mL) (Aladdin Corporation, Shanghai, China) to construct the cell models of osteomyelitis.
RNA Extraction and Quantitative Real-time PCR (RT-qPCR)
The total RNA was extracted from hBMSCs and blood using the TRIzol reagent (Invitrogen, CA, USA). Subsequently, the PrimeScript RT reagent kit (Thermo Fisher Scientific, Inc) was used to reverse transcription of the RNA into the complementary DNA. RT-qPCR was subsequently performed on the Applied Biosystems Prism 7900 system (Thermo Fisher Scientific, Inc) in accordance with the instructions of the SYBR‑Green Master Mix kit (Thermo Fisher Scientific, Inc). The used primers were listed in Table 1.
 |
Table 1 Quantitative Polymerase Chain Reaction Primers for Reverse Transcription |
Cell Transfection
The siRNA oligos for NEAT1 (si-NEAT1) or SPI1 (si-SPI1) were commercially designed and obtained from GenePharma Inc (Shanghai, China).These oligos were then transfected into hBMSCs with Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) for knockdown experiments. The miR-339-5p mimics, miR-339-5p inhibitor and miRnegative controls were commercially designed and obtained from Genepharma Inc (Shanghai, China). They were then directly transfected into hBMSCs with Lipofectamine 3000 (Thermo Fisher Scientific, Inc.).
Western Blot Assay
Western blotting analysis was performed as we previously reported.16 Total proteins were extracted from hBMSCs using RIPA buffer (Biosharp, Chongqing, China) and protein quantitation using Bradford protein assay (Bio-Rad, Hercules, CA, USA). Electrophoresis was performed using a 12% SDS-PAGE gel, which was then transferred to a PVDF membrane and 5% fat-free milk was blocked at room temperature for 2 h. Membranes were incubated with primary antibodies overnight at 4°C. The secondary antibodies were then incubated at room temperature for 1 h. Pierce™ ECL Western Blotting Substrate (cat. no. 32109; Thermo Fisher Scientific, Inc.) was used to visualize the bound antibodies. Protein levels were evaluated using a chemiluminescence detection system (GE Healthcare Biosciences).The antibodies used were listed in Table 2.
 |
Table 2 The List of the Antibodies Used for Western Blotting |
ALP Activity Measurement
After osteogenic induction successfully, 1 million hBMSCs were collected and subjected to 5 min of lysis with PIPA lysis buffer (Beyotime biotechnology, China), then 3 min centrifugation at 4°C to obtain the supernatant. A 5 mL ALP substrate buffer (BD Biosciences Clontech, USA) was then added to the 5 mL supernatant. The detection of ALP activity was performed using a colorimetric analysis of the mixture and a 1h reaction.
ARS Staining
The mineralization was measured using ARS staining. The cells were first fixed with 4% paraformaldehyde for half an hour, and then washed twice with phosphate buffered saline (PBS). Subsequently, the cells were stained with ARS stain solution (Leagene Biotech., China) at room temperature for 10 min. Finally, 10% acetic acid (Sigma, USA) was used to extract ARS and then the OD was measured at an absorbance of 540 nm for quantitative analysis and imaging.
Nuclear-Cytoplasmic Fractionation
PARIS™ Kit (Invitrogen, USA) was applied to separate nuclear and cytoplasmic RNA in hBMSCs in order to explore cellular location of NEAT1. The extracted RNA was analyzed using a qRT-PCR assay.
Fluorescence in situ Hybridization (FISH)
An FISH kit (C002; Gefan Biotechnology, China) was used to perform the hybridization assay. First, 4% paraformaldehyde was used to fix hBMSCs, then 100 nM NEAT1 probes was added and incubated at 37°C for 12 h. Finally, the nuclei were stained with DAPI. The profiles were detected by an inverted fluorescence microscope (Shanghai Dianying Optical Instrument, China).
RNA Immunoprecipitation (RIP)
The RIP kit (Millipore Sigma, USA) was used to detect the ability of NEAT1 to bind SPI1. The hBMSCs were lysed with RIPA lysis (Beyotime Biotechnology, China) in an ice bath for 5 min. The cell extract was incubated with SPI1 (1:1000, ab227835) or IgG (1:100, ab109489) antibodies as input and the remainder was co-precipitation. A magnetic substrate was then used to collect the bead protein complex. Finally, protease K was used to detach the RNA from the co-precipitated bead protein. The expression of NEAT1, miR-339-5p and SPI1 was assessed by RT‐qPCR.
