Back to Journals » OncoTargets and Therapy » Volume 14
Near Complete Pathologic Response to PD-1 Inhibitor and Radiotherapy in a Patient with Locally Advanced Pancreatic Ductal Adenocarcinoma
Authors McCarthy PM , Rendo MJ, Uy MD, Adams AM, O'Shea AE, Nelson DW, Fenderson JL, Cebe KM, Krell RW, Clifton GT, Peoples GE , Vreeland TJ
Received 18 March 2021
Accepted for publication 5 May 2021
Published 1 June 2021 Volume 2021:14 Pages 3537—3544
DOI https://doi.org/10.2147/OTT.S311661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Patrick M McCarthy,1 Matthew J Rendo,2 Matthew D Uy,3 Alexandra M Adams,1 Anne E O’Shea,1 Daniel William Nelson,4 Joshua L Fenderson,2 Katherine M Cebe,3 Robert W Krell,1 Guy T Clifton,1 George E Peoples,5 Timothy J Vreeland1
1Department of Surgery, Brooke Army Medical Center, San Antonio, TX, USA; 2Department of Hematology and Oncology, Brooke Army Medical Center, San Antonio, TX, USA; 3Department of Pathology, Brooke Army Medical Center, San Antonio, TX, USA; 4Department of Surgery, William Beaumont Army Medical Center, El Paso, TX, USA; 5Cancer Vaccine Development Program, San Antonio, TX, USA
Correspondence: Patrick M McCarthy
Department of Surgery, Brooke Army Medical Center, 3551 Roger Brooke Dr., San Antonio, TX, 78234, USA
Tel +1 240 285-0930
Fax +1 210 916-6658
Email [email protected]
Abstract: Pancreatic ductal adenocarcinoma (PDAC) remains deadly despite advances in systemic therapies and surgical techniques. While there is increasing utilization of immune therapies across diverse cancer types, PDAC remains generally resistant to these treatments. We report a case of locally advanced PDAC treated with preoperative radiation and anti-PD-1 immunotherapy guided by preoperative PD-L1 tumor analysis. After 4 months of preoperative therapy, the patient was submitted to resection, demonstrating a near-complete pathologic response on final tumor analysis. We will discuss the relevant literature and current state of immunotherapeutics for PDAC.
Keywords: pancreatic cancer, pembrolizumab, complete response, PD-L1, locally advanced, immune checkpoint inhibitor
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is difficult to treat; the 5 year-survival rate across all stages is 7.9% in the United States.1 Approximately 20% of patients are candidates for surgical intervention at the time of diagnosis.2,3 As a result, there has been increasing interest in expanding the armamentarium of neoadjuvant therapies aimed at decreasing tumor burden and selecting patients with favorable tumor biology prior to curative-intent surgery, particularly for locally advanced4–7 and borderline resectable8–12 PDAC. In contrast to breast, rectal, and esophageal cancers, complete pathologic responses to neoadjuvant chemotherapy occur in only 5–7.5% of cases.13,14
Cancer patients have benefited from the development and expansion of personalized medicine and immunotherapy, particularly with the use of immune checkpoint inhibitors (ICI). Drugs targeting CTLA-4, PD-1, and PD-L1 have been far more effective in the management of melanoma15–17 and lung cancer18–22 than cytotoxic chemotherapy alone. The success of ICI in these settings has led to expanded use of these immunotherapies across diverse tumor types. Recently, pembrolizumab received FDA approval for treating metastatic cancers with either high microsatellite instability (MSI-H)23 or high tumor mutational burden-high (TMB-H), regardless of histology.24,25 These and other trials targeting tumor biology or gene expression over specific histologic origin highlight a major paradigm shift in cancer treatment.
Despite this enthusiasm for immune therapy, outcomes with ICI in PDAC patients have been less impressive. In the KEYNOTE-158 trial, even amongst PDAC tumors that were MSI-H/dMMR, overall response rate to pembrolizumab monotherapy was 18.2%; the lowest of all GI tract malignancies.24 While the results of immunotherapy in PDAC have by and large been unimpressive, it is possible that it may still have a role in correctly selected patients. Here, we present the case of a patient with locally advanced PDAC and near pathologic complete response (pCR) after administration of combination pembrolizumab and radiation therapy (RT) and a review of the available literature on the use of personalized medicine and immunotherapy in PDAC.
