Back to Journals » Infection and Drug Resistance » Volume 16
Nasopharyngeal Mycobacterium abscessus Infection: A Case Report and Literature Review
Authors Kaji M , Namkoong H , Nagao G, Azekawa S, Nakagawara K, Tanaka H , Morita A , Asakura T , Kamata H, Uwamino Y, Yoshida M, Fukunaga K, Hasegawa N
Received 3 April 2023
Accepted for publication 2 June 2023
Published 20 June 2023 Volume 2023:16 Pages 3955—3963
DOI https://doi.org/10.2147/IDR.S415197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Masanori Kaji,1 Ho Namkoong,2 Genta Nagao,1 Shuhei Azekawa,1 Kensuke Nakagawara,1 Hiromu Tanaka,1 Atsuho Morita,1 Takanori Asakura,1,3,4 Hirofumi Kamata,1 Yoshifumi Uwamino,2,5 Mitsunori Yoshida,6 Koichi Fukunaga,1 Naoki Hasegawa2
1Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, Tokyo, Japan; 2Department of Infectious Diseases, Keio University School of Medicine, Tokyo, Japan; 3Department of Clinical Medicine (Laboratory of Bioregulatory Medicine), Kitasato University School of Pharmacy, Tokyo, Japan; 4Department of Respiratory Medicine, Kitasato University, Kitasato Institute Hospital, Tokyo, Japan; 5Department of Laboratory Medicine, Keio University School of Medicine, Tokyo, Japan; 6Department of Mycobacteriology, Leprosy Research Center, National Institute of Infectious Diseases, Tokyo, Japan
Correspondence: Ho Namkoong, Department of Infectious Diseases, Keio University School of Medicine, 35 Shinanomachi, Shinjuku, Tokyo, 160-8582, Japan, Tel + 3-3353-1211 61164, Fax + 3-3353-5958, Email [email protected]
Background: Mycobacterium abscessus (M. abscessus) is a rapidly growing bacterium (RGM) that causes refractory pulmonary and extrapulmonary infections. However, studies investigating pharyngeal and laryngeal M. abscessus infections are limited.
Case Presentation: A 41-year-old immunocompetent woman complaining of bloody sputum was referred to our hospital. Although her sputum culture tested positive for M. abscessus subsp. abscessus, radiological findings were not indicative of pulmonary infection or sinusitis. Further diagnostic workup, including laryngeal endoscopy and positron emission tomography/computed tomography (PET/CT), confirmed the presence of nasopharyngeal M. abscessus infection. The patient was initially treated with intravenous amikacin, imipenem/cilastatin, azithromycin, and clofazimine for 28 days, after which the patient was provided with amikacin, azithromycin, clofazimine, and sitafloxacin for four months. After the completion of antibiotic therapy, the patient showed negative results on sputum smear and culture and normal findings on PET/CT and laryngeal endoscopy. Whole-genome sequencing of this strain revealed that it belonged to the ABS-GL4 cluster, which has a functional erythromycin ribosomal methylase gene, although it is not a major lineage in non-cystic fibrosis (CF) patients in Japan and Taiwan and in CF patients in European countries. We conducted a literature review and identified seven patients who developed pharyngeal/laryngeal non-tuberculous mycobacterium (NTM) infection. Four of the eight patients had a history of immunosuppressant use, including steroids. Seven of the eight patients responded well to their treatment regimens.
Conclusion: Patients whose sputum culture tests are positive for NTM and who meet the diagnostic criteria for NTM infection but do not have intrapulmonary lesions should be evaluated for otorhinolaryngological infections. Our case series revealed that immunosuppressant use is a risk factor for pharyngeal/laryngeal NTM infection and that patients with pharyngeal/laryngeal NTM infections respond relatively well to antibiotic therapy.
Keywords: pharyngeal NTM, laryngeal NTM, whole-genome sequencing
Introduction
Nontuberculous mycobacteria (NTM) are a group of mycobacteria distinct from tuberculosis and leprosy, with approximately 200 species identified to date.1 The incidence of NTM disease has increased in recent years, surpassing that of tuberculosis in Japan.2 NTM are ubiquitous environmental organisms found in various locations, including soil and water. Immunocompromised individuals and individuals with genetic disorders are more prone to acquire NTM infections.3 However, immunocompetent individuals may also acquire NTM infections. NTM primarily cause lung diseases; however, extrapulmonary NTM infections, including skin infections, superficial lymphadenitis, and disseminated lesions, are common in immunosuppressed patients. Although NTM infections of the head and neck region – such as otitis media – have been reported, the number of patients who developed pharyngeal/laryngeal NTM infections were few.