Dual-Luciferase Reporter Assay
After hBMSCs were cultured for 24h, the wild type (wt) and mutant type (mut) NEAT1 sequence were cloned into pmirGLO reporters (GenePharma, China) containing the predicted binding sites of miR-339-5p and SPI1 using a Lipofectamine 2000 kit (GenePharma, China) with renilla luciferase served as an internal normalizer. 48 h post transfection, a dual‑luciferase reporter system (GenePharma, China) was used to examine luciferase activities.
Chromatin Immunoprecipitation (ChIP) Assay
Magna ChIP™ A/G kit (Millipore, USA) was used in hBMSCs to conduct ChIP assay. Formaldehyde was first used to cross-link the cells, followed by 125 mM glycine for 10 min before stopping. After that, the cells were re-suspended and sonicated. Later, anti-SPI1 or anti-IgG was immunoprecipitated to extract chromatin. Finally, qRT-PCR was used to evaluated ChIP DNA after cleaning, eluting and de-crosslinking.
Statistical Analysis
All experiments were repeated three times. GraphPad Prism 8.0 software (GraphPad Software, USA) was used to analyze the data. The comparison between two groups was expressed as mean ± standard deviation and conducted using unpaired t-test. Pearson correlation was used to assess the correlation between two different variables. The diagnostic value of NEAT1 for acute suppurative osteomyelitis in children was analyzed by the receiver operating characteristic (ROC) curve. P < 0.05 was deemed statistically significant.
Results
Low Expression of NEAT1 in Acute Suppurative Osteomyelitis of Children
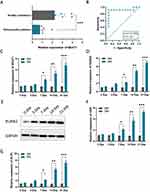
qRT-PCR was performed to explore whether NEAT1 expression level was lower in the whole blood of children with acute suppurative osteomyelitis compared to healthy controls, and it was shown that NEAT1 expression was relatively lower in the blood of children with osteomyelitis compared to health controls (Figure 1A). The results of the ROC analysis, based on the expression level of NEAT1, indicated NEAT1 is a sensitive marker for the diagnosis of acute suppurative osteomyelitis in children (P=0.001, AUC=0.889) (Figure 1B). It was found that the expression of NEAT1 gradually increases during osteogenic differentiation in hBMSCs, which was confirmed by RT-qPCR assay (Figure 1C). RUNX2, OSX, and ALPL are three key osteogenic genes. Their expression levels indicate the degree of osteogenic differentiation in hBMSCs, so we detected their expression levels at days 0, 3, 7, 14 and 21 after incubation. It was found that in hBMSCs incubated in OM, RUNX2, OSX, and ALPL expression levels were progressively higher than that in GM (Figure 1D-G).
Knockdown of NEAT1 Inhibited Osteogenic Differentiation
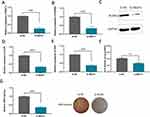
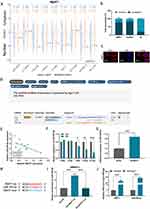
NEAT1 plasmids were transfected into hBMSCs to inhibit NEAT1 expression. Efficiency knockout of NEAT1 was explored using RT-qPCR (Figure 2A). RUNX2, OSX and ALPL were also found to be suppressed when NEAT1 was knockdown (Figure 2B-E). It was also shown that after NEAT1 knockdown, both ALP activity and ARS staining were reduced compared to the control group, suggesting that silencing NEAT1 may inhibit the osteogenesis in hBMSCs (Figure 2F and G). Using the lncLocator website: lncATLAS, NEAT1 was found to have different cellular sublocalization in different cell lines; either in the cytoplasm or in the nucleus (Figure 3A). Next, in hBMSCs, we found that NEAT1 is predominantly located in the cytoplasm, suggesting that post-transcriptional regulation is the key role of NEAT1 in hBMSCs (Figure 3B). In addition, FISH was performed to locate NEAT1. It has also been shown that the expression of NEAT1 is present in the cytoplasm (Figure 3C). The putative binding sites of NEAT1 and miR-339-5p were predicted using the starbase website (Figure 3D). It was found NEAT1 and miR‑339‑5p expression has a significant negative correlation in children with acute suppurative osteomyelitis by pearson’s correlation analysis (P=0.047, r=‑0.581) (Figure 3E). Consistent with this, we found a decrease in miR-339-5p expression levels during osteogenic differentiation (Figure 3F). And miR-339-5p levels were up-regulated upon NEAT1 silencing (Figure 3G).