Materials and Methods
Consents and Permissions
All information in this project was obtained in accordance with the Declaration of Helsinki, HIPAA, and our institutional guidelines. The patient described in this case has given informed consent and consent to publish the clinical details contained within this report. Institutional approval was not required to publish the details of the case included in this report.
Results
Clinical Presentation
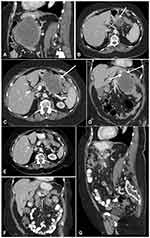
The patient is an 83-year-old female who presented with progressive flank discomfort over two months with multiple comorbidities and an Eastern Cooperative Oncology Group (ECOG) performance status of 3. Physical examination was non-focal and lab workup was notable for an elevated lipase, mild leukocytosis, and normocytic anemia. An abdominal CT revealed a 10.6×8.0×11.7cm mass arising from the pancreatic body, with partial encasement of the splenic artery (Figure 1A). The mass abutted the stomach and splenic flexure of the colon, with evidence of invasion of both organs (Figure 1B–D) without evidence of metastatic disease. Serum cancer antigen 19–9 (CA 19–9) was 3366 U/mL (reference value <36 U/mL) and carcinoembryonic antigen (CEA) was 67.5 ng/mL (reference value <3.5 ng/mL). Endoscopic biopsy of the pancreatic mass revealed adenocarcinoma consistent with pancreatic origin, which was confirmed on colonoscopy. Immunohistochemical analysis revealed a poorly differentiated carcinoma staining positive for CK7, weakly positive for CDX2, and negative for CK20; further supporting upper gastrointestinal origin and a diagnosis of locally advanced pancreas cancer with colonic invasion. Immunohistochemistry analysis for MLH1, PMS2, MSH2, and MSH6 showed intact expression.
Given her locally advanced tumor and the need for multi-visceral resection, the multidisciplinary tumor board recommended neoadjuvant systemic therapy. Due to her multiple comorbidities and poor performance status, however, the patient was not a good candidate for multi-agent cytotoxic chemotherapy. Tumor analysis with FoundationOne CDx (Foundation Medicine, Inc. Cambridge, MA) demonstrated a PD-L1 tumor proportion score of 70%, Microsatellite Instability–High (MSI-H) status, and a tumor mutational burden of 49 mutations per megabase.
Treatment and Outcomes
The patient received stereotactic body radiotherapy (SBRT) (25 Gy/5 five fractions) to the pancreatic mass before commencing pembrolizumab monotherapy at 200mg every three weeks. Interval CT scan after 2 cycles of pembrolizumab showed decreased mass size at 3.6×3.8×3.8cm. Serum CA 19–9 had decreased to 27.7 U/mL. Repeat imaging after a total of 4 cycles showed further tumor volume decrease (3.2×2.7×3.1 cm) and cystic degeneration (Figure 1E–G).
The patient’s favorable response on imaging led to the recommendation for exploration with resection via distal pancreatectomy with splenectomy. In the operating room, the tumor was inseparable from the posterior gastric wall and transverse mesocolon, necessitating en bloc wedge gastrectomy and resection of 6 cm of transverse colon with primary anastomosis. The patient recovered without complications and was discharged on post-operative day five.
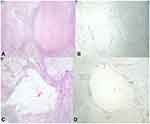
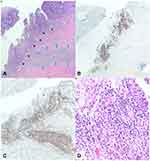
The pancreatic specimen revealed abundant mucin and necrotic tissue surrounded by a prominent histiocytic and lymphocytic infiltrate with patchy neutrophilic inflammation, indicative of treatment effect, with no residual dysplasia or carcinoma in the pancreas (Figure 2). Thirty-nine lymph nodes were negative for carcinoma. The gastric specimen was also negative for carcinoma. The resected colon contained a 4 mm segment of poorly differentiated adenocarcinoma within the lamina propria surrounded by a prominent histiocytic and lymphocytic infiltrate (Figure 3). The margins and all six colonic lymph nodes were negative for carcinoma. Post-operative CA 19–9 was 26.2 U/mL.
The patient has continued to receive pembrolizumab post-operatively, with plans for one year of total therapy. At her most recent follow up five months after surgery she has continued to tolerate therapy well without evidence of disease recurrence.
