Back to Journals » Journal of Inflammation Research » Volume 17
NcRNA Regulated Pyroptosis in Liver Diseases and Traditional Chinese Medicine Intervention: A Narrative Review
Authors Deng J , Qin L , Qin S, Wu R, Huang G , Fang Y, Huang L, Zhou Z
Received 24 November 2023
Accepted for publication 19 March 2024
Published 3 April 2024 Volume 2024:17 Pages 2073—2088
DOI https://doi.org/10.2147/JIR.S448723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Jiasheng Deng,1,* Le Qin,2,* Sulang Qin,3,* Ruisheng Wu,1 Guidong Huang,1 Yibin Fang,4 Lanlan Huang,4 Zhipin Zhou4
1School of Pharmacy, Guangxi University of Chinese Medicine, Nanning, Guangxi, 530200, People’s Republic of China; 2Department of Pharmacy, Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi, 533000, People’s Republic of China; 3School of Graduate Studies, Youjiang Medical University for Nationalities, Baise, Guangxi, 533000, People’s Republic of China; 4Department of Pharmacy, Liuzhou People’s Hospital, Liuzhou, Guangxi, 545006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhipin Zhou, Department of Pharmacy, Liuzhou People’s Hospital, 8 Wenchang Road, Cheng Zhong District, Liuzhou, Guangxi, People’s Republic of China, Tel +86 − 18776921947, Email [email protected]
Abstract: Pyroptosis is a novel pro-inflammatory mode of programmed cell death that differs from ferroptosis, necrosis, and apoptosis in terms of its onset and regulatory mechanisms. Pyroptosis is dependent on cysteine aspartate protein hydrolase (caspase)-mediated activation of GSDMD, NLRP3, and the release of pro-inflammatory cytokines, interleukin-1 (IL-1β), and interleukin-18 (IL-18), ultimately leading to cell death. Non-coding RNA (ncRNA) is a type of RNA that does not encode proteins in gene transcription but plays an important regulatory role in other post-transcriptional links. NcRNA mediates pyroptosis by regulating various related pyroptosis factors, which we termed the pyroptosis signaling pathway. Previous researches have manifested that pyroptosis is closely related to the development of liver diseases, and is essential for liver injury, alcoholic fatty liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), liver fibrosis, and liver cancer. In this review, we attempt to address the role of the ncRNA-mediated pyroptosis pathway in the above liver diseases and their pathogenesis in recent years, and briefly outline that TCM (Traditional Chinese Medicine) intervene in liver diseases by modulating ncRNA-mediated pyroptosis, which will provide a strategy to find new therapeutic targets for the prevention and treatment of liver diseases in the future.
Keywords: non-coding RNA, pyroptosis, liver diseases, inflammasomes, gasdermin, traditional Chinese medicine
Introduction
Data statistics indicate that approximately one in five people in China is affected by some form of liver disease, including viral hepatitis, non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), liver injury, liver fibrosis, liver cancer, etc.1,2 Liver disease has gradually become a serious cause of death in China.3 During the development of these diseases, a variety of infectious or non-infectious activators can stimulate liver cells to produce the corresponding immune response, which can lead to inflammatory changes in liver cells, thus causing liver inflammation.4 In 2005, Fink et al first discovered and defined the phenomenon of cell pyroptosis, also known as cell inflammatory necrosis.5 Acute/chronic hepatic inflammation is associated with pyroptosis in liver injury, NAFLD, ALD, liver fibrosis, and hepatocellular carcinoma. Therefore, pyroptosis may play a pivotal role in the development of liver disease.6 Pyroptosis is a novel pro-inflammatory programmed cell death pathway that depends on the activation of Caspases, including Caspase-1, Caspase-4, Caspase-5 and Caspase-11. It then mediates the hydrolysis of gasdermin D (GSDMD) into GSDMD-N, which is embedded in the plasma membrane and forms soluble membrane pores, leading to ion decompensation, water influx, cell swelling and rupture, release of cell contents, and a large number of inflammatory mediators,7,8 thereby initiating the inflammatory cascade.9,10 Therefore, pyroptosis acts as a key adjustment factor in the immune defense mechanism, and pyroptosis mediated by Caspase-1 activation is an important mode of cell death in the clearance of pathogens and inhibition of pathogen infection.11 Notably, hepatocytes are resistant to pyroptosis primarily with low expression levels of caspase-1 or −11, which are insufficient to cleave sufficient GSDMD to cause cell lysis through membrane pore formation. It is speculated that there are still sufficient activity in hepatocytes such as release of inflammasomes or HMGB1.12 Inflammation caused by pyroptosis is the common basis for the development of liver diseases and is essentially accompanied by the development of malignant liver diseases, culminating in a full-cycle liver disease course.13
Non-coding RNA(ncRNAs) are a class of RNA without protein-coding functions, including microRNAs (miRNAs), long non-coding RNA (lncRNAs), and circular RNA (circRNA).14 Although ncRNAs lack the potential to encode proteins, they can affect the expression of many molecular targets to drive specific cellular biological reactions and destinies.15 Studies have indicated that ncRNAs regulate gene expression at multiple levels, including replication, transcription, and post-transcription,16 and are involved in chromatin modification, cell differentiation, protein function regulation, and disease development.17 An increasing number of studies have found that ncRNAs can mediate the transcriptional or post-transcriptional regulation of pyroptosis-related genes by participating in the regulatory network of pyroptosis, thereby affecting the pathogenesis of certain diseases such as diabetes and diabetic nephropathy,18 cardiovascular disease,19 atherosclerosis,20 and cancer.21 Consequently, pyroptosis regulated by ncRNAs is crucial for the occurrence of liver inflammation, and ncRNAs have become a key factor in liver diseases caused by pyroptosis. They are strongly associated with the occurrence and development of liver diseases, and can be used as biomarkers and potential targets for the diagnosis and treatment of liver diseases. In this study, we reviewed the effects of the ncRNA-mediated pyroptosis signaling pathway on liver diseases and the mechanism by which TCM regulates liver diseases through the ncRNA intervention pyroptosis signaling pathway. It aims to provide a new direction for the treatment of liver diseases.
Signaling Pathways of Pyroptosis
Pyroptosis is a novel pro-inflammatory programmed cell death, and it is accompanied by caspase activation, gasdermin cleavage, and release of the pro-inflammatory cytokines IL-1β and IL-18. Initially, it was suggested that pyroptosis is related to bacterial infection of immune cells. The morphological features and functions of pyroptosis were first observed in macrophages infected with the Gram-negative bacterium Shigella fowleri, but this was mistaken for apoptosis.22 Comparable phenotypes were observed in Salmonella-infected macrophages, but it was found that caspase-1 was not involved in apoptosis,23–25 this also signals the emergence of a new form of cell death. To date, pyroptosis is usually divided into two pathways based on the caspase-1-dependent pathway: canonical and non-canonical pyroptosis pathways. Moreover, Caspase-3 and Caspase-8, as apoptosis-related caspase proteins, can also trigger pyroptosis, which we refer to as other pyroptosis pathways. We summarize the mechanisms of these three pyroptosis pathways in Figure 1 according to the literatures.