Targeting RNA-directed miR degradation (TDMD) is a novel discovery model that can inhibit miR function.17 In this study, we explored the possibility that NEAT1 may inhibit miR-339-5p through this mechanism. Following the successful mutation of NEAT1 (Figure 3H), an RNA pulldown assay was performed and miR-339-5p was subsequently found to be enriched in the Bio-NEAT1-wt group, while no significant change was observed in neither the Bio-NC nor Bio-NEAT1-mut group, (Figure 3I), providing preliminary evidence for our conjecture. Following this, RIP assays were performed, and it was found that both NEAT1 and miR-339-5p were enriched in the anti-AGO2 group compared to the anti-IgG group, suggesting coexistence of RNA-induced silencing complexes (RISCs) in them (Figure 3J). Altogether, NEAT1 may inhibit miR-339-5p in hBMSCs via the TDMD mechanism.
NEAT1 Negatively Regulated miR-339-5p in vitro to Inhibit Osteogenic Differentiation
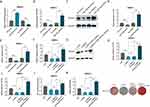
miR-339-5p has been found to attenuate osteogenic differentiation according to a previous research.18 In hBMSCs, the efficiency was confirmed by RT‑qPCR, when miR‑339‑5p mimics or inhibitor was transfected (Figure 4A). We found that RUNX2, OSX and ALPL levels decreased after transfection with miR-339-5p mimics, while they were elevated when transfected with miR-339-5p inhibitor (Figure 4B-E). In addition, rescue assays were performed and it was found that miR-339-5p inhibitor may partially counteract the effect of NEAT1 silencing on RUNX2, OSX, and ALPL expression (Figure 4F-I). Consistently, miR-339-5p inhibition may partially rescue the reduction in ALP activity caused by NEAT1 silencing (Figure 4J). Similarly, ARS staining, previously reduced by NEAT1 depletion, can be restored by miR-339-5p inhibitor (Figure 4K). In other words, NEAT1 miR-339-5p was negatively regulated by NEAT1 to inhibit the osteogenic differentiation in hBMSCs.
The Target of NEAT1/miR-339-5p Axis Was SPI1during Osteogenic Differentiation
The binding site between miR-339-5p and SPI1 was found by bioinformatics analysis (http://213.82.215.117:9999) (Figure 5A). Compared with NC, it was found in hBMCs, when co-transfection with miR‑339‑5p mimics, SPI1 expression decreased, while it increased in the miR‑339‑5p inhibitor (Figure 5B). Afterwards, it was found that, SPI1 and miR-339-5p were enriched in the anti-AGO2 group compared to the anti-IgG group based on an RIP assay, suggesting their coexistence in RISCs (Figure 5C).Then, after a successful mutation of SPI1 (Figure 5D), it was found that the luciferase activity of the cells was altered according to the luciferase reporter assays (Figure 5E). Moreover, based on the results of RT-qPCR assays, SPI1 expression was found to increase when miR-339-5p was silence (Figure 5F). In addition, we confirmed that RUNX2, ALPL and OSX show reduced expression when SPI1 is silenced, whereas their expression is increased by miR-339-5p inhibitor in hBMSCs (Figure 5G-J). Consistently, both ALP activity and ARS staining were reduced upon SPI1 silencing, while they can be enhanced in hBMSCs when co-transfection with miR-339-5p inhibitor (Figure 5K and L). Therefore, it was fair to say that the NEAT1/miR-339-5p axis is targeted by SPI1 in the osteogenic differentiation of hBMSCs.
SPI1 Constituted a Positive Feedback Loop in hBMSCs with NEAT1/miR-339-5p
The binding motif of SPI1 was predicted using JASPAR (Figure 6A). From the ChIP assay, it can be shown that SPI1 has a strong affinity for the NEAT1 promoter in hBMSCs (Figure 6B). Using the HumanTFBD website (http://bioinfo.life.hust.edu.cn/), we obtained the promoter sequence of NEAT1 and searched for potential SPI1 binding sites on the NEAT1 promoter (Figure 6C). Later, luciferase reporter assay results showed that when SPI1 is depressed in hBMSCs, NEAT1 promoter-wt has reduced relative luciferase activity compared to NEAT1 promoter-mut of (Figure 6D). In summary, the up-regulation of NEAT1 in hBMSCs may be caused by SPI1-induced transcriptional activation. Taken together, SPI1 and NEAT1/miR-339-5p formed a positive feedback loop in hBMSCs and contributed to osteogenic differentiation in acute suppurative osteomyelitis of children (Figure 6E).
