Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Naples Prognostic Score is an Independent Prognostic Factor in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma
Authors Xie YM, Lu W, Cheng J, Dai M, Liu SY, Wang DD , Fu TW, Ye TW , Liu JW, Zhang CW, Huang DS, Liang L
Received 19 April 2023
Accepted for publication 29 August 2023
Published 4 September 2023 Volume 2023:10 Pages 1423—1433
DOI https://doi.org/10.2147/JHC.S414789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Ya-Ming Xie,1 Wenfeng Lu,2 Jian Cheng,1 Mugen Dai,3 Si-Yu Liu,4 Dong-Dong Wang,1 Tian-Wei Fu,1 Tai-Wei Ye,1 Jun-Wei Liu,1 Cheng-Wu Zhang,1 Dong-Sheng Huang,1 Lei Liang1
1Department of Hepatobiliary & Pancreatic Surgery and Minimally Invasive Surgery, General Surgery, Cancer Center, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University), Shanghai, People’s Republic of China; 3Department of Gastroenterology, The Fifth Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, People’s Republic of China; 4Department of Laboratory Medicine, The Key Laboratory of Imaging Diagnosis and Minimally Invasive Interventional Research of Zhejiang Province, Zhejiang University Lishui Hospital, Lishui, Zhejiang, People’s Republic of China
Correspondence: Lei Liang; Dong-Sheng Huang, Department of Hepatobiliary & Pancreatic Surgery and Minimally Invasive Surgery, General Surgery, Cancer Center, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, 310014, People’s Republic of China, Email [email protected]; [email protected]
Background: Nutritional and inflammatory status has been reported to be associated with the prognosis of hepatocellular carcinoma (HCC), but many studies did not include all biomarkers simultaneously. The present study aimed to determine the impact of Naples prognostic score (NPS) on the long-term survival in patients undergoing hepatectomy for HCC.
Methods: Patients with HCC after curative resection were eligible. Then, all patients were stratified into three groups according to the NPS. Clinical features and survival outcomes were compared among the three groups. Independent prognostic factors were determined by COX analysis. The time dependent receiver operating characteristic (ROC) curves were used to compare prognostic performance with other immunonutrition scoring systems.
Results: A total of 476 patients were enrolled eventually. Baseline characteristics showed that patients with higher NPS had a higher proportion of poor liver function and advanced tumor features. Accordingly, Kaplan-Meier survival curves showed that patients with higher NPS had a lower rate of overall survival (OS) and recurrence-free survival (RFS). Multivariable COX analysis demonstrated that NPS was an independent risk factor of OS (NPS group 2 vs 1: HR=1.958, 95% CI: 1.038– 3.369, p = 0.038; NPS group 3 vs 1: HR=2.608, 95% CI: 1.358– 5.008, p=0.004, respectively) and RFS (NPS group 2 vs 1: HR=2.014, 95% CI: 1.299-2-3.124, p=0.002; NPS group 3 vs 1: HR=2.002, 95% CI: 1.262– 3.175, p=0.003, respectively). The time-dependent ROC curve showed that NPS was superior to other models in prognostic performance and discriminatory power for long-term survival (median AUC 0.675, 95% CI: 0.586– 0.712, P < 0.05).
Conclusion: The NPS is a simple tool strongly associated with long-term survival in patients undergoing curative hepatectomy for HCC.
Keywords: hepatocellular carcinoma, Naples prognostic score, nutritional indicator, inflammatory indicator, prognostic performance
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death globally, and its rate of incidence is still increasing.1 Hepatectomy is the most commonly chosen radical treatment for patients with removable HCC. However, long-term survival remains far from satisfactory. Postoperative recurrence occurs in almost 70% of the patient,2 with a 5-year survival rate of merely 19.6%.3 Despite the identification of some clinicopathological associated risk factors that are significantly associated with HCC prognosis, their predictive value does not produce the expected results for a variety of reasons.