Rapidly growing mycobacteria (RGM) are more frequently associated with extrapulmonary NTM infections than with pulmonary NTM infections.4 Mycobacterium abscessus (M. abscessus) is a type of RGM and is divided into three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii. Most M. abscessus subsp. abscessus and M. abscessus subsp. bolletii carry the erythromycin ribosomal methylase [erm(41)] gene which confers resistance to macrolide antibiotics and are therefore treatment resistant.5 Mycobacterium abscessus complex (MABC) causes extrapulmonary lesions on the skin (skin and soft tissue infections), bones, central nervous system, eyes, and blood.6
Herein, we report a rare case of a patient who tested positive for M. abscessus on three succeeding sputum culture tests without developing any lung lesions; M. abscessus in the nasopharynx was diagnosed after performing a close examination. In addition, whole-genome sequencing of M. abscessus was performed, and a literature review of NTM infections of the pharynx and larynx was conducted.
Case Presentation
A 41-year-old woman with a history of cough variant asthma and chronic sinusitis visited her primary care physician in October 2020 with a complaint of bloody sputum. Upon consultation, she was not provided with any immunosuppressive medications, including inhaled corticosteroids. The patient repeatedly underwent nasal irrigation and gargling. Despite treatment with clarithromycin, amoxicillin, and amoxicillin/clavulanic acid, she continued to experience symptoms and increased purulent sputum production. In December 2020, the patient underwent further examinations at a general hospital. Chest computed tomography (CT) did not show any obvious abnormalities, while three sputum culture tests consistently detected the presence of M. abscessus.
She was referred to our department in April 2021 and tested positive for M. abscessus subsp. abscessus on sputum culture. She had worsening symptoms of possible posterior rhinorrhoea (phlegm dripping into the throat) and showed mild sinusitis on sinus CT; hence, M. abscessus infection of the sinuses was suspected. Nasal endoscopy did not show any abnormalities in the nasal cavity, such as purulent nasal discharge. Polymerase chain reaction (PCR) tests for Mycobacterium tuberculosis/NTM and acid-fast bacilli (AFB) cultures of the nasal secretions yielded negative results.
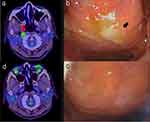
Positron emission tomography/computed tomography (PET/CT), performed in June 2021 to identify the focus of infection, showed 18-fluorodeoxyglucose accumulation with a maximum standardized uptake value of 3.5 in the right nasopharyngeal wall (Figure 1a). Laryngeal endoscopy revealed purulent discharge in the right nasopharynx (Figure 1b). Initially, the purulent nasopharyngeal discharge showed positive results on smear test but negative for AFB on culture test. Repeat culture tests of purulent discharge collected by laryngeal endoscopy were performed; the purulent discharge collected in December 2021 tested positive for M. abscessus subsp. abscessus leading to a diagnosis of M. abscessus disease of the nasopharynx. Drug susceptibility tests indicated that the isolate was susceptible to amikacin and resistant to macrolides (Table 1). Specimens collected from the patient’s kitchen and bathroom tested negative for AFB.
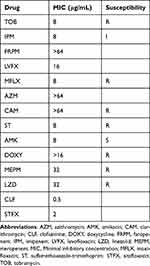 |
Table 1 Antimicrobial Drug Susceptibility |
The patient was hospitalized and treated with amikacin, imipenem/cilastatin, azithromycin, and clofazimine for 28 days. After discharge, the patient was treated with amikacin, azithromycin, clofazimine, and sitafloxacin, a new fluoroquinolone, for four months. Since sitafloxacin’s minimum inhibitory concentration for M. abscessus is reported to be low, it is used in clinical settings in Japan.7 The posterior rhinorrhoea improved, and laryngeal endoscopy performed in February 2022 revealed the absence of purulent discharge in the nasopharynx (Figure 1c). In addition, both sputum smear and culture tests yielded negative results at the end of the patient’s treatment. PET/CT performed in May 2022 confirmed the disappearance of the previously observed abnormal accumulation in the right nasopharyngeal wall (Figure 1d). The patient was followed up in an outpatient clinic and revealed no evidence of recurrence. Whole-genome sequencing of M. abscessus subsp. abscessus constitutively isolated from the patient revealed that all strains belonged to a phylogenetic cluster, ABS-GL4 (Figure 2a). The pairwise SNP distance between these isolates was less than 10 SNPs (Figure 2b). Mutations in the 23S rRNA (rrl) gene conferring macrolide resistance were not detected in these isolates, but they carried the functional erm(41) gene (Figure 2a), which was expected to induce macrolide resistance. In addition, the strains did not harbour the known A1375G substitution in the 16S rRNA (rrs) gene, which confers amikacin resistance (Figure 2a).