Discussion
Osteomyelitis is an infectious disease that causes progressive bone destruction and is primarily caused by S. aureus.1 Bone destruction and bone defects are the main pathological changes in osteomyelitis, where the imbalance between osteoblasts and osteoclasts breaks down during bone remodeling.19 hBMSCs have been reported to possess osteogenic differentiation by generating osteoblasts, the main progenitor cells of bone, which play a crucial role in bone tissue regeneration.20 Currently, the role of miRs and lncRNAs in the process of osteogenesis has been demonstrated, namely by regulating the growth or differentiation of osteoblasts.9,12
According to previous related studies, NEAT1 functions were mostly concentrated in cancer and played a tumor promoting role.21,22 In bone diseases, the function of NEAT1 has also been studied. In malignant bone tumors, NEAT1 has been found to be highly expressed and involved in the formation of osteosarcoma in humans.23 One study found that overexpression of NEAT1 reduced bone mass in mice by stimulating the osteoclast.24 In non-cancerous bone diseases in humans, however, the role of NEAT1 has been controversial. One report showed that NEAT1, as a competing endogenous RNA, can adsorb miR-23b-3p in steroid-induced necrosis of the femoral head, which subsequently suppresses osteogenic differentiation in hBMSCs.25 However, investigations into osteoporosis have revealed that NEAT1 may promote osteogenic differentiation in hBMSCs by regulating the miR-29b-3p-BMP1 axis.13 Our study found that the expression of NEAT1 decreases in osteomyelitis and plays a catalytic role in the process of osteogenesis.
In the present study, qRT-PCR was used to detect NEAT1 expression levels in the whole blood of patients with bacterial osteomyelitis or controls with no osteomyeliti. The results showed that NEAT1 expression levels were significantly down-regulated in osteomyelitis compared to healthy controls. The AUC was found to be 0.889 based on the ROC curve, implying that NEAT1 can be used as a predictor of osteomyelitis diagnosis. After hBMSCs were cultured in OM for 21 days, the expression levels of NEAT1 were found to be significantly up-regulated. And during osteogenic differentiation in hBMSCs, the expression of NEAT1 gradually increased, indicating that NEAT1 play a key role in the process of osteogenic differentiation. At the same time, the expression of osteogenic related genes (RUNX2, ALPL, and OSX) was observed to be up-regulated after osteogenic induction. Similarly, a previous report also found that NEAT1 plays an active regulatory function in the osteogenesis process.26
Our study found that NEAT1 is located in the cytoplasm of hBMSCs, suggesting that NEAT1 is involved in post-transcriptional regulation. We predicted miRs with specific loci to NEAT1 via the Starbase website and then found that miR-339-5p was a target of NEAT1. Later, dual luciferase reporter assay confirmed the interaction between NEAT1 and miR-339-5p. At the same time, a study has demonstrated that miR-339-5p participants in osteogenic differentiation in the bones of osteoporotic patients.18 In agreement with previous studies, we also found a decrease in miR-339-5p levels during osteogenic differentiation compared to OM group. And in acute suppurative osteomyelitis of children, a negative correlation was disclosed between NEAT1 and miR‑339‑5p expression in this study. Here, we confirmed that miR-339-5p levels show a downward trend during osteogenic differentiation in hBMSCs, consistent with the previous studies that have shown a negative correlation between miR-339-5p and osteogenic differentiation.
Later, SPI1 was identified as a putative target gene for miR-339-5p. In addition, it was worth noting that SPI1 can function as a transcription factor according to previous literature.27,28 For example, it has been reported that SPI1 can act as a transcriptional activator of SNHG6, facilitating non-small cell lung cancer cellular processes through its association with SNHG6.29 Here we predict that SPI1 can bind to the NEAT1 promoter. We then experimentally verified the binding capacity between them and found that SPI1 promotes the transcription of NEAT1. Moreover, SPI1 was able to activate the transcription of NEAT1 by binding to its promoter. These data suggested that NEAT1/miR-339-5p/SPI1 may form a positive feedback loop and suppress osteogenic differentiation in hBMSCs.