Inflammatory and nutritional indicators have gained attention in recent years as prognostic correlates for predicting prognosis in a variety of cancers, especially in HCC.4,5 Previous studies indicate that HCC is an inflammation-related malignant tumor, and inflammation is involved in the occurrence and development of HCC.5,6 Meanwhile, the liver is an important organ involved in nutrient metabolism and protein production. For patients with HCC, nutritional metabolism is often affected by cirrhosis, as well as the huge requirements of the tumor itself, which often leads to nutritional deficiency in patients. Previous studies have shown that the nutritional status of patients can be reflected through the assessment of fat and muscle components, such as triceps skinfold thickness measurement,7 and it is related to the prognosis of HCC.8,9 However, this method has high requirements for clinicians, and the assessment is relatively subjective, so it cannot be unified in the clinic.
A series of nutrition-related or inflammation-related indicators based on serological tests, such as the controlled nutritional status score (CONUT),10,11 prognostic nutritional index (PNI),12 systemic inflammation score (SIS),13 neutrophil-lymphocyte ratio (NLR),14–16 platelet-lymphocyte ratio (PLR),17 and lymphocyte-monocyte ratio (LMR)14–16 were reported to be correlated with the prognosis of patients with HCC. These aforementioned indicators of inflammation and/or nutrition are, however, to some extent inadequate and the results remain controversial. Thus, there is an urgent need for a comprehensive prognostic model that integrates indicators related to inflammation and nutrition.
The Naples Prognostic Score (NPS), a new prognostic index combining inflammatory with nutritional biomarkers, has been proposed by Galizia et al, including serum albumin, total cholesterol levels, the NLR, and the LMR.18 These scores have been widely used to study a variety of gastrointestinal and other malignancies.19–21 However, the relevance of NPS to the prognosis of patients with HCC after hepatectomy remains uncertain. Therefore, the present study aimed to evaluate the prognostic significance of preoperative NPS on the long-term survival in patients with HCC.
Patients and Methods
Patients
Patients with HCC after curative hepatectomy (R0) were reviewed between January 2013 and October 2018 at Zhejiang Provincial People’s Hospital. Patients with HCC were confirmed by the pathological diagnosis of postoperative specimens. R0 resection was defined as the sub-endoscopic margin being negative. Exclusion criteria were as follows: (1) other concurrent malignancies; (2) preoperative antitumor therapy, (3) inflammatory diseases or other infection (including glomerulonephritis, arthritis, nervous system infection, pneumonia, acute pancreatitis or cholecystitis, etc.) one month before surgery, (4) received preoperative anti-infection or nutrition. Institutional review board at Zhejiang Provincial People’s Hospital approved this study and waived the requirement for patient informed consent because only deidentified data were used. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This study complies with the Declaration of Helsinki.
NPS and Other Scoring Systems
The definition of NPS is the same as previously reported by Galizia et al18 NPS score is the sum of the four metrics’ scores. NPS = serum albumin (≥4 g/dL = 0, <4 g/dL = 1) + total cholesterol concentrations (≥180 mg/dL = 0, <180 mg/dL = 1) + NLR (<2.96 = 0, ≥2.96 = 1) and LMR (≥4.44 = 0, <4.44 = 1). All patients were subsequently stratified into 3 groups: Group 1 (NPS = 0); Group 2 (NPS = 1 or 2), and Group 3 (NPS = 3 or 4), respectively (Figure 1). SIS score = (serum albumin ≥ 4 g/dL and LMR ≥ 4.44 = 0, either serum albumin < 4 g/dL or LMR < 4.44 = 1, both serum albumin < 4 g/dL and LMR < 4.44 = 2).22 CONUT score was calculated by serum albumin and total cholesterol level and the lymphocyte count, and the cut-off value was set at 2 scores as the previous study reported.10 PNI score = 10 x serum albumin (g/dL) + 0.005 x total lymphocyte count. And the cut-off value was set at 44 according to the time-dependent receiver operating characteristic (ROC) curves.