Discussion
Immunotherapy is not commonly utilized in the neoadjuvant setting for locally advanced PDAC. ICI are more commonly utilized in the metastatic setting as second- or third-line therapies in patients with MSI-H or dMMR tumors, or as an alternative monotherapy in patients with poor performance status.26,27 Within pancreatic cancer there is little data directly comparing these modalities to cytotoxic chemotherapy in the metastatic setting, even in patients with MSI-H tumors, and few if any data comparing them in the perioperative setting.
While chemotherapy for PDAC has improved, dramatic responses remain rare13,14 and the ability to predict treatment response has largely remained elusive. A recent analysis by Perri et al demonstrated an association between pathologic response with radiographic metrics and serum tumor markers, but major and complete responses are quite rare.28 Thus, there is rationale for the expanded use of personalized strategies in the form of targeted therapies and immunotherapies, especially in patients who are expected to not tolerate, or not respond to, cytotoxic chemotherapy. To date, ICI have achieved minimal success.29–31 An early trial including PDAC patients with metastatic disease treated with pembrolizumab published a 0% ORR and a 3.9-month median overall survival (mOS),29 and inclusion of anti-CTLA-4 therapies has produced equally disappointing results. Combinations of ICI and chemotherapy agents have not shown a clear benefit, but some rare reports of dramatic responses are encouraging, such as a patient with metastatic PDAC in a phase Ib trial of ipilimumab and gemcitabine who achieved a durable response of 19.8 months.32 Though there are three ongoing Phase III trials investigating ICI in PDAC (NCT03983057, NCT03977272, NCT03755739), the failure of early phase trials to show substantial treatment effects underscores the immunosuppressive and immune-excluding nature of the tumor microenvironment (TME) and surrounding stroma of PDAC relative to other solid tumor types. Within the TME, multiple cell lines such as macrophages, dendritic cells, and PDAC epithelial cells are manipulated to induce immunosuppressive effects.33 The tumor stroma, consisting of the extracellular matrix, vasculature, and cancer associated fibroblasts, create a mechanical and functional barrier to an effective anti-tumor immune response.34
Despite these challenges, there may be hope for ICI in PDAC with better patient selection. One major issue with these early trials is that patients were enrolled without a requirement for specific mutation burden, PD-L1 expression, or MSI status. Unfortunately, PD-L1 expression as low as 3.9% in all nucleated cells of resected PDAC specimens has been reported in patients who received no NAT.35 There is, however, prospective clinical evidence in multiple solid tumor types indicating that MSI-H and TMB-H tumors may be sensitive to PD-L1 targeted immunotherapy, regardless of PD-L1 expression.36,37 While these markers are also fairly rare in PDAC,38 there is a small subset of patients who may have dramatic responses to immunotherapy. Early trials investigating ICI in PDAC have enrolled patients who have failed initial, cytotoxic chemotherapy treatments. Unfortunately, the recipient of such therapies may experience damage to the immune system that could result in weaker, if any, immunologic response to ICI, potentially altering the results of these studies. Additional research is needed to better understand the complex interaction between the immune system and the TME of a PDAC tumor but, as demonstrated by our case, dramatic effects from immunotherapy are likely to be seen in cases with remarkably high TMB and expression of PD-L1. This is where future research on immunotherapy in PDAC should focus.
Though significant baseline immunogenicity is rare in PDAC, it is possible that another therapy may be able to generate an initial immune response in an otherwise immunologically cold tumor, allowing ICI to exploit and expand this response. One promising strategy that was utilized in our case is the addition of ICI to RT. It has been postulated that the cell lysis caused by ionizing radiation may cause an immune response to a patient’s tumor, leading to an immune response with corresponding upregulation of PD-L1 expression on tumor cells.39,40 Syngeneic mouse tumor models demonstrated improved survival and tumor volume reduction with the combination of RT and PD-L1 blockade compared to single modality, and elevations in tumor cell PD-L1 expression were seen following RT.39,41 Additionally, it has been suggested that RT may have effects on the dense, immunosuppressive tumor microenvironment and stroma of PDAC in a way that increases immune cell response and antigen recognition.42 This promising combination is currently being studied in a number of malignancies,43 but has not been studied extensively in pancreatic malignancies. SBRT followed by durvalumab for pancreatic cancer is currently being studied in the metastatic setting in a Phase I trial; however, early data indicate poor therapy responses, with stable disease marking the best response in 21% of patients.44 There are no studies to date assessing frontline combination radiation and immunotherapy for locally advanced or borderline resectable tumors as successfully implemented in this case; however, there are several promising animal studies and enrolling clinical trials.42 While it is possible that this combination may offer meaningful response in PDAC, it is again likely that patients with some baseline immune response to their tumor will see the most benefit.