Discussion and Conclusions
We report a case of a patient with M. abscessus infection in the nasopharynx. Based on endoscopy and PET/CT findings, the patient was diagnosed with M. abscessus infection confined to the nasopharynx without lung involvement. After initial treatment with amikacin, imipenem/cilastatin, azithromycin, and clofazimine, and maintenance treatment with azithromycin, clofazimine, and sitafloxacin, the patient achieved sputum smear- and culture-negative conversion with improvement of symptoms and endoscopic and PET/CT findings.
Although the precise mode of M. abscessus transmission is not fully understood, M. abscessus, which is prevalent in soil and aquatic environments, is thought to be transmitted to hosts with specific risk factors. These risk factors include being diagnosed with a human immunodeficiency virus infection, having genetic abnormalities in the interferon gamma pathway, using tumour necrosis factor inhibitors, having had previous exposure to broad-spectrum antimicrobials, and smoking. Individuals with lung conditions, such as cystic fibrosis (CF) and bronchiectasis, are also at an increased risk.8 Although none of these risk factors were present in our patient, she repeatedly underwent nasal irrigation and gargling, which may have contributed to the occurrence of infection. However, the tap water sample collected from her bathroom and kitchen showed negative results on smear and culture tests.
M. abscessus causes extrapulmonary lesions on the skin (skin and soft tissue infections), bones, central nervous system, eyes, and blood.6,9 Infections of the upper respiratory tract, such as those of the pharynx, rarely occur, with sinusitis being the most common type of upper respiratory tract infection caused by NTM.10,11 Moreover, the only types of pharyngeal NTM infection identified were nasopharyngitis, reported by Oki et al,12 and hypopharyngitis/laryngitis, reported by Hussin et al.13
The M. abscessus complex is resistant to disinfectants used to minimize the risk of infection during surgery and other procedures. Therefore, they can cause postsurgical and postprocedural infections.5 Oki et al reported a case of M. abscessus nasopharyngitis resulting from the transmission of bacteria from contaminated medical devices that were only washed with tap water, which had been used during repeat nasopharyngeal abrasive therapy for pharyngitis.12 Our patient had no history of dental or otolaryngological treatments.
In cases of NTM infection without lung lesions, the diagnosis of sinusitis should not be based solely on the history of sinusitis or mild CT findings. Instead, a thorough examination of the nasopharyngeal region should be conducted to detect the presence of infection. Moreover, NTM sinusitis should be distinguished from pharyngeal/laryngeal NTM disease, as the treatment approaches for these two conditions differ significantly. Surgical debridement is the preferred treatment for NTM sinusitis,14 while antibiotics are the primary treatment for pharyngeal/laryngeal NTM infection.
We conducted a thorough review of the existing literature. By searching the PubMed database, we identified English articles (Table 2) related to pharyngeal and laryngeal NTM infections. This condition is rare, with only eight cases reported to date, including the case reported here. Of the seven other cases, one involved infection of the pharynx alone,12 one involved infection of both the pharynx and larynx,13 and five involved infection of the larynx alone.15–19 All patients were female, had a median age of 45 years (range: 30–81 years), and only two showed abnormal findings on chest CT.
 |
Table 2 Clinical Characteristics of Eight Patients |
Of the three patients with pharyngeal infection, our patient and that reported by Oki et al12 had a history of physical irritation of the pharynx caused by nasal irrigation and nasopharyngeal abrasive therapy, respectively. Among the five patients with laryngeal lesions only, four had a history of steroid therapy, and two were administered with inhaled steroids. Irritation of the pharyngeal mucosa and the use of immunosuppressive agents, including steroids, may be potential risk factors for pharyngeal and laryngeal NTM infection. However, immunocompetent patients with no history of immunosuppressive therapy can also be susceptible to nasopharyngeal Mycobacterium abscessus infection.