 |
Figure 1 Calculation of the Naples Prognostic Score. |
Followed-Up and Data Collection
After hepatectomy, patients were follow-up visited 2 months per time in the first 2 years, thereafter 3 to 6 months per time. Serum tumor markers (AFP) and abdominal ultrasound were evaluated at each of the follow-up visits. Contrast-enhanced Computed tomography (CT) or magnetic resonance imaging (MRI) was performed every 3 months, or HCC metastasis or recurrence was suspected. Tumor recurrence or metastasis was mainly confirmed on Contrast-enhanced CT or MRI. The most recent follow-up visit took place in December 2022. The following clinical and oncological characteristics of the patients were collected retrospectively from the medical record system at Zhejiang Provincial People’s Hospital. Baseline characteristics: age (>60 vs ≤60 years), sex (male vs female), American Society of Anesthesiologists (ASA) classification, performance status, hepatitis B virus (HBV), cirrhosis, portal hypertension, platelet count (PLT), Child-Pugh A/B, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total cholesterol, neutrophil count, monocyte count lymphocyte count, alpha-fetoprotein (AFP). Pathological manifestations: tumor size (≥5 vs <5 cm), tumor number (solitary vs multiple), surgical margins (≥1 vs <1 cm), microscopic vascular, satellite nodes, tumor differentiation. Intraoperative variables: blood loss and transfusion, and operative time. Portal hypertension was confirmed as the presence of either esophageal varices or splenomegaly along with a decline in platelet count (≤ 100×109/L). All diagnoses of cirrhosis were based on pathological examinations, with the criteria being the presence of diffuse liver fibrosis and pseudolobule formation observed in liver tissue histopathology.
Statistical Methods
Frequencies and percentages were used to express the distribution of each categorical variable, and Pearson’s chi-square or Fisher’s exact tests were adopted to estimate the differences among the variables, as appropriate. Kaplan-Meier survival curves were used to estimate the OS and RFS in each group and compared by Log rank tests. Variables with P < 0.1 in the univariate analysis were enrolled into the forward stepwise multivariate Cox proportional hazard regression analysis. ROC curves and the areas under the curve (AUC) were estimated to evaluate the discriminatory and predictive ability of each scoring system, respectively. P values <0.05 were set as statistically significant. The statistical analysis in this study was performed by using the software of R 4.2.3 (http://www.r-project.org/).
Results
Clinicopathological Characteristics
The baseline characteristics of the 476 patients were shown in Table 1. According to preoperative NPS, all patients were stratified into 3 groups (group 1: NPS = 0, n = 53; group 2: NPS = 1 or 2, n = 297; group 3: NPS = 3 or 4, n = 126). As shown in Table 1, patients with a higher score of NPS often have a higher proportion of ASA > 2, performance status ≥ 1, cirrhosis and portal hypertension, Child-Pugh B, multiple tumors, intraoperative blood loss > 600 mL (all P < 0.05). Meanwhile, Table 1 showed that NPS was positively correlated with CONUT, SIS, and NLR values, and had a negative correlation with PNI and LMR values.
 |
Table 1 Comparisons of Clinical Characteristics Among the Three Groups According to Preoperative Naples Prognostic Score |
Overall Survival and Recurrence-Free Survival
The median follow-up time was 68.0 months. During the follow-up, 301 (63.2%) and 182 (38.2%) patients developed HCC recurrence and death, respectively. The 1-, 3-, and 5- years OS among each NPS group were 96.2%, 84.8%, and 79.5% in group 1; 91.9%, 82.2%, and 80.4% in group 2; and 87.3%, 66.9%, and 66.0% in group 3, respectively (Figure 2A). Accordingly, the 1-, 3-, and 5- years RFS among each NPS group were 86.8%, 67%, and 51.8% in group 1; 73.7%, 47.5%, and 34.2% in group 2; and 65.1%, 40.5%, and 23.9% in group 3, respectively (Figure 2B). The K-M curves showed that NPS had a negative effect on OS and RFS for HCC after hepatectomy (all P < 0.001).
Univariable and Multivariable Analyses
Variables with P < 0.1 in the univariate Cox regression analysis were enrolled into the forward stepwise multivariate Cox proportional hazard regression analysis. And the results were shown in Table 2 and Table 3. The results demonstrated that NPS was an independent risk factor of OS (NPS group 2 vs 1: HR=1.958, 95% CI: 1.038–3.369, p = 0.038; NPS group 3 vs 1: HR=2.608, 95% CI: 1.358–5.008, p=0.004, respectively). Meanwhile, the result also showed that NPS was an independent risk factor of RFS (NPS group 2 vs 1: HR=2.014, 95% CI: 1.299-2-3.124, p=0.002; NPS group 3 vs 1: HR=2.002, 95% CI: 1.262–3.175, p=0.003, respectively).
