Back to Journals » OncoTargets and Therapy » Volume 11
Natural antisense transcript of hypoxia-inducible factor 1 regulates hypoxic cell apoptosis in epithelial ovarian cancer
Authors Qiu J, Lin X, Zheng T, Tang X, Hua K
Received 11 May 2018
Accepted for publication 27 August 2018
Published 14 December 2018 Volume 2018:11 Pages 9101—9110
DOI https://doi.org/10.2147/OTT.S173816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Jun-jun Qiu,1–3 Xiao-jing Lin,1–3 Ting-ting Zheng,1–3 Xiao-yan Tang,1–3 Ke-qin Hua1–3
1Department of Gynecology, Obstetrics and Gynecology Hospital, Fudan University, Shanghai 200011, China; 2Obstetrics and Gynecology Department of Shanghai Medical College, Fudan University, Shanghai 200032, China; 3Department of Gynecology, Shanghai Key Laboratory of Female Reproductive Endocrine-Related Diseases, Shanghai 200011, China
Purpose: Hypoxia is a key stress that triggers apoptosis in various tumors, including epithelial ovarian cancer (EOC). Previous researches identified a hypoxia-upregulated lncRNA named “a natural antisense transcript of hypoxia-inducible factor 1 (aHIF)” in some tumors. However, the contribution of aHIF to EOC remains unclear. Here, we aimed to investigate the expression, function, and underlying mechanisms of aHIF in EOC progression under hypoxia.
Materials and methods: Expression levels of aHIF in EOC tissues were tested. In vitro and in vivo assays were conducted to explore the function and mechanism of aHIF in hypoxia-induced EOC progression.
Results: aHIF levels were increased in EOC tissues and were upregulated by hypoxia in EOC cells. Functional data revealed that aHIF knockdown accelerated cell apoptosis under hypoxia and inhibited EOC tumorigenesis and tumor growth in vivo. Additionally, aHIF overexpression inhibited cell apoptosis and enhanced cell proliferation under hypoxia in EOC. Mechanistically, the dysregulation of certain key mitochondrial apoptosis pathway-related genes, including Bcl-2, Bax, Caspase-7, and Caspase-9, may partially explain aHIF-regulated EOC apoptosis and growth under hypoxia.
Conclusion: These data provide the first convincing evidence that aHIF may inhibit EOC apoptosis and thereby promote tumor growth through activation of the mitochondrial apoptosis pathway under hypoxia. Our findings help clarify the role of lncRNA in hypoxia-induced EOC progression.
Keywords: lncRNA, hypoxia, microenvironment, tumor growth
Introduction
Epithelial ovarian cancer (EOC) is an aggressive female reproductive tumor characterized by uncontrollable proliferation and invasion and high mortality and recurrence rates. Although therapeutic options have improved, the prognosis remains uniformly poor.1–3 Recent evidence suggests that hypoxia is a pathophysiological hallmark that commonly exists in solid tumors and plays a critical role in tumor progression.4–6 As a solid tumor, EOC occurrence and progression may be mediated by hypoxia. Therefore, fully elucidating the contribution of hypoxia to EOC is of great importance, which may help provide promising therapeutic options.
In hypoxic microenvironments, tumor cells must overcome certain obstacles such as cell apoptosis, cell division, growth, and progression.7 This process can be accomplished by the activation of hypoxia-responsive target genes, which play key roles in cancer progression. Previous studies have mainly focused on the roles of hypoxia-responsive coding RNAs and miRNAs in cancer progression.8–10 However, in addition to these coding RNAs and miRNAs, a limited number of lncRNAs (>200 nucleotides), such as H19,11 UCA1,6 HOTAIR,12 NEAT1,13 linc-RoR,14 EFNA3,15 lncRNA-LET,16 and MALAT1,17 have also been reported to be differentially expressed in response to hypoxia and to critically impact hypoxic tumor progression. Therefore, elucidating the roles of hypoxia-responsive lncRNAs and lncRNA-related mechanisms may help fully clarify the molecular biology underlying hypoxia-induced cancer progression.