The strains identified included Mycobacterium kansasii in two patients, M. abscessus subsp. abscessus in one patient, Mycobacterium abscessus subspecies massiliense in one patient, Mycobacterium malmoense in one patient, Mycobacterium fortuitum in one patient, and Mycobacterium avium in one patient. Three patients were infected with RGM.
Of the eight patients, seven responded well to treatment, while one died. The treatment duration varied widely among the patients, ranging from 12 to 17 months.
Although NTM infections are traditionally thought to be acquired from the environment, previous studies have suggested that they may be transmitted directly or indirectly to patients with cystic fibrosis.20 According to Bryant et al,20 identical or nearly identical M. abscessus clones (clusters) have been isolated from patients with cystic fibrosis in a wide range of locations across the United States, Europe, and Australia, thus suggesting the possibility of a recent outbreak of patient-to-patient transmission of M. abscessus within the cystic fibrosis patient community. More than ten such clones, also referred to as clusters, were identified in M. abscessus subsp. abscessus and five in M. abscessus subsp. massiliense. Among these clusters, M. abscessus subsp. abscessus clusters 1 and 2 and M. abscessus subsp. massiliense cluster 1, which were isolated from patients in the United States, Europe, and Australia, were regarded as the dominant circulating clones (DCC) 1, 2, and 3 owing to their capacity for intercontinental transmission.20
In the present study, whole-genome sequencing of M. abscessus isolates from our patient was performed. All strains analysed in this study (KO1157_R1, KO1157_S1, KO1216, KO4696, and KO4697) belonged to the ABS-GL4 cluster. The ABS-GL4 strains were widely isolated from non-CF patients in East Asia and from CF patients in European countries but have not been reported to be the major lineage in either region.20,21 Analysis of drug resistance genes revealed that our strains lacked T28C sequevar and had no rrl and rrs mutation.22–24 The profile of drug resistance genes predicts inducible macrolide resistance and amikacin sensitivity, which corresponds to the clinical course of the present case and the drug susceptibility test.
ABS-GL4 is a lineage that differs from DCC1 (Absc. 1) and DCC2 (Absc. 2) described by Bryant et al in 2016,19 and is closely related to Absc. 8 and 13 reported in the same study. The ABS-GL4 cluster has spread to East Asia and Europe and has no erm(41) T28C sequence.21 A T-to-C substitution at position 28 (T28C) in erm(41) makes the gene non-functional, thus leading to the recovery of macrolide susceptibility.25,26 Therefore, more intensive treatments should be considered for patients infected with ABS-GL4 strains lacking the erm(41) T28C sequence.
The maximum SNP distances between KO1157_R1, KO1157_S1, KO1216, KO4696, and KO4697 were approximately 10. The SNP distance between KO1157_R1 (rough morphotype) and KO1157_S1 (smooth morphotype) was 7 SNPs (Figure 2), suggesting that these strains are identical clones despite having different morphotypes.
M. abscessus complex pulmonary disease (MABC-PD) is a severe infection. According to the report by Kwak et al, the overall treatment success rate for MABC-PD is 45.6%, with success rates of 33.0% for M. abscessus subsp. abscessus and 56.7% for M. abscessus subsp. massiliense.27
Because M. abscessus infection in the nasopharyngeal region rarely occurs, and no clinical guidelines have been established for managing this disease, our patient was treated in accordance with the guidelines for treating M. abscessus pulmonary disease.
The ATS/ERS/ESCMID/IDSA (American Thoracic Society/European Respiratory Society/European Society of Clinical Microbiology and Infectious Diseases/Infectious Diseases Society of America) clinical practice guidelines recommend a regimen consisting of three or more antimicrobial agents for the initial phase of therapy based on in vitro drug susceptibility. Even in patients with macrolide-resistant M. abscessus pulmonary disease, the guidelines suggest a macrolide-containing regimen, although macrolides should be used because of their immunomodulatory properties and are not considered active drugs in the multidrug regimen.