Canonical Pyroptosis Pathway
The canonical pyroptosis pathways is defined as pyroptosis mediated by Caspase-1-dependent. The activation of this pathway is mainly triggered by pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). After receiving danger signal molecules through the pattern recognition receptor (PRR), apoptosis-associated spot protein (ASC) is recruited, and pro-caspase-1 is assembled to form an inflammasome.26 These inflammasomes stimulate the activation of caspase-1, finally leads to pyroptosis. PRRs (also known as inflammasome sensors) are widely expressed in immune cells and are classified into four categories: Toll-like receptors (TLRs), C-type lectin receptors (CLRs), AIM2-like receptors (ALRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs), which are widely expressed in immune cells.27 Among them, TLRs and NLRs both play direct or indirect roles in pyroptosis. Nuclear factor kappa-B (NF-κB) is an essential signaling molecule in the pyroptosis pathway. During the activation of inflammatory factors, endogenous cytokines or microbial components in the cell membrane stimulate other factors, such as TLRs or tumor necrosis factor (TNF), on the cell surface to activate NF-κB.28 Further, NLRP3 protein synthesis and pro-IL-1βand pro-IL-18 expression were promoted. Currently, it has been found that inflammasome associated with pyroptosis include NLRP1, NLRP3, NLRC4, and AIM2 inflammasome,29 of which the most classical and intensively investigated is the pyroptosis mediated by the NLRP3 inflammasome, such as depressive symptoms,30 sepsis-associated encephalopathy31 and myocardial injury.32 NLRP3 protein binds to ASC through the PYD-PYD interactions, and ASC connects NLRP3 to Pro-caspase1 via the caspase activation and recruitment domains (CARD), and the three together form the NLRP3 inflammasome.33 Next, the NLRP3 inflammasome activates caspase-1 to cleave GSDMD proteins into GSDMD-N and GSDMD-C, from which GSDMD-N forms membrane pores and releases contents, such as interleukin-1 alpha (IL-1α) and HMGB1, inducing pyroptosis. Simultaneously, activated caspase-1 cleaves pro-IL-1β and pro-IL-18 to form active IL-1β and IL-18, which are released into the extracellular space, inducing pyroptosis and causing inflammation.34,35
Non-Canonical Pyroptosis Pathway
Unlike canonical pyroptosis, which relies on caspase-1, the non-canonical pyroptosis process refers to pyroptosis that is dependent on the activation of caspase-4/5 or caspase-11. Amusingly, caspase4/5 can only mediate pyroptosis in human cells, whereas caspase-11 can only mediate pyroptosis in mice, indicating that there are species differences in non-canonical pyroptosis pathways.36 In contrast to canonical pyroptosis, caspase-4/5/11 can directly bind bacterial lipopolysaccharide (LPS) through its CARD domain to promote oligomerization and activation. Activated caspase-4/5/11 cleave GSDMD to produce GSDMD-N, leading to pore formation in the cell membrane. It induces the release of cellular contents and a large number of pro-inflammatory factors, IL-18, and IL-1β, thereby inducing pyroptosis.37,38 With the further research, it was found that activated caspase-4/5/11 could not only directly induce pyroptosis, but also indirectly causes K+ efflux / pannexin-1 cleavage /ATP release or P2X7 release through the pore previously formed by GSDMD-N, which in turn mediates NLRP3 inflammasome activation.39,40 Whether it’s the canonical or the non-canonical pyroptosis pathways, it can be seen that GSDMD proteins play crucial roles in both of the above.
Other Pyroptosis Pathways
In addition, evidence demonstrated that gasdermin family protein E (GSDME) also mediates pyroptosis,41,42 chemotherapy drugs activate caspase-3 in tumor cells, then caspase-3 cleave GSDME to form GSDME-N active fragment. Since GSDME-N is similar to GSDMD-N, both can perforate the cell membrane, leading to cell swelling and rupture, ultimately promoting tumor cell pyroptosis.43 Meanwhile, NLRP3 inflammasome can also be activated by caspase-8, suggesting that caspase-8 may induce pyroptosis.44 Further exploration revealed that extrinsic and intrinsic apoptosis both activate pannexin-1 to drive NLRP3 inflammasome assembly, and caspase-1 and caspase-8 cleave GSDMD to trigger pyroptosis.45 Among several caspase families, caspase-1 appears to be the strongest driver of GSDMD cleavage and caspase-8 is the weakest, it is probable that caspase-8 act as a fallback measure when other members of the caspase family are damaged.46 Excitingly, researchers have found that PD-L1 transforms TNFα-induced apoptosis into pyroptosis in cancer cells, leading to tumor necrosis. Under hypoxia, p-Stat3 physically interacts with PD-L1 and promotes its nuclear translocation, thereby enhancing the transcription of the GSDMC gene. GSDMC is specifically cleaved by caspase-8, generating the GSDMC N-terminal structural domain, which forms a pore in the cell membrane and induces pyroptosis.47 Of interest, Orning et al found that the activity of TGF-β activated kinase-1 (TAK1) and IκB kinase (IKK) was blocked in the immune response to Yersinia infection, which activated the caspase-8 pathway, leading to cleavage and activation of the GSDMD at the same location, ultimately causing pyroptosis.48 Hence, these results indicate that the Caspase-8/GSDMD/GSDMC and Caspase-3/GSDME pathways may be other mechanisms causing pyroptosis. These results provide new directions for the study of pyroptosis. Importantly, clarifying the changes in these mechanisms may be a key point for understanding the different pyroptosis pathways.
Nc-RNA Mediated Pyroptosis Pathway in Liver Injury
Liver injury was defined as the onset of all chronic liver diseases. There are many causes of liver injury, including viral infections, drug abuse, long-term alcoholism, and liver surgery. Serious liver injury may develop into liver fibrosis, cirrhosis, or even liver cancer if no intervention is made.49 Pyroptosis-related proteins and pro-inflammatory factors are secreted in the process of pyroptosis, which can induce an inflammatory response and aggravate liver injury. An in-depth study of the mechanism revealed that ncRNAs affect hepatic inflammation through the regulation of the pyroptosis signaling pathway, thereby affecting the occurrence and development of liver injury.
Several miRNAs can cause pyroptosis in the liver by regulating the abnormal expression of pyroptotic factors such as NLRP3, GSDMD, and caspase-1, which trigger an inflammatory response and worsen the liver. The expression level of miR-494 in hepatocytes gradually increased with alleviation of liver injury. In vivo experiments have shown that upregulation of miR-494 expression in hepatocytes can activate the PI3K/AKT/Nrf2 pathway to inhibit the expression of the NLRP3 inflammasome, reduce pyroptosis and inflammation, and alleviate liver injury.50 MiR-4057 is up-regulated in acute liver injury induced by LPS and galactoside (D-GalN), and miR-4057 targets to inhibit the activation of the NLRP3 inflammasome and the release of pro-inflammatory factors IL-1β and IL-18.51 Similarly, compared to normal mice, the expression of XBP1, NLRP3, caspase-1, and other pyroptotic proteins was increased in mice with liver injury, indicating that pyroptosis aggravated liver injury in mice. Further exploration determined that raising miR-182 expression suppression XBP1 / NLRP3 pathways activated, which in turn alleviated the occurrence of pyroptosis and attenuated liver injury in mice.52 Interestingly, miR-182-5p plays a bidirectional regulatory role in pyroptosis-induced liver injury. FoxO3a, a miR-182-5p target involved in hepatocyte pyroptosis, reduces FoxO3a expression and can increase the expression of the pro-inflammatory factors caspase-1, IL-1, and IL-18. Overexpression of miR-182-5p reduced the expression of FoxO3a; conversely, it increases the expression of FoxO3a, and miR-182-5p interfered with hepatocyte pyroptosis by directly targeting FoxO3a. Therefore, upregulation of miR-182-5p exacerbates liver injury, whereas downregulation of miR-182-5p could mitigate liver injury.53 Another study indicated that upregulation of miR-330-3p reduces cleaved caspase-1 and GSDMD protein expression in mouse hepatic tissues by targeting phosphoglycerate mutase 5(PGAM5)-mediated caspase-1 and GSDMD expression,54 leading to ameliorat the extent of liver injury in mice. Moreover, Yang et al demonstrated that overexpression of miR-30b-5p could target cannabinoid receptor 1 (CB1) to indirectly inhibit NLRP3 mRNA expression, thereby reducing NLRP3 inflammasome activation and pyroptosis.55 Similarly, miR-23a-3p downregulation attenuated NLRP3 inflammasome-induced pyroptosis, mechanistically, miR-23a-3p targeted NIMA associated kinase 7 (NEK7) to inhibit NLRP3 inflammasome activation.56 In the LPS- and ATP-induced hepatocyte injury models, exosomes containing miR-233 were found to significantly reverse liver injury and downregulate the expression of NLRP3 and caspase-1 at both the protein and mRNA levels in hepatocytes, resulting in protection against liver injury.57 According to research, the expression of miR-297 is significantly upregulated in vitro cell model of liver injury. In vitro transfection of miR-297 antagonist targeting SIRT3 inhibited activation of the NLRP3 inflammasome and alleviated hepatic ischemia/reperfusion injury.58
In addition, a few lncRNAs have also been found to affect the development of liver injury by regulating the pyroptosis pathway, which are directly or indirectly involved in the process of pyroptosis by participating in mRNA degradation, competitive binding of mi-RNA, or regulating the translation of mRNA.59 It has been shown that lncRNA-KCNQ1OT1 participates in liver injury pyroptosis signaling through the miR-142a-3p/HMGB1 axis, competitively binds to miR-142a-3p, upregulates the expression of the downstream target gene High Mobility Group Protein B1 (HMGB1), and activates the downstream TLR4/NF-κB pyroptosis pathway. Therefore, the miR-142a-3p/HMGB1 axis is regulated by silencing lncRNA-KCNQ1OT1 to reduce pyroptosis to improve liver injury.60 Furthermore, knockdown of lncRNA-AK139328 inhibited macrophage infiltration and caspase-3 activation in the liver. Silencing of lncRNA-AK139328 improved liver injury by inhibiting NF-κB activity and inflammatory cytokine expression. These results suggest that lncRNA-AK139328 inhibits the production of downstream pyroptotic proteins and pro-inflammatory factors via the NF-κB pathway to repair liver injury.61 Meanwhile, LncRNA-Gm15441 was abnormally upregulated in mice, and lncRNA-Gm15441 overexpression inhibited its antisense transcription encoding thioredoxin interacting protein (TXNIP), which in turn reduced TXNIP-induced NLRP3 inflammasome activation, caspase-1 cleavage, and pro-IL-1β maturation, ultimately reducing the inflammatory response and the level of damage in the liver.62 Therefore, by targeting and regulating lncRNA-Gm15441, new insights into the treatment of metabolic stress-induced liver inflammation and injury can be obtained. The pharmacological mechanisms of ncRNA-mediated pyroptosis signaling against liver injury are summarized in Table 1.