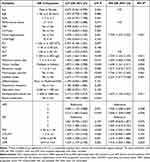 |
Table 2 Univariable and Multivariable Cox Regression Analyses of Risk Factors Associated with Overall Survival for HCC After Hepatectomy |
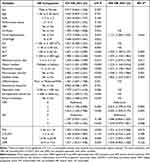 |
Table 3 Univariable and Multivariable Cox Regression Analyses of Risk Factors Associated with Recurrence-Free Survival for HCC After Hepatectomy |
To avoid collinearity with NPS, SIS, PNI, COUNT, NLR, and LMR were analyzed separately with other variables. And the results showed that SIS (group 2 vs 0: HR: 1.467, 95% CI: 1.013–2.123, P = 0.042), PNI (HR: 1.436, 95%:1.043–1.976, P = 0.026), NLR (HR:1.376, 95% CI: 1.004–1.886, P = 0.047) and LMR (HR:1.416, 95% CI: 1.052–1.906, P = 0.022) were independent risk factors of OS. Meanwhile, PNI (HR:1.351, 95% CI: 1.214–2.068, P = 0.019) and NLR (HR:1.371, 95% CI: 1.052–1.787, P = 0.020) were also independent risk factors of RFS. SIS (group 2 vs 0: HR: 1.335, 95% CI: 0.979–1.821, P = 0.068) and LMR (HR:1.045, 95% CI: 0.902–1.428, P = 0.126) were not prognosis factors of RFS. Moreover, COUNT is neither an independent risk factor for OS (HR:1.237, 95% CI: 0.906–1.689, P = 0.181) nor RFS (HR:0.976, 95% CI: 0.754–1.239, P = 0.789).
Prognostic Performance
The area under the time-dependent ROC was calculated to determine which indicator was good at predicting survival. Firstly, we calculated the model’s ability to predict overall survival at 5 years (Figure 3A). The AUC value of the NPS was 0.692, which is higher than SIS (AUC: 0.621), PNI (AUC: 0.603), COUNT (AUC: 0.526), NLR (AUC: 0.589), and LMR (AUC: 0.574) (all P < 0.05). Meanwhile, we used time-dependent ROC curves to calculate the estimated AUC at different time points. Results showed that the AUC was stable (median AUC 0.687, range 0.648–0.716), and the diagnostic capacity of the model was higher than that of any other indicators, including SIS (median AUC: 0.651, Range: 0.605–0.676), PNI (median AUC: 0.638, range: 0.581–0.695), COUNT (median AUC: 0.565, range: 0.518–0.612), NLR (median AUC: 0.603, range: 0.568–0.659) and LMR (median AUC: 0.588, range: 0.536–0.651) (Figure 3B).
Discussion
This study mainly aimed to evaluate the impact of NPS on survival in patients undergoing hepatectomy for HCC. Eventually, 476 patients were included and stratified into 3 groups based on the NPS. Baseline characteristics showed that patients with high NPS scores are more likely to be associated with poor performance status, poor liver function, advanced tumor stage, and more prone to intraoperative bleeding. Of note, the multivariable cox-regression analysis indicated that NPS was an independent prognosis factor of OS and RFS. In other words, compared to group 1 (NPS = 0), patients in group 2 (NPS = 1/2) had a nearly 1.5-fold increased risk of HCC recurrence and death, and patients in group 3 (NPS = 3/4) had a nearly 2-fold increased risk of HCC recurrence and death. Furthermore, compared with previously reported prognostic models, the NPS showed the best performance of discriminatory and predictive ability (median AUC 0.687, range 0.648–0.716). To our knowledge, the present study is the first report to determine the prognostic significance of NPS on long-term outcomes in patients with HCC.