For the initial phase of therapy, the recommended parenteral agents are amikacin, imipenem (or cefoxitin), and tigecycline, whereas the recommended oral agents are azithromycin (or clarithromycin), clofazimine, and linezolid. The recommended agents for continuation-phase therapy are azithromycin (or clarithromycin), clofazimine, linezolid, and inhaled amikacin.9 Our patient received amikacin, imipenem, azithromycin, and clofazimine in the initial phase of therapy, and azithromycin, clofazimine, and sitafloxacin in the maintenance phase.
The incidence of pharyngeal and laryngeal NTM is expected to increase with the increasing use of immunosuppressive drugs. If sputum is positive for NTM and chest imaging shows an absence of abnormal findings, NTM infection of the upper respiratory tract, including the pharynx and larynx, should be considered as the differential diagnosis. As NTM infection of the upper respiratory tract may respond well to antimicrobial therapy, aggressive treatment rather than follow-up should be considered.
We encountered a case of M. abscessus infection localized to the nasopharynx in an adult with no history of immunosuppression or use of immunosuppressive drugs, which was completely cured with medical therapy alone. As no guidelines have been established for the treatment of NTM disease of the upper respiratory tract, the patient was treated in accordance with the treatment guidelines for M. abscessus pulmonary disease. Further studies on NTM infections of the upper respiratory tract are needed to clarify the pathogenesis of the disease and establish more effective therapies.
Abbreviations
AFB, acid-fast bacilli; CT, computed tomography; DCC, dominant circulating clones; erm, erythromycin ribosomal methylase; MABC, Mycobacterium abscessus complex; MABC-PD, Mycobacterium abscessus complex pulmonary disease; NTM, non-tuberculous mycobacterium; PCR, polymerase chain reaction; PET/CT, positron emission tomography/computed tomography; PET, positron emission tomography; RGM, rapidly growing mycobacterium.
Authors’ Information
Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, Tokyo, Japan. Masanori Kaji, Genta Nagao, Syuhei Azekawa, Kensuke Nakagawara, Hiromu Tanaka, Atsuho Morita, Takanori Asakura, Hirofumi Kamata, and Koichi Fukunaga. Department of Infectious Diseases, Keio University School of Medicine, Tokyo, Japan. Ho Namkoong and Naoki Hasegawa. Department of Laboratory Medicine, Keio University School of Medicine, Tokyo, Japan. Yoshifumi Uwamino. Department of Mycobacteriology, Leprosy Research Center, National Institute of Infectious Diseases, Tokyo, Japan. Mitsunori Yoshida.
Data Sharing Statement
The datasets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the ethics review boards of Keio University Hospital.
Consent for Publication
Written informed consent was obtained from each patient for publication of this study.
Acknowledgments
The authors would like to thank Kei Yamato and Kumiko Matsuzaki for aiding in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was funded by AMED (Japan Agency for Medical Research and Development) (JP22wm0325044, JP21fk0108621, and JP22wm0325055), a JSPS Grant-in-Aid for Young Scientists (21K15667), Grant-in-Aid for Scientific Research (21KK0148 and 22H03122), a JAID Clinical Research Promotion Grant, The Mitsubishi Foundation (Research Grants in the Natural Sciences), and JST PRESTO (JPMJPR21R7).
Disclosure
The authors declare no conflict of interest.
References
1. Matsumoto Y, Kinjo T, Motooka D, et al. Comprehensive subspecies identification of 175 nontuberculous mycobacteria species based on 7547 genomic profiles. Emerg Microbes Infect. 2019;8(1):1043–1053. doi:10.1080/22221751.2019.1637702
2. Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis. 2016;22(6):1116–1117. doi:10.3201/eid2206.151086
3. Honda JR, Alper S, Bai X, Chan ED. Acquired and genetic host susceptibility factors and microbial pathogenic factors that predispose to nontuberculous mycobacterial infections. Curr Opin Immunol. 2018;54:66–73. doi:10.1016/j.coi.2018.06.001
4. Henkle E, Hedberg K, Schafer SD, Winthrop KL. Surveillance of extrapulmonary nontuberculous mycobacteria infections, Oregon, USA, 2007–2012. Emerg Infect Dis. 2017;23(10):1627–1630. doi:10.3201/eid2310.170845
5. Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21(9):1638–1646. doi:10.3201/2109.141634
6. Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49–56. doi:10.1016/j.ijid.2017.05.007
7. Asami T, Aono A, Chikamatsu K, et al. Efficacy estimation of a combination of triple antimicrobial agents against clinical isolates of Mycobacterium abscessus subsp. abscessus in vitro. JAC Antimicrob Resist. 2021;3(1):dlab004. doi:10.1093/jacamr/dlab004
8. Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi:10.1038/s41579-020-0331-1
9. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
10. Nabi S, Marglani OA, Javer AR. Mycobacterium Avium-intracellulare Sinusitis. J Otolaryngol Head Neck Surg. 2010;39(5):E51–E55.