 |
Table 1 NcRNA Regulated Pyroptosis to Attenuate Liver Injury |
Nc-RNA Mediated Pyroptosis Pathway in Liver Fibrosis
Liver fibrosis is a wound-healing response caused by various chronic liver injury factors, including inflammation, viral infection, bacterial infection, and alcohol abuse. It is characterized by necrosis of liver parenchyma cells and abnormal deposition of collagen fibers, which mainly manifests as an imbalance in extracellular matrix (ECM) degradation and production, leading to abnormal proliferation of connective tissue in the liver and subsequent development of liver fibrosis.63 Without timely intervention in the evolutionary process, liver fibrosis may develop into liver failure, cirrhosis, and even liver cancer at the end stage of liver disease. Activated hepatic stellate cells (HSC) are the main source of ECM production, and α-smooth muscle actin (α-SMA) and collagen secreted by HSC are the major components of ECM.64,65 In recent years, increasing evidences have show that ncRNAs not only bind to specific mRNA targets to affect HSC activation but also participate in liver fibrosis by regulating pyroptosis signaling pathways.
Jimenez et al reported that in mice with liver fibrosis, miR-223 3p disrupted the activation of the NLRP3 inflammasome and inhibited pyroptosis by impairing the mature IL-1β and, NLRP3 proteins, and the activation of caspase-1 p10. Engagingly, miR-223 3p can also inhibit the release of IL-1β from liver macrophages, thereby inhibiting the activation of HSCs.66 Furthermore, another exploration suggested that early fibrosis occurred in the livers of knockout miR-223 mice, and the degree of injury correlated with the expression level of NLRP3, whereas restoration of miR-223 levels by injection of miR-223 mimics into neutrophils reversed fibrosis, implying that miR-223 is a key negative regulator of the inflammasome of NLRP3.67 Therefore, the upregulation of miR-223 could be used as a novel anti-hepatic fibrosis treatment. Besides, miR-379-5p regulates arsenate-induced HSC activation by targeting GSDMD. Transfection of miR-379-5p in vitro blocked the increase in GSDMD levels and the release of IL-1β, thereby reducing the secretion of collagen type I protein and α-SMA and inhibiting HSC activation. These results indicate that miR-379-5p inhibits pyroptosis by reducing GSDMD expression and inhibiting liver fibrosis.68 Researchers on liver fibrosis demonstrated that miR-21 expression was significantly increased in patients with liver fibrosis and in animal models, indicating that miR-21 overexpression promoted the activation of the NLRP3 inflammasome in HSCs. The above phenomenon was found to be induced by two pathways: miR-21 activates the NLRP3 inflammasome and releases IL-1βby targeting the Spry1-ERK-NF-κB pathway, and miR-21 activates the NLRP3 inflammasome and releases IL-1β in HSCs. Both pathways result in enhanced NLRP3 expression, which promotes HSC activation and collagen synthesis, leading to the exacerbation of hepatic fibrosis.69
Moreover, dysregulation of lncRNAs is an important trigger of hepatic fibrosis, and some lncRNAs can influence the development of hepatic fibrosis by regulating the pyroptosis signaling pathway. Current study found that interfering with macrophage pyroptosis can also affect liver fibrosis, and silencing lncRNA-Lfar1 attenuated M1 macrophage pro-inflammatory activation and inhibited NLRP3 inflammasome-mediated pyroptosis in vivo. Simultaneously, knockdown of lncRNA-Lfar1 could block the activation of the NLRP3 inflammasome and thus reduce the release of pro-inflammatory factors IL-1β and IL-18 to inhibit pyroptosis and liver fibrosis.70 Early research suggested that the positive regulation of lncRNA-p21 could promote liver fibrosis and increases the levels of fibrosis-related proteins (collagen I, α-SMA, and TIMP-1). In vitro, lncRNA-p21 was significantly upregulated in HSCs, which further activated the PI3K-AKT-NF-κB pathway and induced pyroptotic factors, thereby promoting HSC activation and α-SMA secretion.71 More attractively, Wang et al examined that salvianolic acid B (Sal B) could improve liver fibrosis by regulating the NF-κB signaling pathway. Further mechanism found that Sal B can downregulate lncRNA-ROR by targeting the NF-κB signaling pathway to downregulate the expression of inflammatory factors, thereby inhibiting the proliferation and activation of HSCS. Not only that, Sal B can also upregulate miR-6499-3p to competitively target and degrade lncRNA-ROR, which can also play a role in downregulating lncRNA-ROR, and finally play a key in anti-liver fibrosis.72 It is interesting to note that lncRNA-GAS5 also plays an important role in the regulation of liver fibrosis, lncRNA-GAS5 was revealed to be significantly downregulated in induced activated HSC, whereas in vitro experiments transfected with upregulated lncRNA-GAS5 inhibited the downstream NF-κB pyroptosis signaling pathway by binding to the 3’-UTR of TLR10, which in turn hindered HSC activation.73 In general, these studies revealed that ncRNAs regulate pyroptosis and expanded the research field of pyroptosis in liver fibrosis. The association between ncRNAs and pyroptosis is summarized in Table 2.