Prior studies have reported that immunonutritional status is associated with the postoperative prognosis of malignancy.23–25 Immunonutritional status, on the other hand, is usually reflected in blood parameters such as cholesterol concentrations, albumin, and leukocyte count. Among them, serum albumin is present in each of these scoring systems. Because the albumin concentrations can be decreased by proinflammatory substances, hypoalbuminemia not only indicates a nutrient deficiency but also indicate systemic inflammation. However, due to changes in liver function and the volume of body fluids, albumin concentrations can also be affected.26 It has also been reported that serum cholesterol levels correlate with cancer progression.27 Since hypocholesterolemia affects the mobility of cell membranes, which affects cell surface receptor mobility and transmembrane signal transduction.28 Resulting in an inability of immunoreactive cells to destroy cancer cells through alterations in their cellular membranes,29 leading to its correlation with a worse prognosis HCC.30
In cancer, the immune system is critical because it either kills tumor cells or forms a tumor microenvironment that supports tumor progression31 In turn, the cytomediated immune response relies heavily on lymphocytes, which inhibit cancer cell multiplication, invasion, and metastasis by stimulating immune response through cytotoxicity.31,32 It has been reported that extensive lymphocyte invasion is associated with a favorable prognosis. In inclusion, substances produced by neutrophils, such as reactive oxygen species and arginase can decrease the activation and proliferation of T lymphocytes,33,34 while lymphopenia is associated with reduced survival in cancer.35,36 Neutrophilia has been consistently associated with disease severity, whereas a low absolute neutrophil count has been associated with an improved prognosis following tumor treatment.37 Furthermore, related molecules such as intercellular adhesion molecules and chemokines contribute to neutrophil and monocyte recruitment to primary tumors, neutrophils as important inflammatory cells, in turn, release quantities of chemokines and cytokines, which are implicated in cancer-associated vasculogenesis.38,39 In addition, monocytes can differentiate into tumor-associated macrophages (TAMs) within tumor tissues,40 and these macrophages promote vasculogenesis, tumor progression, and metastasis.41 Monocytes therefore also play a crucial role in the tumor microenvironment and may predict tumor prognosis. On the other hand, single indicators can be influenced by host conditions and other factors, ie they can even be misleading when using threshold values. LMR and NLR thus combine the importance of monocytes, lymphocytes, and neutrophils in tumor development and are better prognostic indicators of prognosis than the single endpoints noted above.37,42
While, as noted above, total cholesterol and albumin levels, as well as immune inflammatory cells, both predict the prognosis of patients with cancer, the use of only one nutritional biomarker or one or two types of inflammatory cells to assess the long-term prognosis of HCC patients after hepatectomy may be insufficient. Thus, the true predictive ability of these markers in the postoperative prognosis of HCC warrants further study. In contrast, NPS incorporates multiple factors and has been found to better predict tumor prognosis than other single markers.18 NPS values have previously been shown to correlate with poor prognosis of multiple tumors after surgical clipping and to have different prognostic values.20,43,44 Studies of the postoperative HCC, however, remain lacking. In this study, NPS was validated by multivariate Cox analysis to be an independent risk factor for OS and RFS in patients undergoing hepatectomy. In addition, K-M curves of OS and RFS showed that higher NPS scores corresponded with a worse prognosis. Analysis by time-dependent ROC also showed the NPS showed superiority at each time point when compared to the other immunonutrition scoring systems.
Based on the aforementioned studies, it appears that preoperative nutritional status and immune status are both associated with postoperative tumor prognosis. It has been previously reported in the literature that as nutritional status and immune status improve, the prognosis after hepatectomy may also improve as a result.10,45 Despite this, there is a lack of prospective randomized studies to demonstrate whether an improvement in inflammatory and nutritional status is of prognostic benefit after HCC, it has been suggested that nutritional support can improve prognosis and tolerance in patients with HCC4 and that anti-inflammatories, such as aspirin or other NSAIDs, can reduce inflammation and exert antitumor effects.46 Thus, the use of NPS to evaluate the inflammatory and nutritional status of patients with HCC after hepatectomy may aid in determining individualized treatment plans and provide a foundation for the determination of nonsurgical treatment choices.
There are still some limitations in this study. Although the AUC value (0.687) is not enough high, this is the impact of only one variable on prognosis. To better predict prognosis, it is important to consider other independent risk factors in combination. However, the purpose of this study is to explore the prognostic value of NPS. Therefore, in future studies, we will incorporate NPS as an independent factor into other models to improve the predictive ability of prognosis. In addition, we discussed the potential mechanisms by which NPS may affect prognosis in the discussion section, particularly the pathological features of inflammation. This includes the direct or indirect influence of inflammatory cells and factors through the tumor microenvironment on tumor occurrence and development. Regarding the correlation between NPS and microenvironmental factors in the specimen, we will further explore this in targeted investigations in future studies. As a retrospective study, there is some inherent bias, including variables that could not be standardized or identified, patients lost to follow-up, etc. in addition, some inflammatory indicators, such as CRP and heparin, were not included. Because we think it’s collinearity with inflammatory cells. Further validation, especially multicenter RCT, still needed to be conducted.