Recently, a natural antisense transcript of hypoxia-inducible factor 1 (aHIF), which is complementary to the 3′-untranslated region of HIF-1α mRNA,18 has garnered a substantial amount of attention. aHIF has been found in renal cancer cells and lymphocytes18 and reportedly facilitates cancer cell survival and adaptation under hypoxia.18,19 In addition, aHIF overexpression was reported to be correlated with aggressive proliferation and poor prognosis in breast cancer20 and with metastatic potential in paraganglioma.21 However, despite these findings, the involvement of aHIF in EOC and the relationship between hypoxia, aHIF, and EOC development remain undetermined.
In this study, we found that aHIF was elevated in EOC tissues and was induced by hypoxia in EOC cells. Functional data revealed that aHIF knockdown accelerated cell apoptosis under hypoxia and inhibited EOC tumorigenesis and tumor growth in vivo. Additionally, aHIF overexpression inhibited EOC cell apoptosis and enhanced cell proliferation under hypoxia. Mechanistically, the dysregulation of certain key mitochondrial apoptosis pathway-related genes, including Bcl-2, Bax, Caspase-7, and Caspase-9, may partially explain aHIF-regulated EOC apoptosis and growth under hypoxia. These results provide strong evidence that aHIF may inhibit EOC apoptosis and thereby promote tumor growth through the activation of the mitochondrial apoptosis pathway under hypoxia. Our findings help clarify the function of lncRNA in hypoxia-induced EOC progression.
Materials and methods
Cell lines and hypoxic treatment
Human EOC SKOV3 and HO8910 cells were gifts from the University of Texas MD Anderson Cancer Center (Houston, TX, USA). Roswell Park Memorial Institute 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) was used to culture EOC cells in a humidified 5% CO2 atmosphere at 37°C. For hypoxic treatment, cells were incubated with a gas mixture (1% O2, 5% CO2, and 94% N2) in a three-chamber air incubator for 2, 4, 6, or 12 hours.
Tissue samples
The study was approved by the research ethics committee of Obstetrics and Gynecology Hospital, Fudan University, China (Kyy2015-1). Written informed consent was obtained from each patient. Forty EOC tissues, including 12 early-stage EOC tissues (5 Federation of Gynecology and Obstetrics [FIGO] stage I tumors and 7 FIGO stage II tumors) and 28 advanced-stage EOC tissues (24 FIGO stage III tumors and 4 FIGO stage IV tumors), were obtained during surgical operations from the Obstetrics and Gynecology Hospital of Fudan University from January 2012 to December 2014. All tissues were pathologically confirmed and were selected from patients with no history of any preoperative therapies. Additionally, 20 normal ovarian epithelial tissues were collected during hysterectomy and oophorectomy from patients with uterine fibroids. Tissues were selected from participants with no history of ovarian pathology or ovarian surgery. All fresh tissues were immediately stored at −80°C.
Quantitative reverse transcriptase-PCR (qRT-PCR)
RNA extraction and qRT-PCR were conducted as previously described.22 Primer sequences are listed in Table 1. All assays were performed in triplicate.
  | Table 1 Primer sequences of the studied genes |
Establishment of stable aHIF knockdown or overexpression cell lines
The target sequences of the two aHIF-shRNAs were as follows: 5′-GGCACTTCCTACATAATTT-3′ (shRNA1) and 5′-TCTGTTAATGGGAACAGAT-3′ (shRNA2). Based on these sequences, lentiviral vectors (aHIF-knockdown [KD] 1 and aHIF-KD2) were designed by Hanyin Co. (Shanghai, China). The negative control (NC) lentivirus was also provided by Hanyin Co. To stably overexpress aHIF, the full-length aHIF was synthesized by Hanyin Co. and cloned into a lentiviral vector, named aHIF-overexpression (OE); the control viruses (named LV-Vector) were also provided by Hanyin Co. After infection with virus and polybrene, puromycin was used to select the positive clones for 14 days and the following stable cell lines were established: SKOV3-NC, SKOV3-KD1, SKOV3-KD2, HO8910-NC, HO8910-KD1, HO8910-KD2, SKOV3-Vector, SKOV3-OE, HO8910-Vector, and HO8910-OE.