 |
Table 2 NcRNA Regulated Pyroptosis to Attenuate Liver Fibrosis |
Nc-RNA Mediated Pyroptosis Pathway in ALD and NAFLD
ALD is characterized by liver tissue damage and inflammation caused by excessive alcohol consumption. It is mainly characterized by structural changes and dysfunction of hepatocytes.74 The initial clinical manifestation is steatosis, which then develops into liver fibrosis, alcoholic hepatitis (AH), cirrhosis and liver cancer.75 NAFLD is characterized by the accumulation of lipids in the liver caused by factors other than high alcohol intake, drug use, or other factors that contribute to hepatic steatosis,76 which can be classified pathologically as varying in severity, ranging from simple steatosis to NASH and liver fibrosis.77,78 Increasing number of studies have found that pyroptosis is the inflammatory link between simple steatosis and NASH.79,80 Specifically, pyroptosis is rarely observed in animal models of simple steatosis without inflammation, and pyroptosis has been suggested to occur in NASH in humans and animal models, implying that the pro-inflammatory factors released by pyroptosis process are key molecules in the development of NAFLD.81 Similarly, pyroptosis is a key driver of ALD in patients and animal models, and inhibition of pyroptosis prevents ALD progression and has beneficial effects on liver injury and steatosis.82
Recently, growing works have suggested that ncRNAs are involved in the development of ALD and NAFLD by regulating the pyroptosis signaling pathways. Bala et al reported that miR-155 knockout NASH mice exhibited less liver injury, steatosis, and fibrosis than normal NASH mice. In miR-155 knockout mice, pyroptotic proteins such as NLRP3, ASC, and caspase-1 were downregulated, and collagen deposition was also indirectly attenuated.83 Therefore, miR-155 knockout could alleviated steatosis and liver fibrosis in NASH mice, thereby mitigating the development of NAFLD. Similarly, another finding suggested that the overexpression of ATG2B (an autophagy-related protein) was shown to attenuates lipid droplet accumulation and reduces ALT, AST, inflammatory cytokines, and pyroptosis in established mouse and cellular models of NASH. Thus, by knocking down miR-375-3p, its specific binding to ATG2B is reduced, thereby promoting autophagy, inhibiting pyroptosis, and improving the occurrence and development of NASH.84 Pan et al applied that liver Kupffer cells are activated in ASH mice with alcoholic steatohepatitis, contributing to alcoholic liver injury. miR-34a-5p can target and inhibit the expression of SIRT1 to promote Kupffer cells pyroptosis. When SIRT1 was knocked down, the protein expression of NLRP3, GSDMD, IL-1β, and IL-18 was significantly increased.85 In addition, miR-148a levels were significantly reduced in patients with ALD and mouse models. Forkhead box protein O1 (FoxO1), as a transcription factor for miR-148a transcription activation, inhibits the expression of FoxO1 with alcohol and then reduces the expression of miR-148a. Reduction in miR-148a expression induced upregulation of TXNIP expression. TXNIP overexpression activates NLRP3 inflammasome, caspase-1 and promoted pyroptosis.86 Therefore, upregulation of miR-148a levels by hepatocyte-specific transfection can inhibit TXNIP overexpression and inflammasome activation, which can be used as a new research direction for the treatment of ALD. Furthermore, lncRNA-Platr4 is an lncRNA associated with steatohepatitis, whose expression is driven by NF-κB, and upregulation of lncRNA-Platr4 repairs NASH in mice. Mechanistically, lncRNA-Platr4 prevents the NF-κB/Rxrα complex from binding to the κB site through physical interactions, which inhibits the transcriptional activation of NLRP3 and ASC, which in turn inactivates the NLRP3 inflammasome, reduces pyroptosis, and improves NASH.87 Another outcome in NAFLD indicated that lncRNA-GAS5 as a sponge, can bind to miR-28a-5p, and miR-28a-5p enhanced pyroptosis by targeting the 3’-untranslated region (UTR) of the E3 ligase MARCH7 during NAFLD development. With the negative regulation of miR-28a-5p, MARCH7 overexpression could cause NLRP3 proteasomal degradation, thereby inhibiting pyroptosis.88 Above all, these studies not only further determine the vital role of ncRNAs in pyroptosis but also provide a new direction for the identification of fatty liver markers. (Table 3)
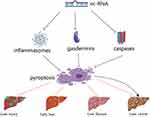 |
Figure 2 NcRNA regulates major pyroptosis signaling molecules to influence liver disease. |
 |
Table 3 NcRNA Regulated Pyroptosis to Attenuate ALD and NAFLD |
Nc-RNA Mediated Pyroptosis Pathway in Liver Cancer
Liver cancer is one of the most common malignant tumors worldwide, and hepatic malignancies originates from the epithelial or mesenchymal tissues of the liver. Its pathogenesis of hepatocellular carcinoma has been reported to be related to pyroptosis.89 In particular, it has been reported that the expression level of the NLRP3 inflammasome is significantly reduced in hepatocellular carcinoma (HCC) compared to normal liver tissues, and the levels of pyroptosis-related proteins are also decreased.90 Additionally, berberine activated caspase-1 and triggered pyroptosis, showing a strong inhibitory effect on the proliferation and metastasis of HCC cells in vitro. Interestingly, similar results were observed for 17βestradiol-induced NLRP3 inflammasome activation and caspase-1-dependent pyroptosis, which suppressed HCC progression by inhibition autophagy.91 In Recent years, certain ncRNAs have been detected to influence the pathological process of liver cancer through mediating the pyroptosis pathway. However, the mechanisms underlying ncRNA-mediated pyroptosis in HCC remain unclear.
Du et al investigated that miRNA-30a-3p mediates pyroptosis by targeting caspase-1. Upregulation of miRNA-30a-3p could reduce the expression level of caspase-1, thereby inhibiting the release of the pro-inflammatory cytokines IL-18, IL-1β, and pyroptosis, implying that the reduction of pyroptosis in HCC cells can inhibit the proliferation, invasion, and metastasis of HCC cells.92 Dramatically, lncRNA-SNHG7 expression was upregulated in liver cancer tissues and HCC cells, which inhibited NLRP3, caspase-1, and IL-1β, suggesting that lncRNA-SNHG7 may play an oncogenic role in liver cancer by inhibiting pyroptosis.93 Vitro experiments showed that lncRNA-SNHG7 knockdown increased the expression levels of NLRP3, caspase-1, and IL-1β, caused caspase-1-dependent pyroptosis in liver cancer cells, and then inhibited the proliferation and metastasis of cancer cells. It has been demonstrated that the inhibition of lncRNA-SNHG7 may promote pyroptosis to aggravate the death of HCC cells and inhibit the growth of liver tumors.93 In summary, these studies illustrate that pyroptosis plays a dual regulatory role in liver cancer development. Pyroptosis may promote the proliferation and metastasis of liver cancer tissues and aggravate liver cancer, which must be reduced to play an anti-liver cancer role. In contrast, pyroptosis may also inhibit the proliferation and invasion of liver cancer tissues through a certain mechanism, and by promoting pyroptosis, it may play a direct role in the anti-liver cancer effect. Until now, researches that investigate the role of pyroptosis in liver cancer are very rare, but pyroptosis seems to be crucial for liver cancer development and therapeutic treatment. Extensive studies have shown that HCC is still more related to apoptosis,94,95 and the pyroptosis pathway can provide a new approach to treat HCC. (Table 4)
 |
Table 4 NcRNA Regulated Pyroptosis to Attenuate Liver Cancer |
Traditional Chinese Medicine and Its Active Ingredients Influence ncRNA-Mediated Pyroptosis Pathway in Liver Disease
Increasing reports denoted that traditional Chinese medicine (TCM) in the clinical treatment of liver disease plays a unique efficacy in the treatment of multiple targets, multiple components, and multi-level outstanding advantages because it contains many bioactive components, such as alkaloids, flavonoids, steroids, glycosides and phenylpropanoids.96–100 Previous studies have found that Isodon ternifolius can reduce the release of fibrotic and inflammatory cytokines by inhibiting the activation of the TLR4/NF-κB/NLRP3 pathway, which downregulates the expression of NF-κB p65, NLRP3, GSDMD, ASC proteins, and mRNAs. This leads to the attenuation of hepatocyte inflammation, inhibition of HSC activation, and have the effect of anti fibrosis.101,102 In the aspect of experiment reveals that TCM via signaling pathways and more targets way to explore new strategies for the treatment of liver disease. With the in-depth exploration of TCM, it can be observed that Chinese herbal compounds and their active ingredients regulate pyroptosis at the ncRNA level, impacting the development process of liver diseases. Although many researches have reported that Chinese medicines and their active ingredients are involved in pyroptosis to influence liver disease, there are very few studies on the role of ncRNAs in the regulation of pyroptosis to influence liver disease. Therefore, we have summarized this information as follows.