There were also some limitations in our study, where we only discussed the effects of NEAT1 in vitro. Therefore, in vivo assays should be performed in our future studies and the function of NEAT1 in osteogenesis should be directly validated. Despite the shortcomings, our study shed light on the novel mechanism of the NEAT1 for osteogenic differentiation in hBMSCs. Taken together, our research has identified a novel competing endogenous RNA pathway (NEAT1-miR-339-5p-SPI1) in hBMSCs. Our findings may contribute to the understanding of the pathogenesis in children with acute suppurative osteomyelitis and hopefully provide novel diagnostic and therapeutic targets for the treatment of osteomyelitis.
Funding
No funding was received.
Disclosure
Dongsheng Zhu and Zhitao Zhu are co-first authors for this study. The authors declare that they have no competing interests in this work.
References
1. Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–379. doi:10.1016/S0140-6736(04)16727-5
2. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203–218. doi:10.1038/s41579-018-0147-4
3. Wang Y, Liu X, Dou C, et al. Staphylococcal protein A promotes osteoclastogenesis through MAPK signaling during bone infection. Journal of Cellular Physiology. 2017;232(9):2396–2406. doi:10.1002/jcp.25774
4. Liu H, Zhong L, Yuan T, et al. MicroRNA-155 inhibits the osteogenic differentiation of mesenchymal stem cells induced by BMP9 via downregulation of BMP signaling pathway. Int J Mol Med. 2018;41(6):3379–3393. doi:10.3892/ijmm.2018.3526
5. Mahmoudian-Sani MR, Forouzanfar F, Asgharzade S. Overexpression of MiR-183/96/182 Triggers Retina-Like Fate in Human Bone Marrow-Derived Mesenchymal Stem Cells (hBMSCs) in Culture. Journal of Ophthalmology. 2019;2019:2454362. doi:10.1155/2019/2454362
6. Murugan Girija D, Kalachaveedu M, Subbarayan R. Osteogenic differentiation of human gingival mesenchymal stem cells by Aristolochia bracteolata supplementation through enhanced Runx2 expression. J Cell Physiol. 2017;232(7):1591–1595. doi:10.1002/jcp.25835
7. Cristofaro F, Pani G, Pascucci B, Mariani A, Balsamo M. The NATO project: nanoparticle-based countermeasures for microgravity-induced osteoporosis. Scientific Reports. 2019;9(1):17141. doi:10.1038/s41598-019-53481-y
8. Wen H, Chen Z, Cui Y, Xu Y. LncRNA NONHSAT009968 inhibits the osteogenic differentiation of hBMMSCs in SA-induced inflammation via Wnt3a. Biochem Biophys Res Commun. 2021;577:24–31. doi:10.1016/j.bbrc.2021.08.086
9. Ju C, Liu R, Zhang YW, et al. Mesenchymal stem cell-associated lncRNA in osteogenic differentiation. Biomed Pharmacother. 2019;115:108912. doi:10.1016/j.biopha.2019.108912
10. Prinz F, Kapeller A, Pichler M, Klec C. The Implications of the Long Non-Coding RNA NEAT1 in Non-Cancerous Diseases. International Journal of Molecular Sciences. 2019;20(3). doi:10.3390/ijms20030627
11. Wang Z, Li K, Huang W. Long non-coding RNA NEAT1-centric gene regulation. Cell Mol Life Sci. 2020;77(19):3769–3779. doi:10.1007/s00018-020-03503-0
12. Zhu Z, Zhang X, Jiang Y, et al. NEAT1 functions as a key mediator of BMP2 to promote osteogenic differentiation of renal interstitial fibroblasts. Epigenomics. 2021;13(15):1171–1186. doi:10.2217/epi-2021-0212
13. Zhang Y, Chen B, Li D, Zhou X, Chen Z. LncRNA NEAT1/miR-29b-3p/BMP1 axis promotes osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Pathol Res Pract. 2019;215(3):525–531. doi:10.1016/j.prp.2018.12.034
14. Zhou J, Nie H, Liu P, Wang Z, Yao B, Yang L. Down-regulation of miR-339 promotes differentiation of BMSCs and alleviates osteoporosis by targeting DLX5. Eur Rev Med Pharmacol Sci. 2019;23(1):29–36. doi:10.26355/eurrev_201901_16744
15. Wang X, Pei Z, Hao T, et al. Prognostic analysis and validation of diagnostic marker genes in patients with osteoporosis. Front Immunol. 2022;13:987937. doi:10.3389/fimmu.2022.987937
16. Zhu D, Xu X, Zhang M, Wang T. Enhanced expression of KIF4A in osteosarcoma predicts a poor prognosis and facilitates tumor growth by activation of the MAPK pathway. Exp Ther Med. 2021;22(5):1339. doi:10.3892/etm.2021.10774