Cell apoptosis analysis
SKOV3-NC, SKOV3-KD1, SKOV3-KD2, HO8910-NC, HO8910-KD1, HO8910-KD2, SKOV3-Vector, SKOV3-OE, HO8910-Vector, and HO8910-OE cells were maintained under hypoxia for 12 hours. Cell apoptosis was tested using Annexin V and a 7-aminoactinomycin D staining kit (BD Pharmingen) following the manufacturer’s instructions. The cell apoptosis rate was analyzed by flow cytometry. All assays were performed in triplicate.
Cell viability assay
SKOV3-Vector, SKOV3-OE, HO8910-Vector, and HO8910-OE cells were cultured. Cell viability assays were conducted with a Cell Counting Kit-8 (Dojindo, Japan). The cell numbers per well were measured by the absorbance (450 nm) of reduced 2-(2-methoxy-4-nitrophenyl)–3-(4-nitrophenyl)–5-(2,4-isulfophenyl)–2H-tetrazolium, monosodium salt at 1, 2, 3, 4, and 5 days. Each experiment was repeated thrice.
Western blot assay
The Western blot assay was conducted as previously described.22 The primary antibodies were as follows: anti-Bcl-2 (Santa Cruz), anti-Bax (Cell Signaling Technology), anti-Caspase-9 (Abways), anti-Caspase-7 (Abways), and β-actin (Proteintech). The loading control was β-actin.
Xenograft tumors in nude mice
Four- to six-week-old (weighing 20–22 g) female BALB/c athymic nude mice were provided by Slac Laboratory Animal Co. Ltd (Shanghai, China). The mice were subcutaneously injected with SKOV3-KD1, SKOV3-KD2, and SKOV3-NC cells (1×106) and divided into three groups (n=6 in each group). After all the mice were euthanized, the tumors were weighed. The animal experiments were approved by the Institutional Animal Care and Use Committee of Fudan University (20150557A185).
Statistical analysis
Data were shown as the mean±standard error and analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). A Tukey’s test was used for multiple comparisons in conjunction with one-way ANOVA. All results were considered significant at two-sided P-values <0.05 (P<0.05).
Results
aHIF is highly expressed in EOC tissues
To first investigate whether aHIF has any clinical implications in EOC, we performed qRT-PCR to test the expression pattern of aHIF in EOC. We found that aHIF expression was significantly elevated in 40 EOC tissues compared to that in 20 normal controls (Figure 1A). Moreover, aHIF levels were substantially increased in patients at advanced FIGO stages compared with patients at early FIGO stages (Figure 1B). These data suggest that increased aHIF expression may contribute to EOC aggressiveness.
aHIF is upregulated by hypoxia in EOC cell lines
Based on the above clinical observations and the commonness of hypoxia in solid tumors, it is logical to hypothesize that increased aHIF expression may contribute to EOC aggressiveness under hypoxic conditions. To confirm this hypothesis, we initially performed qRT-PCR to examine the expression pattern of aHIF in EOC SKOV3 and HO8910 cells under hypoxia. We observed that aHIF levels were significantly increased after hypoxic culture for 2 hours and was maintained over the next 12 hours in both SKOV3 and HO8910 cells (Figure 2). These data suggest that hypoxia can induce aHIF upregulation in EOC cells.
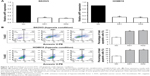
AHIF knockdown promotes cell apoptosis under hypoxia in EOC
Since tumor cells must overcome certain obstacles, such as cell apoptosis, under hypoxia, we wondered whether aHIF influenced EOC cell apoptosis to grow and develop under hypoxic conditions. Initially, we constructed aHIF-knockdown SKOV3 and HO8910 cell lines and found that both aHIF-KD1 and aHIF-KD2 efficiently silenced aHIF expression (Figure 3A). Flow cytometry results revealed that aHIF suppression enhanced the total number of apoptotic cells in SKOV3 and HO8910 cells compared with their corresponding controls under hypoxia (Figure 3B), suggesting that aHIF knockdown promoted EOC cell apoptosis under hypoxia.