Theaflavin is a compound derived from black tea and has a variety of pharmacological effects, including hypolipidemic, hypotensive, antioxidant, antiradical, antibacterial, and anti-cancer.103–106 A study demonstrated that theaflavin-3,3 ‘-digallate (TFDG) in this group of compounds showed stronger biological activity. TFDG not only ameliorated learning and memory dysfunction,107 but also effectively diminished high-fat diet-induced liver injury and inflammatory response by inhibiting the NLRP3/IL-1β pathway through upregulation of miR-223, which reduced the excessive activation of the NLRP3 inflammasome as well as the release of large amounts of cytokines, such as IL-1βand IL-18, and alleviated high-fat diet-induced liver injury.108 Zhao et al examined that polydatin inhibited ROS-driven TXNIP activation of the NLRP3 inflammasome by upregulating miR-200a, decrease pyroptosis, and improving fructose-induced liver inflammation and lipid deposition.109 Similarly, curcumin, a polyphenolic compound extracted and isolated from turmeric, exhibits strong biological activity and alleviates fructose-induced liver inflammation. It also works through the miR-200a-mediated TXNIP/NLRP3 pathway, directly targeting the inhibition of TXNIP and reducing the activation of NLRP3 inflammasome, which ultimately plays a role in liver protection.110 Ginseng saponin Rg1 (Rg1) is separated from the TCM-ginseng, which possesses a variety of biological activities such as anti-inflammatory and anti-cancer properties.111–113 In another probe on NASH, researchers discovered that Rg1 participates in autophagy and pyroptosis through the miR-375-3p/ATG2B/PTEN-AKT axis, which mainly manifests as knocking down miR-375-3p, reducing ALT, AST, IL-1β, IL-18, and pyroptosis protein NLRP3 and caspase 1 expression, promoting autophagy, and inhibiting pyroptosis, thus attenuating NASH.84
According to previous research, Atractylodes macrocephala Koidz (AMK) has plentiful pharmacological effects, including anti-tumor, anti-inflammatory, anti-oxidation, and hypoglycemic.114 The polysaccharide components of AMK play a crucial role in immune regulation of the body.115 Experimental studies have demonstrated that AMK polysaccharides alleviate LPS-induced inflammatory liver tissue injury and act as anti-hepatic injury agents by regulating the miR-223/NLRP3 axis, thus significantly reducing the expression levels of IL-1β and IL-18.116 Additionally, Silibinin is an effective component of traditional Chinese medicine, used as a hepatoprotective agent in clinical therapy, and its phospholipid complex has been used in the clinical treatment of acute liver injury.117 Further finding found that the Silibinin-phospholipid complex significantly diminished d-GalN/LPS-induced liver injury by enhancing the expression of miR-223-3p, and miR-223-3p directly targeted the 3’ UTR of NLRP3, thereby inhibiting the activation of the NLRP3 inflammasome and the expression of pyroptotic proteins to achieve hepatoprotective effects.118 Curcumol is an effective active ingredient extracted from the genuine medicinal herb Curcuma kwangsiensis S. G. Lee et C. F. Liang in Guangxi, China, which is known for its ability to invigorate blood circulation and remove blood stasis, protect the liver, and have anti-inflammatory, cholagogic and antioxidant effects.119,120 Curcumol inhibits miR-125b/NLRP3 pathway to prevent liver fibrosis. Mechanistically, it can upregulate the expression of miR-125b, which decreases the downstream NLRP3 inflammasome and the expression of Caspase-1, GSDMD, IL-1β, and IL-18, reduces the onset of pyroptosis, and inhibits the activation of HSC to prevent liver fibrosis.121 Evidences have implied that Salvianolic acid B (Sal B), an active ingredient in Salvia miltiorrhiza extract, blocks the NF-κB signaling pathway by downregulating lnc-ROR and inhibiting the expression of pyroptotic proteins and inflammatory cytokines, which in turn hinders the proliferation and activation of HSC. Meanwhile, Sal B could also target the degradation of lnc-ROR through the regulation of miR-6499-3P, which can also play an anti- hepatic fibrosis effect.72 As a Chinese medicine compound formula, the main effects of Shugan Jianpi formula are to relieve liver and depression, nourish blood and strengthen spleen.122 Studies on liver fibrosis have demonstrated that this formula reduces pyroptosis and inhibits HSC activation by regulating the lnc-ECONEXIN/miR-26b-5p/TLR4 signaling axis. Mechanistically, it can downregulate lncECONEXIN, which acts as a ceRNA to competitively bind to miR-26b-5p. MiR-26b-5p is indirectly upregulated, which reduces hepatocyte pyroptosis and inhibits HSC activation to achieve anti-hepatic fibrosis effects.123 Analogously, Chaihu Shugan powder is also a liver protection formula in TCM, which has the effect of soothing the liver, regulating qi, promoting blood circulation and relieving pain. It is mainly used for the treatment of chronic hepatitis, chronic gastritis, intercostal neuralgia, and liver qi depression.124 Further studies indicated that the formula increased miR-155 expression, inhibiting the TLR4 /NF-κB signaling pathway, activated the NLRP3 inflammasome, and reduced TLR4, NF-κB, NLRP3, Caspase-1, ASC and IL - 1β expression.125 Amusingly, researchers have also presented new insights into TCM-ncRNA-pyroptosis in liver diseases. Although there have been few reports in this area, the role of TCM in pyroptosis is worthy of further investigation. These results indicate that the active ingredients and compounds of TCM can against liver disease by regulating the pyroptosis signaling pathway through ncRNA. Therefore, it is necessary to explore key ncRNAs that could provide potential targets for the future treatment of liver diseases. The pharmacological mechanisms of TCM against liver disease are summarized in Table 5.
 |
Table 5 TCM Regulated Pyroptosis by ncRNA to Treat Liver Diseases |
Conclusion and Future Perspectives
Liver diseases appear in an increasing proportion of current clinical diseases, including liver injury, ALD, NAFLD, liver fibrosis, and liver cancer. During the process of liver lesions, the pyroptosis associated protein abnormal expression, such as NLRP3, caspase-1, and GSDMD, which subsequently causes the release of the downstream proinflammatory factor IL-1βand IL - 18. These findings suggest that pyroptosis plays a key role in hepatocyte inflammation, lipid accumulation, fibropathy, and carcinogenesis. Accordingly, the pyroptosis signaling pathway is closely related to the pathological process of liver disease and is emerging as one of the most important avenues for the treatment and prevention of liver disease. Obviously, the final manifestation of pyroptosis in the liver is the cascade response of inflammation, generating a massive storm of inflammatory factors that exacerbate hepatocyte inflammation, and ncRNAs are involved in the progression of these liver diseases through the regulation of the pyroptosis signaling pathway. Furthermore, this review summarizes the available evidence on the mechanism of ncRNA-mediated pyroptosis in liver disease (Figure 2). In liver injury, ALD, and NAFLD, ncRNAs mediate the pyroptosis pathway by regulating the expression of pyroptotic factors, such as NLRP3, GSDMD, caspase-1, and thus inhibiting the release of pro-inflammatory factors IL-1β and IL-18. It is well known that IL-1β, IL-18 are pivotal molecules in the inflammatory response, steatosis, and fibrosis that occur in the liver, and play an important role in the pathogenesis of above three of these liver diseases.
Amusingly, for ncRNA anti-liver fibrosis via pyroptosis signaling pathway, although pyroptosis does not play a direct regulatory role in it, the inflammatory factor storm caused by pyroptosis can indirectly promote the activation of HSCs, thereby aggravating liver fibrosis. Moreover, the NLRP3 inflammasome, a crucial key in the development of pyroptosis, was found to be persistently activated, causing an increase in the expression of the HSC activation markers α-SMA and type I collagen in mice,126 which implies that the NLRP3 inflammasome may play a straightforward role in HSC activation. Overall, the ncRNA-regulated pyroptosis signaling pathway mediates the inflammasome that induces HSC activation and exacerbates liver fibrosis. To date, a few ncRNAs have been found to mediate the pyroptosis signaling pathway in HCC, but it is not difficult to conclude that pyroptosis is also closely related to the development of HCC. What is remarkable is that pyroptosis plays a dual regulatory role in the development of liver cancer. First, pyroptosis may promote the proliferation and metastasis of liver tumor tissues and aggravate liver cancer. Second, pyroptosis may also inhibit the proliferation and metastasis of liver tumor tissues, and by promoting pyroptosis, it may also play a role in anti-hepatocellular carcinoma. Unfortunately, there are no in-depth studies on this two-sided regulatory mechanism for the time being. Furthermore, how to trigger pyroptosis in tumor cells by regulating ncRNAs is still facing a huge challenge.
Notably, TCM has shown great advantages in the prevention and treatment of chronic liver diseases127,128 because it contains a large number of effective active ingredients to protect the liver. In recent years, ncRNAs regulation of post-transcriptional gene expression has become a hot topic in the field of biomedical research. Concurrently, with the in-depth exploration of TCM-target-disease mechanisms based on the regulation of ncRNAs, the multi-level and multi-target therapeutic characteristics of TCM have been scientifically expounded. Such qualities broaden the way and provide new research strategies for the molecular mechanisms of TCM anti-liver diseases. However, due to few studies have been conducted on Chinese medicines against liver disease by modulating the ncRNA-mediated pyroptosis signaling pathway, it can be used as a breakthrough point to continue intensive research to provide new ideas for the treatment of liver disease with TCM. In the future, with the development of bioinformatics, high-throughput sequencing, and metabolomics, it will be possible to screen and predict ncRNAs for disease-drug-target associations, which will further clarify the molecular mechanisms of ncRNAs and active ingredients of TCM against liver disease.