11. Hicks MD, Karempelis PS, Janus SC. Mycobacterium chelonae sinusitis in an immunocompetent adult. JAMA Otolaryngol Head Neck Surg. 2016;142(8):805–806. doi:10.1001/jamaoto.2016.1865
12. Oki Y, Hatakeyama H, Komatsu M, et al. A first case report of nasopharyngeal Mycobacterium abscessus subspecies massiliense infection. Eur J Med Res. 2021;26(1):109. doi:10.1186/s40001-021-00578-8
13. Hussin N, Mat Baki M, Sani A. Chronic large non healing ulcer: non-tuberculous mycobacterial infection of the laryngopharynx. Korean J Fam Med. 2017;38(5):303–306. doi:10.4082/kjfm.2017.38.5.303
14. Mullin D, Jothi S, Healy D. Mycobacterium chelonae infections involving the head and neck. Ann Otol Rhinol Laryngol. 2009;118(10):714–720. doi:10.1177/000348940911801006
15. Lehman B, Procop GW, Silva Merea V, Harrington SM, Mawhorter SD, Benninger MS. Chronic laryngitis caused by Mycobacterium kansasii in a traveler. Laryngoscope. 2019;129(11):2534–2536. doi:10.1002/lary.27952
16. McEwan JA, Mohsen AH, Schmid ML, McKendrick MW. A hoarse voice: atypical mycobacterial infection of the larynx. J Laryngol Otol. 2001;115(11):920–922. doi:10.1258/0022215011909369
17. Yan K, Taxy JB, Paintal A, Friedman AD. Atypical laryngeal infections: localized lesions from unusual organisms may simulate malignancy. Ann Otol Rhinol Laryngol. 2020;129(1):82–86. doi:10.1177/0003489419875755
18. Al-Zahid S, Wright T, Reece P. Laryngeal Inflammatory Pseudotumour Secondary to Mycobacterium kansasii. Case Rep Pathol. 2018;2018:9356243. doi:10.1155/2018/9356243
19. Wang BY, Amolat MJ, Woo P, Brandwein-Gensler M. Atypical mycobacteriosis of the larynx: an unusual clinical presentation secondary to steroids inhalation. Ann Diagn Pathol. 2008;12(6):426–429. doi:10.1016/j.anndiagpath.2007.04.011
20. Bryant JM, Grogono DM, Rodriguez-Rincon D, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science. 2016;354(6313):751–757. doi:10.1126/science.aaf8156
21. Yoshida M, Chien JY, Morimoto K, et al. Molecular epidemiological characteristics of Mycobacterium abscessus complex derived from non-cystic fibrosis patients in Japan and Taiwan. Microbiol Spectr. 2022;10(3):e0057122. doi:10.1128/spectrum.00571-22
22. Boeck L, Burbaud S, Skwark M, et al. Mycobacterium abscessus pathogenesis identified by phenogenomic analyses. Nat Microbiol. 2022;7(9):1431–1441. doi:10.1038/s41564-022-01204-x
23. Lipworth S, Hough N, Leach L, et al. Whole-genome sequencing for predicting clarithromycin resistance in Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63(1):e01204–e01218.
24. Lipworth S, Hough N, Buchanan R, et al. Improved performance predicting clarithromycin resistance in Mycobacterium abscessus on an independent data set. Antimicrob Agents Chemother. 2019;63(8):e00400–e00419.
25. Nash KA, Brown-Elliott BA, Wallace RJ. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367–1376. doi:10.1128/AAC.01275-08
26. Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol. 2015;53(4):1211–1215. doi:10.1128/JCM.02950-14
27. Kwak N, Dalcolmo MP, Daley CL, et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J. 2019;54(1):1801991. doi:10.1183/13993003.01991-2018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.