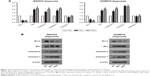
aHIF regulates EOC cell apoptosis under hypoxia partly through Bcl-2, Bax, Caspase-7, and Caspase-9
To investigate the possible mechanisms through which aHIF influences EOC cell apoptosis under hypoxia, we performed qRT-PCR and Western blot to test the levels of certain key mitochondrial apoptosis pathway-related genes, including Bcl-2, Bax, Caspase-3, Caspase-7, and Caspase-9. qRT-PCR results revealed that Bax, Caspase-7, and Caspase-9 mRNA expression levels were upregulated, whereas Bcl-2 expression was downregulated after aHIF knockdown in hypoxic SKOV3 and HO8910 cells (Figure 4A). Consistent with the qRT-PCR results, upregulation of Bax, Caspase-7, and Caspase-9 and downregulation of Bcl-2 protein levels were observed in hypoxic SKOV3 and HO8910 cells following aHIF knockdown (Figure 4B). These results indicate that under hypoxia, the mitochondrial apoptosis pathway may be activated by aHIF, and that aHIF regulates EOC cell apoptosis under hypoxia partly through Bcl-2, Bax, Caspase-7, and Caspase-9.
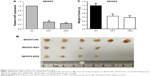
AHIF knockdown inhibits EOC tumor growth in vivo
To extend our in vitro findings, we established a mouse xenograft model using SKOV3-KD1, SKOV3-KD2, and SKOV3-NC cells. After the mice were sacrificed, we first confirmed that aHIF expression was reduced in SKOV3-KD cell tumors compared with SKOV3-NC cell tumors (Figure 5A). Furthermore, we observed that aHIF knockdown reduced tumor formation frequency (SKOV3-NC, 6/6; SKOV3-KD1, 4/6; and SKOV3-KD2, 4/6), as shown in Figure 5B. Additionally, the average tumor weight was much lower in the SKOV3-KD1 and SKOV3-KD2 groups than in the control group (Figure 5C). Collectively, these data confirm that aHIF knockdown inhibits EOC growth in vivo and imply that aHIF demonstrates oncogenic activities in hypoxic ovarian tumors.
Overexpression of aHIF inhibits cell apoptosis and enhances cell proliferation under hypoxia in EOC
To further confirm the roles of aHIF in regulating EOC proliferation and apoptosis under hypoxia, we conducted gain-of-function experiments. As shown in Figure 6A, aHIF overexpression was confirmed using qRT-PCR. Cell Counting Kit-8 assays showed that SKOV3-OE and HO8910-OE cells had increased cell viability compared with their controls under hypoxia (Figure 6B). Apoptosis assays showed that SKOV3-OE and HO8910-OE cells had decreased number of apoptotic cells compared with their controls under hypoxia (Figure 6C). Together, these results suggest that aHIF overexpression inhibits cell apoptosis and enhances cell proliferation under hypoxia in EOC and indicate that aHIF promotes EOC progression under hypoxia.
Discussion
Hypoxia is a pathophysiological hallmark that commonly exists in tumor tissues, including EOC.6 Hypoxia is known to promote cancer progression by activating hypoxia-responsive target genes. Numerous hypoxia-induced protein-coding genes and miRNAs have been reported to contribute to this property,8–10 but the involvement of hypoxia-induced lncRNAs in cancer progression remains limited. In this study, we observed that aHIF is upregulated in EOC cells under hypoxia, and that aHIF plays important roles in hypoxia-induced EOC apoptosis and growth by regulating certain mitochondrial apoptosis pathway-related genes. These results help clarify the role of lncRNA in hypoxia-induced EOC progression.
The lncRNAs have been a popular topic in cancer research.23–25 The exploration of lncRNAs has greatly expanded our understanding of cancer biology. Previous studies discovered a hypoxia-upregulated lncRNA, aHIF, and demonstrated that aHIF contributed to an aggressive malignant phenotype in renal cancer, breast cancer, and paraganglioma.18–21 However, the involvement of aHIF in hypoxia-induced EOC progression remains unclear. Here, we found that aHIF is overexpressed in EOC tissues, especially in the tissue at an advanced FIGO stage, and that high aHIF expression is induced by hypoxia in SKOV3 and HO8910 EOC cells. These results imply that increased aHIF expression may contribute to hypoxia-induced EOC aggressiveness.