Acknowledgments
This work was supported by grants of the National Natural Scientific Foundation of China (No.81760751), Guangxi Provincial Natural Scientific Foundation (No. 2021GXNSFAA075020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi:10.1002/hep.27406
2. Xiao J, Wang F, Wong NK, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71(1):212–221. doi:10.1016/j.jhep.2019.03.004
3. Zhu J, Wang P, Ye H, et al. Trend of the mortality of major liver diseases and its impact on life expectancy in China from 2006 to 2017. J Public Health (Oxf). 2022;44(1):100–110. doi:10.1093/pubmed/fdaa261
4. Sato K, Kennedy L, Liangpunsakul S, et al. Intercellular Communication between Hepatic Cells in Liver Diseases. Int J Mol Sci. 2019;20(9). doi:10.3390/ijms20092180
5. Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi:10.1128/IAI.73.4.1907-1916.2005
6. Wree A, Holtmann TM, Inzaugarat ME, Feldstein AE. Novel Drivers of the Inflammatory Response in Liver Injury and Fibrosis. Semin Liver Dis. 2019;39(3):275–282. doi:10.1055/s-0039-1685515
7. DiPeso L, Ji DX, Vance RE, Price JV. Cell death and cell lysis are separable events during pyroptosis. Cell Death Discov. 2017;3:17070. doi:10.1038/cddiscovery.2017.70
8. Wang F, Gómez-Sintes R, Boya P. Lysosomal membrane permeabilization and cell death. Traffic. 2018;19(12):918–931. doi:10.1111/tra.12613
9. Guo H, Xie M, Zhou C, Zheng M. The relevance of pyroptosis in the pathogenesis of liver diseases. Life Sci. 2019;223:69–73. doi:10.1016/j.lfs.2019.02.060
10. Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–157. doi:10.1038/s41577-019-0228-2
11. Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi:10.1111/imr.12534
12. Sun P, Zhong J, Liao H, et al. Hepatocytes Are Resistant to Cell Death From Canonical and Non-Canonical Inflammasome-Activated Pyroptosis. Cell Mol Gastroenterol Hepatol. 2022;13(3):739–757. doi:10.1016/j.jcmgh.2021.11.009
13. Al Mamun A, Wu Y, Jia C, et al. Role of pyroptosis in liver diseases. Int Immunopharmacol. 2020;84:106489. doi:10.1016/j.intimp.2020.106489
14. Liu Y, Liu X, Lin C, et al. Noncoding RNAs regulate alternative splicing in Cancer. J Exp Clin Cancer Res. 2021;40(1):11. doi:10.1186/s13046-020-01798-2
15. Seal RL, Chen LL, Griffiths-Jones S, et al. A guide to naming human non-coding RNA genes. EMBO J. 2020;39(6):e103777. doi:10.15252/embj.2019103777
16. Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15:R17–29. doi:10.1093/hmg/ddl046
17. Gao L, Jiang Z, Han Y, Li Y, Yang X. Regulation of Pyroptosis by ncRNA: a Novel Research Direction. Front Cell Dev Biol. 2022;10:840576. doi:10.3389/fcell.2022.840576
18. Cao Z, Huang D, Tang C, et al. Pyroptosis in diabetes and diabetic nephropathy. Clin Chim Acta. 2022;531:188–196. doi:10.1016/j.cca.2022.04.011
19. Gao J, Chen X, Wei P, Wang Y, Li P, Shao K. Regulation of pyroptosis in cardiovascular pathologies: role of noncoding RNAs. Mol Ther Nucleic Acids. 2021;25:220–236. doi:10.1016/j.omtn.2021.05.016
20. Yuan Y, Xu L, Geng Z, et al. The role of non-coding RNA network in atherosclerosis. Life Sci. 2021;265:118756. doi:10.1016/j.lfs.2020.118756
21. Zhang M, Dang P, Liu Y, Qiao B, Sun Z. Noncoding RNAs in pyroptosis and cancer progression: effect, mechanism, and clinical application. Front Immunol. 2022;13:982040. doi:10.3389/fimmu.2022.982040
22. Sansonetti PJ, Phalipon A, Arondel J, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12(5):581–590. doi:10.1016/s1074-7613(00)80209-5
23. Li P, Allen H, Banerjee S, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80(3):401–411. doi:10.1016/0092-8674(95)90490-5
24. Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96(5):2396–2401. doi:10.1073/pnas.96.5.2396
25. Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 2001;3(12):825–837. doi:10.1046/j.1462-5822.2001.00162.x
26. Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front Immunol. 2018;9:2379. doi:10.3389/fimmu.2018.02379
27. Liao Z, Su J. Progresses on three pattern recognition receptor families (TLRs, RLRs and NLRs) in teleost. Dev Comp Immunol. 2021;122:104131. doi:10.1016/j.dci.2021.104131
28. Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–159. doi:10.1038/cmi.2015.95
29. Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15–27. doi:10.2147/JIR.S51250
30. Cao H, Yang D, Nie K, et al. Hesperidin may improve depressive symptoms by binding NLRP3 and influencing the pyroptosis pathway in a rat model. Eur J Pharmacol. 2023;952:175670. doi:10.1016/j.ejphar.2023.175670
31. Zhou S, Li Y, Hong Y, Zhong Z, Zhao M. Puerarin protects against sepsis-associated encephalopathy by inhibiting NLRP3/Caspase-1/GSDMD pyroptosis pathway and reducing blood-brain barrier damage. Eur J Pharmacol. 2023;945:175616. doi:10.1016/j.ejphar.2023.175616
32. Liu Y, Shu J, Liu T, et al. Nicorandil protects against coronary microembolization-induced myocardial injury by suppressing cardiomyocyte pyroptosis via the AMPK/TXNIP/NLRP3 signaling pathway. Eur J Pharmacol. 2022;936:175365. doi:10.1016/j.ejphar.2022.175365
33. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
34. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi:10.1038/nature10759
35. Liston A, Masters SL. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol. 2017;17(3):208–214. doi:10.1038/nri.2016.151
36. Jin Y, Li H, Xie G, Chen S, Wu S, Fang X. Sevoflurane combined with ATP activates caspase-1 and triggers caspase-1-dependent pyroptosis in murine J774 macrophages. Inflammation. 2013;36(2):330–336. doi:10.1007/s10753-012-9550-6
37. Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi:10.1111/imr.12287
38. Vanaja SK, Russo AJ, Behl B, et al. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell. 2016;165(5):1106–1119. doi:10.1016/j.cell.2016.04.015
39. Gao YL, Zhai JH, Chai YF. Recent Advances in the Molecular Mechanisms Underlying Pyroptosis in Sepsis. Mediators Inflamm. 2018;2018:5823823. doi:10.1155/2018/5823823
40. Zhao Y, Shi J, Shao F. Inflammatory Caspases: activation and Cleavage of Gasdermin-D In Vitro and During Pyroptosis. Methods Mol Biol. 2018;1714:131–148. doi:10.1007/978-1-4939-7519-8_9
41. Feng S, Fox D, Man SM. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol. 2018;430(18 Pt B):3068–3080. doi:10.1016/j.jmb.2018.07.002
42. Xia W, Li Y, Wu M, et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12(2):139. doi:10.1038/s41419-021-03431-2
43. Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
44. Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016;73(11–12):2349–2367. doi:10.1007/s00018-016-2205-2
45. Chen KW, Demarco B, Heilig R, et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38(10):e101638. doi:10.15252/embj.2019101638
46. Newton K, Wickliffe KE, Maltzman A, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575:679–682. doi:10.1038/s41586-019-1752-8
47. Hou J, Zhao R, Xia W, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z
48. Orning P, Weng D, Starheim K, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi:10.1126/science.aau2818
49. Hernandez C, Huebener P, Pradere JP, Antoine DJ, Friedman RA, Schwabe RF. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J Clin Invest. 2018;128(6):2436–2451. doi:10.1172/JCI91786
50. Wu Y, Qiu G, Zhang H, et al. Dexmedetomidine alleviates hepatic ischaemia-reperfusion injury via the PI3K/AKT/Nrf2-NLRP3 pathway. J Cell Mol Med. 2021;25(21):9983–9994. doi:10.1111/jcmm.16871
51. Chen X, Liu B, Li X, et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J Extracell Vesicles. 2021;10(4):e12069. doi:10.1002/jev2.12069