Conclusion
The presence of preoperative NPS in patients with HCC after hepatectomy is an independent risk factor for both OS and RFS, and NPS has superior prognostic performance compared with other immunonutrition scoring systems.
Abbreviations
HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, alpha-fetoprotein.
Acknowledgment
Yaming Xie, Wenfeng Lu and Jian Cheng contributed equally to this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding for the study was provided by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ23H160049, Health Commission of Zhejiang Province (No.2022KY532), Zhejiang Provincial People’s Hospital (No. ZRY2020A004), and Lishui Public welfare technology application research project (No. 2022GYX50). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
2. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi:10.1097/SLA.0000000000000710
3. Lewis DR, Chen HS, Cockburn MG, et al. Early estimates of SEER cancer incidence, 2014. Cancer. 2017;123(13):2524–2534. doi:10.1002/cncr.30630
4. Ruiz-Margain A, Roman-Calleja BM, Moreno-Guillen P, et al. Nutritional therapy for hepatocellular carcinoma. World J Gastrointest Oncol. 2021;13(10):1440–1452. doi:10.4251/wjgo.v13.i10.1440
5. Yang YM, Kim SY, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis. 2019;39(1):26–42. doi:10.1055/s-0038-1676806
6. van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–1360. doi:10.1002/hep.29914
7. Alberino F, Gatta A, Amodio P, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17(6):445–450. doi:10.1016/S0899-9007(01)00521-4
8. Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–140. doi:10.1016/j.jhep.2015.02.031
9. Perisetti A, Goyal H, Yendala R, Chandan S, Tharian B, Thandassery RB. Sarcopenia in hepatocellular carcinoma: current knowledge and future directions. World J Gastroenterol. 2022;28(4):432–448. doi:10.3748/wjg.v28.i4.432
10. Harimoto N, Yoshizumi T, Sakata K, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma. World J Surg. 2017;41(11):2805–2812. doi:10.1007/s00268-017-4097-1
11. Tsunematsu M, Haruki K, Fujiwara Y, et al. Preoperative controlling nutritional status (CONUT) score predicts long-term outcomes in patients with non-B non-C hepatocellular carcinoma after curative hepatic resection. Langenbecks Arch Surg. 2021;406(1):99–107. doi:10.1007/s00423-020-01987-9
12. Liang X, Liangliang X, Peng W, et al. Combined prognostic nutritional index and albumin-bilirubin grade to predict the postoperative prognosis of HBV-associated hepatocellular carcinoma patients. Sci Rep. 2021;11(1):14624. doi:10.1038/s41598-021-94035-5
13. Fan X, Chen G, Li Y, et al. The preoperative prognostic nutritional index in hepatocellular carcinoma after curative hepatectomy: a retrospective cohort study and meta-analysis. J Invest Surg. 2021;34(8):826–833. doi:10.1080/08941939.2019.1698679
14. Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167–6179. doi:10.2147/CMAR.S171035
15. Feng JF, Sheng C, Zhao Q, Chen P. Prognostic value of mean platelet volume/platelet count ratio in patients with resectable esophageal squamous cell carcinoma: a retrospective study. PeerJ. 2019;7:e7246. doi:10.7717/peerj.7246
16. Castillo-Martinez L, Castro-Eguiluz D, Copca-Mendoza ET, et al. Nutritional assessment tools for the identification of malnutrition and nutritional risk associated with cancer treatment. Rev Invest Clin. 2018;70(3):121–125. doi:10.24875/RIC.18002524
17. Lucca I, de Martino M, Hofbauer SL, Zamani N, Shariat SF, Klatte T. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World J Urol. 2015;33(12):2045–2052. doi:10.1007/s00345-015-1559-7
18. Galizia G, Lieto E, Auricchio A, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. 2017;60(12):1273–1284. doi:10.1097/DCR.0000000000000961
19. Feng JF, Zhao JM, Chen S, Chen QX. Naples prognostic score: a novel prognostic score in predicting cancer-specific survival in patients with resected esophageal squamous cell carcinoma. Front Oncol. 2021;11:652537. doi:10.3389/fonc.2021.652537