Hypoxia is an essential feature of tumor microenvironment.6,7 Under hypoxic conditions, cancer cells must overcome certain obstacles in order to divide, spread, and grow. One of the most important obstacles is to overcome apoptosis.7 Once apoptosis is suppressed, cancer cell and tissue growth increases. Therefore, determining how apoptosis is regulated in hypoxic EOC is important for understanding how this tumor grows and develops. Inspired by this need and based on our findings that aHIF expression is upregulated in EOC tissues and induced by hypoxia, we speculated that aHIF may play certain roles in regulating EOC cell apoptosis under hypoxia. For this, we conducted flow cytometry assays and found that aHIF knockdown promoted cell apoptosis in both hypoxic SKOV3 and HO8910 EOC cells. To extend the in vitro findings, we established a mouse xenograft model and observed that aHIF knockdown inhibited EOC tumorigenesis and tumor growth in vivo. Moreover, to further confirm the effects of aHIF on EOC progression under hypoxia, we performed the gain-of-function experiments and observed that aHIF overexpression inhibited EOC cell apoptosis and enhanced cell proliferation under hypoxia. Collectively, these data indicated that aHIF suppressed EOC cell apoptosis, thereby promoting EOC growth, and highlighted that aHIF exhibits oncogenic activity during hypoxia-induced EOC growth.
Furthermore, to investigate the preliminary mechanism underlying aHIF-mediated EOC apoptosis under hypoxic conditions, we detected the expression levels of the key pro-(Caspase-9, Caspase-7, Caspase-3, Bax) and antiapoptotic genes (Bcl-2).26–28 The qRT-PCR and Western blot assay results both showed that upregulation of Caspase-9, Caspase-7, and Bax and downregulation of Bcl-2 expression were observed in hypoxic SKOV3 and HO8910 cells following aHIF knockdown. These results indicated that under hypoxic conditions, the mitochondrial apoptosis pathway may be activated by aHIF, and aHIF-induced Bcl-2, Bax, Caspase-7, and Caspase-9 dysregulation may partially explain aHIF-mediated EOC apoptosis and proliferation under hypoxia.
A limitation of the present study is that the exact mechanism through which aHIF regulates Bcl-2, Bax, Caspase-7, and Caspase-9 expression was not explored. Future studies should focus on exploring the exact molecular biology of the “hypoxia–aHIF–Caspase-9/Caspase-7/Bax/Bcl-2” pathway.
Conclusion
Our data demonstrated that aHIF was elevated in EOC tissues, and that aHIF was upregulated by hypoxia in EOC cells. Functional data showed that aHIF knockdown accelerated cell apoptosis under hypoxia and inhibited EOC tumorigenesis and tumor growth in vivo. Moreover, aHIF overexpression inhibited cell apoptosis and enhanced cell proliferation under hypoxia in EOC. Mechanistically, the dysregulation of mitochondrial apoptosis pathway-related genes, including Bcl-2, Bax, Caspase-7, and Caspase-9, may partially explain aHIF-mediated EOC apoptosis and growth under hypoxia. These findings provide strong evidence that under hypoxia, aHIF may inhibit EOC apoptosis and thereby promote tumor growth through the activation of the mitochondrial apoptosis pathway. These results help clarify the function of lncRNA in hypoxia-induced EOC malignant development.
Acknowledgments
This study was funded by the National Natural Science Foundation for Young Scholars of China (to J-JQ; 81502240), the National Natural Science Foundation of China (to K-QH; 81571404), and the Shanghai Science and Technology Innovation Foundation (to K-QH; 16411950500).