Finally, the discovery of frequent somatic mutations within pancreatic cancers has also led to a broadening array of directed therapies. In addition to PD-1/PD-L1 inhibitors, recent trials have begun to enroll patients for treatment with RAS inhibitors, specifically a small-molecule KRASG12C inhibitor, AMG 510.45 At the most recent ASCO scientific assembly, researchers presented a cohort of patients diagnosed with KRASG12C-mutated tumors, including eight with pancreatic cancer. Of these patients, six had achieved stable disease and three had a 30% reduction in tumor burden.46 Given the frequency of KRAS mutations in pancreatic cancer, the promise of RAS inhibitors provides hope for patients with this malignancy. In addition, there is hope that combining ICI with small molecule inhibitors, whether targeting RAS or other common mutations, will lead to synergistic anti-cancer effects.47 While these therapies are in their infancy, especially with regards to use in pancreatic cancer, they demonstrate where the field of oncology is going: towards personalized medicine, with each patient’s treatment being chosen based on their specific tumor’s genetic signature.
Conclusion
The future of oncologic care is personalized medicine, and here we present remarkable success utilizing neoadjuvant pembrolizumab for a PD-L1 high, TMB-H tumor, with radiation that potentially enhanced PD-L1 blockade. This case report adds to the growing body of evidence that RT may alter the immunogenicity of the PDAC TME and allow for the synergistic use of ICI. Furthermore, this case highlights the impressive efficacy of this combination that may be possible in correctly selected patients. This calls for further study on application of ICI in PDAC, particularly in appropriately selected patients. Routine testing for targets of immunotherapies should be strongly considered for frail patients with unresectable or metastatic PDAC.
Disclosure
The authors declare no potential conflicts of interest.
References
1. Surveillance Research Program NCI. SEER*Explorer: an interactive website for SEER cancer statistics; 2020. Available from: https://seer.cancer.gov/explorer/.
2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.can-14-0155
3. Müller PC, Frey MC, Ruzza CM, et al. Neoadjuvant chemotherapy in pancreatic cancer: an appraisal of the current high-level evidence. Pharmacology. 2020;1–11. doi:10.1159/000510343.
4. Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270(2):340–347. doi:10.1097/sla.0000000000002753
5. Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer. Ann Surg. 2016;264(3):457–463. doi:10.1097/sla.0000000000001850
6. Kunzmann V, Algül H, Goekkurt E, et al. Conversion rate in locally advanced pancreatic cancer (LAPC) after nab-paclitaxel/gemcitabine- or FOLFIRINOX-based induction chemotherapy (NEOLAP): final results of a multicenter randomised Phase II AIO trial. Ann Oncol. 2019;30(Supplement_5):v253. doi:10.1093/annonc/mdz247
7. Michelakos T, Pergolini I, Castillo CF-D, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269(4):733–740. doi:10.1097/sla.0000000000002600
8. Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma. JAMA Oncol. 2018;4(7):963. doi:10.1001/jamaoncol.2018.0329
9. Jang J-Y, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer. Ann Surg. 2018;268(2):215–222. doi:10.1097/sla.0000000000002705
10. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763–1773. doi:10.1200/JCO.19.02274
11. Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49(2):190–194. doi:10.1093/jjco/hyy190
12. Ghaneh P, Palmer DH, Cicconi S, et al. ESPAC-5F: four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J Clin Oncol. 2020;38(15_suppl):4505. doi:10.1200/JCO.2020.38.15_suppl.4505
13. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi:10.1371/journal.pmed.1000267
14. Mellon EA, Jin WH, Frakes JM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncologica. 2017;56(3):391–397. doi:10.1080/0284186x.2016.1256497
15. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:10.1056/NEJMoa1504030
16. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label Phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–1862. doi:10.1016/S0140-6736(17)31601-X
17. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. doi:10.1016/S1470-2045(19)30388-2
18. Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter Phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi:10.1093/annonc/mds213
19. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. doi:10.1200/jco.2016.67.6601
20. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/nejmoa1809064
21. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
22. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi:10.1200/jco.20.00793
23. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–3758. doi:10.1158/1078-0432.ccr-18-4070
24. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/jco.19.02105
25. FDA. FDA approves pembrolizumab for adults and children with TMB-H solid tumors; 2020. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors.
26. Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38(27):3217–3230. doi:10.1200/jco.20.01364
27. Network NCC. Pancreatic adenocarcinoma (version 1.2020), 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf.
28. Perri G, Prakash L, Wang H, et al. Radiographic and serologic predictors of pathologic major response to preoperative therapy for pancreatic cancer. Ann Surg. 2021;273(4):806–813. doi:10.1097/SLA.0000000000003442
29. Ott PA, Bang Y-J, Piha-Paul SA, et al. T-cell–inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi:10.1200/jco.2018.78.2276
30. Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi:10.1097/cji.0b013e3181eec14c
31. O’Reilly EM, Oh D-Y, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma. JAMA Oncol. 2019;5(10):1431. doi:10.1001/jamaoncol.2019.1588
32. Kamath SD, Kalyan A, Kircher S, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist. 2020;25(5):2. doi:10.1634/theoncologist.2019-0473
33. Fan JQ, Wang MF, Chen HL, Shang D, Das JK, Song J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol Cancer. 2020;19(1):32. doi:10.1186/s12943-020-01151-3
34. Henke E, Nandigama R, Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2020;6. doi:10.3389/fmolb.2019.00160.
35. Stromnes IM, Hulbert A, Pierce RH, Greenberg PD, Hingorani SR. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2017;5(11):978–991. doi:10.1158/2326-6066.cir-16-0322
36. Lal N, Beggs AD, Willcox BE, Middleton GW. An immunogenomic stratification of colorectal cancer: implications for development of targeted immunotherapy. OncoImmunology. 2015;4(3):e976052. doi:10.4161/2162402x.2014.976052
37. Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–2970. doi:10.1158/1055-9965.epi-14-0654
38. Eso Y, Seno H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Therap Adv Gastroenterol. 2020;13:175628482094877. doi:10.1177/1756284820948773
39. Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6(1). doi:10.1186/s40425-018-0361-7
40. Wang Y, Kim TH, Fouladdel S, et al. PD-L1 expression in circulating tumor cells increases during radio(chemo)therapy and indicates poor prognosis in non-small cell lung cancer. Sci Rep. 2019;9(1). doi:10.1038/s41598-018-36096-7
41. Azad A, Yin Lim S, D’Costa Z, et al. PD‐L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med. 2017;9(2):167–180. doi:10.15252/emmm.201606674
42. Cellini F, Arcelli A, Simoni N, et al. Basics and frontiers on pancreatic cancer for radiation oncology: target delineation, SBRT, SIB technique, MRgRT, particle therapy, immunotherapy and clinical guidelines. Cancers. 2020;12(7):1729. doi:10.3390/cancers12071729
43. Talimogene laherparepvec and radiation therapy in treating patients with newly diagnosed soft tissue sarcoma that can be removed by surgery; 2021. Available from: https://ClinicalTrials.gov/show/NCT02923778.
44. Duffy AG, Makarova-Rusher OV, Kleiner DE, et al. A pilot study of immune checkpoint inhibition in combination with radiation therapy in patients with metastatic pancreatic cancer. J Clin Oncol. 2017;35(15_suppl):e15786. doi:10.1200/JCO.2017.35.15_suppl.e15786
45. Bar-Sagi D, Knelson EH, Sequist LV. A bright future for KRAS inhibitors. Nat Cancer. 2020;1(1):25–27. doi:10.1038/s43018-019-0016-8
46. Fakih M, Desai J, Kuboki Y, et al. CodeBreak 100: activity of AMG 510, a novel small molecule inhibitor of KRASG12C, in patients with advanced colorectal cancer. J Clin Oncol. 2020;38(15_suppl):4018. doi:10.1200/JCO.2020.38.15_suppl.4018
47. Ward AB, Keeton AB, Chen X, et al. Enhancing anticancer activity of checkpoint immunotherapy by targeting RAS. MedComm. 2020;1(2):121–128. doi:10.1002/mco2.10
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.