20. Nakagawa N, Yamada S, Sonohara F, et al. Clinical Implications of Naples Prognostic Score in Patients with Resected Pancreatic Cancer. Ann Surg Oncol. 2020;27(3):887–895. doi:10.1245/s10434-019-08047-7
21. Xuan J, Peng J, Wang S, Cai Y. Prognostic significance of Naples prognostic score in non-small-cell lung cancer patients with brain metastases. Future Oncol. 2022;18(13):1545–1555. doi:10.2217/fon-2021-1530
22. Inokuchi S, Itoh S, Yoshizumi T, et al. Prognostic significance of systemic inflammation score in patients who undergo hepatic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2021;406(3):773–779. doi:10.1007/s00423-021-02103-1
23. Abe T, Nakata K, Kibe S, et al. Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(13):3996–4003. doi:10.1245/s10434-018-6761-6
24. Pokharel N, Katwal G, Adhikari SK. Comparison of preoperative nutritional risk index and body mass index for predicting immediate postoperative outcomes following major gastrointestinal surgery: cohort-study. Ann Med Surg. 2019;48:53–58. doi:10.1016/j.amsu.2019.10.011
25. Wang D, Hu X, Xiao L, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. 2021;25(2):421–427. doi:10.1007/s11605-019-04492-7
26. Tokunaga R, Sakamoto Y, Nakagawa S, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32(1):99–106. doi:10.1007/s00384-016-2668-5
27. Cengiz O, Kocer B, Surmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;12(6):CR240–247.
28. Oliver MF. Serum cholesterol--The knave of hearts and the joker. Lancet. 1981;2(8255):1090–1095. doi:10.1016/S0140-6736(81)91286-1
29. Kritchevsky SB, Kritchevsky D. Serum cholesterol and cancer risk: an epidemiologic perspective. Annu Rev Nutr. 1992;12:391–416. doi:10.1146/annurev.nu.12.070192.002135
30. Jiang SS, Weng DS, Jiang L, et al. The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. J Cancer. 2016;7(6):626–632. doi:10.7150/jca.13837
31. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of the lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. doi:10.1016/j.ctrv.2015.10.003
32. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
33. Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849. doi:10.1158/0008-5472.CAN-04-0465
34. Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122(1):327–336. doi:10.1172/JCI57990
35. Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol. 2010;15(6):544–551. doi:10.1007/s10147-010-0130-1
36. Schobert IT, Savic LJ, Chapiro J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol. 2020;30(10):5663–5673. doi:10.1007/s00330-020-06931-5
37. Johnson PJ, Dhanaraj S, Berhane S, Bonnett L, Ma YT. The prognostic and diagnostic significance of the neutrophil-to-lymphocyte ratio in hepatocellular carcinoma: a prospective controlled study. Br J Cancer. 2021;125(5):714–716. doi:10.1038/s41416-021-01445-3
38. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83–91. doi:10.1158/2326-6066.CIR-15-0313
39. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
40. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
41. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor-associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404–1412. doi:10.1002/jcp.24260
42. Lin ZX, Ruan DY, Li Y, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21(38):10898–10906. doi:10.3748/wjg.v21.i38.10898
43. Jin J, Wang H, Peng F, et al. Prognostic significance of preoperative Naples prognostic score on short- and long-term outcomes after pancreatoduodenectomy for ampullary carcinoma. Hepatobiliary Surg Nutr. 2021;10(6):825–838. doi:10.21037/hbsn-20-741
44. Li Q, Cong R, Wang Y, et al. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: results from a retrospective cohort study. Gynecol Oncol. 2021;160(1):91–98. doi:10.1016/j.ygyno.2020.10.013
45. Kong W, Yang M, Zhang J, et al. Prognostic value of inflammation-based indices in patients with resected hepatocellular carcinoma. BMC Cancer. 2021;21(1):469. doi:10.1186/s12885-021-08153-4
46. Tao Y, Li Y, Liu X, Deng Q, Yu Y, Yang Z. Nonsteroidal anti-inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:2695–2709. doi:10.2147/CMAR.S167560
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