Author contributions
K-QH was involved in the study design. J-JQ was a major contributor in writing the manuscript. X-YT analyzed the patient data. X-JL and T-TZ conducted the in vitro and in vivo experiments. All authors approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Physician. 2016;93(11):937–944. | ||
Palmirotta R, Silvestris E, D’Oronzo S, Cardascia A, Silvestris F. Ovarian cancer: novel molecular aspects for clinical assessment. Crit Rev Oncol Hematol. 2017;117:12–29. | ||
Hollis RL, Gourley C. Genetic and molecular changes in ovarian cancer. Cancer Biol Med. 2016;13(2):236–247. | ||
Shih JW, Kung HJ. Long non-coding RNA and tumor hypoxia: new players ushered toward an old arena. J Biomed Sci. 2017;24(1):53. | ||
Parks SK, Cormerais Y, Pouysségur J. Hypoxia and cellular metabolism in tumour pathophysiology. J Physiol. 2017;595(8):2439–2450. | ||
Xue M, Li X, Li Z, Chen W. Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1α-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol. 2014;35(7):6901–6912. | ||
Liu M, Fu Z, Wu X, du K, Zhang S, Zeng L. Inhibition of phospholipaseD2 increases hypoxia-induced human colon cancer cell apoptosis through inactivating of the PI3K/AKT signaling pathway. Tumour Biol. 2016;37(5):6155–6168. | ||
Baker AF, Malm SW, Pandey R, et al. Evaluation of a hypoxia regulated gene panel in ovarian cancer. Cancer Microenviron. 2015;8(1):45–56. | ||
Kinose Y, Sawada K, Nakamura K, et al. The hypoxia-related microRNA miR-199a-3p displays tumor suppressor functions in ovarian carcinoma. Oncotarget. 2015;6(13):11342–11356. | ||
Bertero T, Rezzonico R, Pottier N, Mari B. Impact of microRNAs in the cellular response to hypoxia. Int Rev Cell Mol Biol. 2017;333:91–158. | ||
Wu W, Hu Q, Nie E, et al. Hypoxia induces H19 expression through direct and indirect Hif-1α activity, promoting oncogenic effects in glioblastoma. Sci Rep. 2017;7:45029. | ||
Zhou C, Ye L, Jiang C, Bai J, Chi Y, Zhang H. Long noncoding RNA HOTAIR, a hypoxia-inducible factor-1α activated driver of malignancy, enhances hypoxic cancer cell proliferation, migration, and invasion in non-small cell lung cancer. Tumour Biol. 2015;36(12):9179–9188. | ||
Choudhry H, Albukhari A, Morotti M, et al. Tumor hypoxia induces nuclear paraspeckle formation through HIF-2α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene. 2015;34(34):4482–4490. | ||
Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127(Pt 7):1585–1594. | ||
Gómez-Maldonado L, Tiana M, Roche O, et al. EFNA3 long noncoding RNAs induced by hypoxia promote metastatic dissemination. Oncogene. 2015;34(20):2609–2620. | ||
Yang F, Huo XS, Yuan SX, et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell. 2013;49(6):1083–1096. | ||
Sallé-Lefort S, Miard S, Nolin MA, et al. Hypoxia upregulates Malat1 expression through a CaMKK/AMPK/HIF-1α axis. Int J Oncol. 2016;49(4):1731–1736. | ||
Thrash-Bingham CA, Tartof KD. aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst. 1999;91(2):143–151. | ||
Choudhry H, Harris AL, Mcintyre A. The tumour hypoxia induced non-coding transcriptome. Mol Aspects Med. 2016;47–48:3535–3553. | ||
Cayre A, Rossignol F, Clottes E, Penault-Llorca F. aHIF but not HIF-1alpha transcript is a poor prognostic marker in human breast cancer. Breast Cancer Res. 2003;5(6):R223–RR230. | ||
Span PN, Rao JU, Oude Ophuis SB, et al. Overexpression of the natural antisense hypoxia-inducible factor-1alpha transcript is associated with malignant pheochromocytoma/paraganglioma. Endocr Relat Cancer. 2011;18(3):323–331. | ||
Qiu J, Ye L, Ding J, et al. Effects of oestrogen on long noncoding RNA expression in oestrogen receptor alpha-positive ovarian cancer cells. J Steroid Biochem Mol Biol. 2014;141:60–70. | ||
Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981. | ||
Hosseini ES, Meryet-Figuiere M, Sabzalipoor H, Kashani HH, Nikzad H, Asemi Z. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol Cancer. 2017;16(1):107. | ||
Rao A, Rajkumar T, Mani S. Perspectives of long non-coding RNAs in cancer. Mol Biol Rep. 2017;44(2):203–218. | ||
Li P, Zhou L, Zhao T, et al. Caspase-9: structure, mechanisms and clinical application. Oncotarget. 2017;8(14):23996–24008. | ||
Lakhani SA, Masud A, Kuida K, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311(5762):847–851. | ||
Li H, Lv B, Kong L, et al. Nova1 mediates resistance of rat pheochromocytoma cells to hypoxia-induced apoptosis via the Bax/Bcl-2/caspase-3 pathway. Int J Mol Med. 2017;40(4):1125–1133. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.