17. Shi CY, Kingston ER. The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science. 2020;370(6523):eabc9359.
18. Li M, Li C, Zheng H, et al. CircRNA_0001795 sponges miRNA-339-5p to regulate yes-associated protein 1 expression and attenuate osteoporosis progression. Bioengineered. 2022;13(2):2803–2815. doi:10.1080/21655979.2021.2022074
19. Wang X, Zheng R, Huang X, et al. Effects of alkaloids from Sophora flavescens on osteoblasts infected with Staphylococcus aureus and osteoclasts. Phytotherapy Research: PTR. 2018;32(7):1354–1363. doi:10.1002/ptr.6069
20. Zeng X, Wang Y, Dong Q, Ma MX, Liu XD. DLX2 activates Wnt1 transcription and mediates Wnt/β-catenin signal to promote osteogenic differentiation of hBMSCs. Gene. 2020;744:144564. doi:10.1016/j.gene.2020.144564
21. Cheng H, Malhotra A. Evaluation of Potential of Long Noncoding RNA NEAT1 in Colorectal Cancer. J Environ Pathol Toxicol Oncol. 2020;39(2):101–111. doi:10.1615/JEnvironPatholToxicolOncol.2020032508
22. Marzano F, Caratozzolo MF, Consiglio A, et al. Plant miRNAs Reduce Cancer Cell Proliferation by Targeting MALAT1 and NEAT1: a Beneficial Cross-Kingdom Interaction. Front Genet. 2020;11:552490. doi:10.3389/fgene.2020.552490
23. Ji S, Wang S, Zhao X, Lv L. Long noncoding RNA NEAT1 regulates the development of osteosarcoma through sponging miR-34a-5p to mediate HOXA13 expression as a competitive endogenous RNA. Molecular Genetics & Genomic Medicine. 2019;7(6):e673. doi:10.1002/mgg3.673
24. Zhang Y, Chen XF, Li J, He F, Li X, Guo Y. lncRNA Neat1 Stimulates Osteoclastogenesis Via Sponging miR-7. J Bone Miner Res. 2020;35(9):1772–1781. doi:10.1002/jbmr.4039
25. Zhou Y, Zhang F, Xu F, et al. lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis. Open Life Sci. 2021;16(1):969–980. doi:10.1515/biol-2021-0097
26. Mo C, Huang B, Zhuang J, Jiang S, Guo S, Mao X. LncRNA nuclear-enriched abundant transcript 1 shuttled by prostate cancer cells-secreted exosomes initiates osteoblastic phenotypes in the bone metastatic microenvironment via miR-205-5p/runt-related transcription factor 2/splicing factor proline- and glutamine-rich/polypyrimidine tract-binding protein 2 axis. Clinical and Translational Medicine. 2021;11(8):e493. doi:10.1002/ctm2.493
27. Du B, Gao W, Qin Y, Zhong J, Zhang Z. Study on the role of transcription factor SPI1 in the development of glioma. Chinese Neurosurgical Journal. 2022;8(1):7. doi:10.1186/s41016-022-00276-2
28. He Y, Lin L, Ou Y, Hu X, Xu C, Wang C. Endothelial cell-specific molecule 1 (ESM1) promoted by transcription factor SPI1 acts as an oncogene to modulate the malignant phenotype of endometrial cancer. Open Med. 2022;17(1):1376–1389. doi:10.1515/med-2022-0529
29. Gao N, Ye B. SPI1-induced upregulation of lncRNA SNHG6 promotes non-small cell lung cancer via miR-485-3p/VPS45 axis. Biomed Pharmacother. 2020;129:110239. doi:10.1016/j.biopha.2020.110239
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.