52. Li F, Zhang L, Xue H, Xuan J, Rong S, Wang K. SIRT1 alleviates hepatic ischemia-reperfusion injury via the miR-182-mediated XBP1/NLRP3 pathway. Mol Ther Nucleic Acids. 2021;23:1066–1077. doi:10.1016/j.omtn.2020.11.015
53. Du C, Song H, Wang X, Wang Z. miR-182-5p regulates pyroptosis involving liver ischemia reperfusion injury. Tianjin Med J. 2018;46(10):1045–1050. doi:10.11958/20180682
54. Li S, Zhu Z, Sun L, Zhang H. Effect of miR-330-3p on hepatic ischemia-reperfusion injury in mice. Chin J Hepatobiliary Surg. 2021;27(5):371–376. doi:10.3760/cma.j.cn113884-20200915-00491
55. Yang L, Tian L, Zhang Z, et al. Cannabinoid Receptor 1/miR-30b-5p Axis Governs Macrophage NLRP3 Expression and Inflammasome Activation in Liver Inflammatory Disease. Mol Ther Nucleic Acids. 2020;20:725–738. doi:10.1016/j.omtn.2020.04.010
56. Chang H, Chang H, Cheng T, Lee GD, Chen X, Qi K. Micro-ribonucleic acid-23a-3p prevents the onset of type 2 diabetes mellitus by suppressing the activation of nucleotide-binding oligomerization-like receptor family pyrin domain containing 3 inflammatory bodies-caused pyroptosis through negatively regulating NIMA-related kinase 7. J Diabetes Investig. 2021;12(3):334–345. doi:10.1111/jdi.13396
57. Chen L, Lu FB, Chen DZ, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93:38–46. doi:10.1016/j.molimm.2017.11.008
58. Yue Y, Du Z, Tao J, Shi L. Inhibition of microRNA-297 alleviates THLE-2 cell injury induced by hypoxia/reoxygenation by inhibiting NLRP3 inflammasome activation via sirtuin 3. Can J Physiol Pharmacol. 2022;100(2):125–133. doi:10.1139/cjpp-2021-0287
59. Menon MP, Hua KF. The Long Non-coding RNAs: paramount Regulators of the NLRP3 Inflammasome. Front Immunol. 2020;11:569524. doi:10.3389/fimmu.2020.569524
60. Liang C, Peng Y, Sun H, Wang L, Jiang L, Zou S. Silencing lncRNA KCNQ1OT1 reduced hepatic ischemia reperfusion injury-induced pyroptosis by regulating miR-142a-3p/HMGB1 axis. Mol Cell Biochem. 2022. doi:10.1007/s11010-022-04586-y
61. Chen Z, Jia S, Li D, et al. Silencing of long noncoding RNA AK139328 attenuates ischemia/reperfusion injury in mouse livers. PLoS One. 2013;8(11):e80817. doi:10.1371/journal.pone.0080817
62. Brocker CN, Kim D, Melia T, et al. Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat Commun. 2020;11(1):5847. doi:10.1038/s41467-020-19554-7
63. Oakley F. Interrogating mechanisms of liver fibrosis with omics. Nat Rev Gastroenterol Hepatol. 2022;19(2):89–90. doi:10.1038/s41575-021-00567-6
64. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi:10.1016/j.addr.2017.05.007
65. Du G, Wang J, Zhang T, et al. Targeting Src family kinase member Fyn by Saracatinib attenuated liver fibrosis in vitro and in vivo. Cell Death Dis. 2020;11(2):118. doi:10.1038/s41419-020-2229-2
66. Jimenez Calvente C, Del Pilar H, Tameda M, Johnson CD, Feldstein AE. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol Ther. 2020;28(2):653–663. doi:10.1016/j.ymthe.2019.09.013
67. Calvente CJ, Tameda M, Johnson CD, et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest. 2019;129(10):4091–4109. doi:10.1172/JCI122258
68. Li J, Xue J, Wang D, et al. Regulation of gasdermin D by miR-379-5p is involved in arsenite-induced activation of hepatic stellate cells and in fibrosis via secretion of IL-1β from human hepatic cells. Metallomics. 2019;11(2):483–495. doi:10.1039/c8mt00321a
69. Ning ZW, Luo XY, Wang GZ, et al. MicroRNA-21 Mediates Angiotensin II-Induced Liver Fibrosis by Activating NLRP3 Inflammasome/IL-1β Axis via Targeting Smad7 and Spry1. Antioxid Redox Signal. 2017;27(1):1–20. doi:10.1089/ars.2016.6669
70. Zhang K, Shi Z, Zhang M, et al. Silencing lncRNA Lfar1 alleviates the classical activation and pyoptosis of macrophage in hepatic fibrosis. Cell Death Dis. 2020;11(2):132. doi:10.1038/s41419-020-2323-5
71. Yang L, Fu WL, Zhu Y, Wang XG. Tβ4 suppresses lincRNA-p21-mediated hepatic apoptosis and fibrosis by inhibiting PI3K-AKT-NF-κB pathway. Gene. 2020;758:144946. doi:10.1016/j.gene.2020.144946
72. Wang R, Li S, Chen P, et al. Salvianolic acid B suppresses hepatic stellate cell activation and liver fibrosis by inhibiting the NF-κB signaling pathway via miR-6499-3p/LncRNA-ROR. Phytomedicine. 2022;107:154435. doi:10.1016/j.phymed.2022.154435
73. Su SB, Tao L, Liang XL, Chen W. Long noncoding RNA GAS5 inhibits LX-2 cells activation by suppressing NF-κB signalling through regulation of the miR-433-3p/TLR10 axis. Dig Liver Dis. 2022;54(8):1066–1075. doi:10.1016/j.dld.2021.11.002
74. Hyun J, Han J, Lee C, Yoon M, Jung Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int J Mol Sci. 2021;22(11):5717. doi:10.3390/ijms22115717
75. Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–242. doi:10.1038/nrgastro.2015.35
76. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
77. Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2):e9302. doi:10.15252/emmm.201809302
78. Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. 2021;50:101167. doi:10.1016/j.molmet.2021.101167
79. Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 2014;20(26):8525–8534. doi:10.3748/wjg.v20.i26.8525
80. Beier JI, Banales JM. Pyroptosis: an inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 2018;68(4):643–645. doi:10.1016/j.jhep.2018.01.017
81. Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi:10.1038/nature10809
82. Petrasek J, Iracheta-Vellve A, Saha B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98(2):249–256. doi:10.1189/jlb.3AB1214-590R
83. Bala S, Ganz M, Babuta M, et al. Steatosis, inflammasome upregulation, and fibrosis are attenuated in miR-155 deficient mice in a high fat-cholesterol-sugar diet-induced model of NASH. Lab Invest. 2021;101(12):1540–1549. doi:10.1038/s41374-021-00626-1
84. Chen X, Xue W, Zhang J, Peng J, Huang W. Ginsenoside Rg1 attenuates the NASH phenotype by regulating the miR-375-3p/ATG2B/PTEN-AKT axis to mediate autophagy and pyroptosis. Lipids Health Dis. 2023;22(1):22. doi:10.1186/s12944-023-01787-2
85. Pan XS, Li BW, Wang LL, et al. Kupffer cell pyroptosis mediated by METTL3 contributes to the progression of alcoholic steatohepatitis. FASEB J. 2023;37(6):e22965. doi:10.1096/fj.202300059RR
86. Heo MJ, Kim TH, You JS, Blaya D, Sancho-Bru P, Kim SG. Alcohol dysregulates miR-148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression. Gut. 2019;68(4):708–720. doi:10.1136/gutjnl-2017-315123
87. Lin Y, Wang S, Gao L, et al. Oscillating lncRNA Platr4 regulates NLRP3 inflammasome to ameliorate nonalcoholic steatohepatitis in mice. Theranostics. 2021;11(1):426–444. doi:10.7150/thno.50281
88. Chen T, Meng Y, Zhou Z, et al. GAS5 protects against nonalcoholic fatty liver disease via miR-28a-5p/MARCH7/NLRP3 axis-mediated pyroptosis. Cell Death Differ. 2023;30(7):1829–1848. doi:10.1038/s41418-023-01183-4
89. Chu Q, Jiang Y, Zhang W, et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget. 2016;7(51):84658–84665. doi:10.18632/oncotarget.12384
90. Wei Q, Mu K, Li T, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94(1):52–62. doi:10.1038/labinvest.2013.126
91. Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol Res. 2019;27(7):827–834. doi:10.3727/096504018X15462920753012
92. Du C, Song H, Wang X, Wang Z. MiRNA-30a-3p inhibits proliferation and metastasis of hepatocellular carcinoma cells by regulating caspase 1-mediated pyroptosis. Chinese J of General Sur. 2018;33(11):920–923. doi:10.3760/cma.j.issn.1007-631X.2018.11.007
93. Chen Z, He M, Chen J, Li C, Zhang Q. Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in liver cancer. Oncol Lett. 2020;20(1):893–901. doi:10.3892/ol.2020.11635
94. Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15(12):738–752. doi:10.1038/s41575-018-0065-y
95. Harakeh S, Saber SH, Al-Raddadi R, et al. Novel curcumin nanoformulation induces apoptosis, and reduces migration and angiogenesis in liver cancer cells. Artif Cells Nanomed Biotechnol. 2023;51(1):361–370. doi:10.1080/21691401.2023.2238756
96. Mai W, Xu Y, Xu J, et al. Berberine Inhibits Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation and Pyroptosis in Nonalcoholic Steatohepatitis via the ROS/TXNIP Axis. Front Pharmacol. 2020;11:185. doi:10.3389/fphar.2020.00185
97. Shi H, Qiao F, Lu W, et al. Baicalin improved hepatic injury of NASH by regulating NRF2/HO-1/NRLP3 pathway. Eur J Pharmacol. 2022;934:175270. doi:10.1016/j.ejphar.2022.175270
98. Ye H, Ma S, Qiu Z, et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J Ethnopharmacol. 2022;296:115457. doi:10.1016/j.jep.2022.115457
99. Han H, Li J, Tian L, Pei L, Zheng M. Through regulation of the SIRT1 pathway plant sterol ester of α-linolenic acid inhibits pyroptosis thereby attenuating the development of NASH in mice. J Nutr Biochem. 2023;119:109408. doi:10.1016/j.jnutbio.2023.109408
100. Song A, Ding T, Wei N, et al. Schisandrin B induces HepG2 cells pyroptosis by activating NK cells mediated anti-tumor immunity. Toxicol Appl Pharmacol. 2023;472:116574. doi:10.1016/j.taap.2023.116574
101. Qin L, Chen Y, Huang G, Wu R, Liu D. Effects of Isodon ternifolius extract on TLR4/NF-κB/NLRP3 pathway in hepatic stellate cells and hepatocytes. China Pharm. 2022;33(20):2448–2453. doi:10.6039/j.issn.1001-0408.2022.20.04
102. Huang GD, Zhou ZP, Pang Z, et al. Effect of Isodon ternifolius-medicated serum on hepatic stellate cells based on TLR4/NF-κB/NLRP3 signaling pathway. Zhongguo Zhong Yao Za Zhi. 2023;48(14):3913–3921. doi:10.19540/j.cnki.cjcmm.20230306.704
103. Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131(9):2248–2251. doi:10.1093/jn/131.9.2248
104. Cai X, Liu Z, Dong X, et al. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. Food Funct. 2021;12(20):9922–9931. doi:10.1039/d1fo01966j
105. Alam M, Ali S, Ashraf GM, Bilgrami AL, Yadav DK, Hassan MI. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. 2022;379:132135. doi:10.1016/j.foodchem.2022.132135
106. Yussof A, Cammalleri B, Fayemiwo O, Lopez S, Chu T. Antibacterial and Sporicidal Activity Evaluation of Theaflavin-3,3’-digallate. Int J Mol Sci. 2022;23(4):2153. doi:10.3390/ijms23042153
107. Cao Y, Zhang Y, Jia Z, et al. Theaflavin-3,3’-digallate ameliorates learning and memory impairments in mice with premature brain aging induced by D-galactose. Physiol Behav. 2023;261:114077. doi:10.1016/j.physbeh.2023.114077
108. Zhang G, Fu H, Zou Y, Deng Z. Effects of theaflavin-3,3’-digallate on high-fat diet-induced liver injury in mice and its mechanism. J Third Military Med Univ. 2022;44(2):147–154. doi:10.16016/j.2097-0927.202107051
109. Zhao XJ, Yu HW, Yang YZ, et al. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124–137. doi:10.1016/j.redox.2018.07.002
110. Ding XQ, Wu WY, Jiao RQ, et al. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol Res. 2018;137:64–75. doi:10.1016/j.phrs.2018.09.021
111. Gao Q, Li G, Zu Y, et al. Ginsenoside Rg1 alleviates ANIT-induced cholestatic liver injury by inhibiting hepatic inflammation and oxidative stress via SIRT1 activation. J Ethnopharmacol. 2023;319(Pt 1):117089. doi:10.1016/j.jep.2023.117089
112. Hsieh CC, Chang CY, Yar Lee TX, et al. Longevity, tumor, and physical vitality in rats consuming ginsenoside Rg1. J Ginseng Res. 2023;47(2):210–217. doi:10.1016/j.jgr.2021.04.006
113. Wang Z, Du K, Hou J, et al. Rg1 alleviates oxidative stress and spermatogonium apoptosis in D-gal-induced testicular toxicity by activating Akt. Redox Rep. 2023;28(1):2206197. doi:10.1080/13510002.2023.2206197
114. Yang L, Yu H, Hou A, et al. A Review of the Ethnopharmacology, Phytochemistry, Pharmacology, Application, Quality Control, Processing, Toxicology, and Pharmacokinetics of the Dried Rhizome of Atractylodes macrocephala. Front Pharmacol. 2021;12:727154. doi:10.3389/fphar.2021.727154
115. Guo S, Li W, Chen F, et al. Polysaccharide of Atractylodes macrocephala Koidz regulates LPS-mediated mouse hepatitis through the TLR4-MyD88-NFκB signaling pathway. Int Immunopharmacol. 2021;98:107692. doi:10.1016/j.intimp.2021.107692
116. Chen F, Li B, Li W, et al. Polysaccharide of Atractylodes macrocephala Koidz alleviate lipopolysaccharide-stimulated liver inflammation injury of goslings through miR-223/NLRP3 axis. Poult Sci. 2023;102(1):102285. doi:10.1016/j.psj.2022.102285
117. Gür FM, Bilgiç S. Silymarin, an antioxidant flavonoid, protects the liver from the toxicity of the anticancer drug paclitaxel. Tissue Cell. 2023;83:102158. doi:10.1016/j.tice.2023.102158
118. Tang S, Zhang X, Duan Z, et al. The novel hepatoprotective mechanisms of silibinin-phospholipid complex against d-GalN/LPS-induced acute liver injury. Int Immunopharmacol. 2023;116:109808. doi:10.1016/j.intimp.2023.109808
119. Li ZY, Hao EW, Du ZC, et al. Research progress of Curcuma kwangsiensis root tubers and analysis of liver protection and anti-tumor mechanisms based on Q-markers. Zhongguo Zhong Yao Za Zhi. 2022;47(7):1739–1753. doi:10.19540/j.cnki.cjcmm.20211220.203
120. Sun S, Huan S, Li Z, et al. Curcumol alleviates liver fibrosis by inducing endoplasmic reticulum stress-mediated necroptosis of hepatic stellate cells through Sirt1/NICD pathway. PeerJ. 2022;10:e13376. doi:10.7717/peerj.13376
121. Wang J, Guo X, Zheng B, Liang X. Mechanism of curcumol against liver fibrosis based on miR-125b/NLRP3 signaling pathway. Chinese Archives Traditional Chin Med. 2022;40(11):95–99. doi:10.13193/j.issn.1673-7717.2022.11.023
122. Liu X, Lv M, Wang Y, et al. Anti-depressive effects of Xiaoyaosan, Shugan and Jianpi herbal treatments: role on the gut microbiome of CUMS rats. Phytomedicine. 2021;87:153581. doi:10.1016/j.phymed.2021.153581
123. Fan C. Mechanism of Lnc ECONEXIN/miR-26-b-5p/TLR4 signal axis promotes the activation of HSC by regulating pyroptosis and the intervention effect of Shugan Jianpi formula. Anhui Univ Chine Med. 2023. doi:10.26922/d.cnki.ganzc.2022.000320
124. Wang Y, Wang X, Jiang K, Yang K, Ling J. Network pharmacology and experimental studies for deciphering the molecular targets and mechanisms of Chaihu Shugan powder in the treatment of functional dyspepsia. Technol Health Care. 2023;31(S1):449–462. doi:10.3233/THC-236039
125. Jia KK, Pan SM, Ding H, et al. Chaihu-shugan san inhibits inflammatory response to improve insulin signaling in liver and prefrontal cortex of CUMS rats with glucose intolerance. Biomed Pharmacother. 2018;103:1415–1428. doi:10.1016/j.biopha.2018.04.171
126. Inzaugarat ME, Johnson CD, Holtmann TM, et al. NLR Family Pyrin Domain-Containing 3 Inflammasome Activation in Hepatic Stellate Cells Induces Liver Fibrosis in Mice. Hepatology. 2019;69(2):845–859. doi:10.1002/hep.30252
127. Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 Suppl:E67–70. doi:10.1046/j.1440-1746.2000.02100.x
128. Chen M, Xie Y, Gong S, et al. Traditional Chinese medicine in the treatment of nonalcoholic steatohepatitis. Pharmacol Res. 2021;172:105849. doi:10.1016/j.phrs.2021.105849
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

