Back to Journals » International Journal of Nanomedicine » Volume 18
Nanoscale Morphologies on the Surface of 3D-Printed Titanium Implants for Improved Osseointegration: A Systematic Review of the Literature
Authors Yang S , Jiang W, Ma X , Wang Z, Sah RL , Wang J , Sun Y
Received 19 March 2023
Accepted for publication 10 July 2023
Published 26 July 2023 Volume 2023:18 Pages 4171—4191
DOI https://doi.org/10.2147/IJN.S409033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Shiyan Yang,1,* Weibo Jiang,1,* Xiao Ma,2,* Zuobin Wang,3 Robert L Sah,4,5 Jincheng Wang,1 Yang Sun1
1Orthopedic Medical Center, the Second Hospital of Jilin University, Changchun, Jilin, 130000, People’s Republic of China; 2Department of Orthopedics, the China-Japan Union Hospital of Jilin University, Changchun, Jilin, 130000, People’s Republic of China; 3International Research Centre for Nano Handling and Manufacturing of China, Changchun University of Science and Technology, Changchun, Jilin, 130000, People’s Republic of China; 4Department of Bioengineering, University of California–San Diego, La Jolla, CA, 92037, USA; 5Center for Musculoskeletal Research, Institute of Engineering in Medicine, University of California–San Diego, La Jolla, CA, 92037, USA
*These authors contributed equally to this work
Correspondence: Yang Sun, Orthopedic Medical Center, the Second Hospital of Jilin University, Changchun, 130000, Jilin, People’s Republic of China, Tel/Fax +86 18744000871, Email [email protected]
Abstract: Three-dimensional (3D) printing is serving as the most promising approach to fabricate personalized titanium (Ti) implants for the precise treatment of complex bone defects. However, the bio-inert nature of Ti material limits its capability for rapid osseointegration and thus influences the implant lifetime in vivo. Despite the macroscale porosity for promoting osseointegration, 3D-printed Ti implant surface morphologies at the nanoscale have gained considerable attention for their potential to improve specific outcomes. To evaluate the influence of nanoscale surface morphologies on osseointegration outcomes of 3D-printed Ti implants and discuss the available strategies, we systematically searched evidence according to the PRISMA on PubMed, Embase, Web of Science, and Cochrane (until June 2022). The inclusion criteria were in vivo (animal) studies reporting the osseointegration outcomes of nanoscale morphologies on the surface of 3D-printed Ti implants. The risk of bias (RoB) was assessed using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE’s) tool. The quality of the studies was evaluated using the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. (PROSPERO: CRD42022334222). Out of 119 retrieved articles, 9 studies met the inclusion criteria. The evidence suggests that irregular nano-texture, nanodots and nanotubes with a diameter of 40– 105nm on the surface of porous/solid 3D-printed Ti implants result in better osseointegration and vertical bone ingrowth compared to the untreated/polished ones by significantly promoting cell adhesion, matrix mineralization, and osteogenic differentiation through increasing integrin expression. The RoB was low in 41.1% of items, unclear in 53.3%, and high in 5.6%. The quality of the studies achieved a mean score of 17.67. Our study demonstrates that nanostructures with specific controlled properties on the surface of 3D-printed Ti implants improve their osseointegration. However, given the small number of studies, the variability in experimental designs, and lack of reporting across studies, the results should be interpreted with caution.
Keywords: nano-pattern, 3D-printed, titanium implant, surface modification, osseointegration
Introduction
In the past decade, additive manufacturing, also known as three-dimensional (3D) printing, has become a promising technology for the production of orthopedic implants due to its ability to produce customized shapes for the precise treatment of complex bone defects, which are difficult to conquer with traditional methods.1–4 Based on the computer-aided manufacturing, laser technology, numerical control technology, and polymer materials, 3D printing has shown remarkable advantages, including single-piece customization, adjustment of mechanical strength, and high process efficiency.5 Selective laser melting (SLM), selective laser sintering (SLS), electron beam melting (EBM), laser engineered net shaping (LENS), and direct metal laser sintering (DMLS) are the main technologies for manufacturing 3D-printed orthopedic implants, with commercially pure titanium (cp Ti) and Ti alloys (mainly Ti6Al4V) serving as the most common materials being used due to their biocompatibility, low toxicity, high corrosion resistance, high-strength-to-weight ratio, resistance to fatigue deformation and excellent mechanical properties.6–8 However, as a bio-inert material, surface-unmodified Ti lacks biointegration capability and has a far higher elastic modulus (110–120 GPa) than that of cortical bone (10–30 GPa), which limits the long-term fixation and lifespan of Ti implants.9
Homogeneous interconnected porous structures within the 3D-printed implants can promote the growth of bone tissue into them, and optimize the elastic modulus by changing the porosity, hole shape, diameter, and connectivity.10,11 It has been reported that rhombic dodecahedron- or diamond-shaped pores with a pore size of 300–600µm and 60–78% porosity can sufficiently promote bone ingrowth in 3D-printed Ti implants in preclinical studies.12,13 Wang et al14 and Yu et al15 suggested that such shapes of pores in 3D-printed Ti implants were ideal choices for closely resembling cancellous bone and had a higher yield stress in bone defect animal models. Ran et al16 and Han et al17 found that a 3D-printed Ti scaffold with a pore size of 600 µm has more osteogenic capability than 400, 500, 700, and 800 µm in rabbit. Luan et al12 suggested that 78% porosity had higher osteogenic capacity than 65% and 55%. In addition to micro scale design on the structures of Ti implants, micro-nano and nanoscale surface modification have been reported to further enhance osteogenesis and osseointegration by mimicking the heterogeneous structure of the natural bone tissues.18–20 Nanostructured surface engineering to enhance implant osteointegration properties is considered one of the most promising strategies to achieve next-generation 3D-printed Ti implants.21
Nanoscale surface morphologies regulate osteogenic differentiation of stem cells, tissue regeneration, cell adhesion, and gene expression better than that of microscale surface structures,22–24 for their size more closely resembles that of collagen, protein, and cell membrane receptors in the extracellular matrix (ECM).25,26 Moreover, they help lower the contact angles at the surface, making it hydrophilic and further improving early-stage osseointegration.27,28 Laser etching, acid etching, acid–alkali treatment, anodic oxidation, sandblasting, alkali–acid–heat treatment, and a broad range of other approaches have been explored with demonstrated ability to fabricate different forms of nanomorphology such as nanotubes, nanocolloids, nanopits, nanofibers, nanodots, nanopillars and nanogrooves.29,30 As the nanoscale surface morphology features may further facilitate the bone growth into microscale porous structures, the past decade has witnessed a surge of investigations evaluating the effects of various 3D printing and nanostructured surface engineering technologies on osseointegration.31–34 The results of in vitro studies have demonstrated the osteogenic properties of surface nanomorphologies on 3D-printed Ti implants and have been further validated in the in vivo studies, however, with heterogeneous results.35–37 The relationship between surface nanoscale features and in vivo osseointegration outcomes of 3D-printed Ti implants remains challenging to interpret, owing to the highly variable study designs and the influence of multiple experimental variables on outcome measures. This review study aims to evaluate the effect of nanoscale surface morphologies on osseointegration outcomes of 3D-printed Ti implants in the preclinical studies and summary the strategies to pave the way of translating those from research to clinical application.
Methods
Systematic Literature Search
A comprehensive systematic review was performed independently following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Figure 1).38 The focused question was “Does the nanomorphology on the surface of 3D-printed Ti implants enhance the osseointegration?” and was conceived according to Participants, Interventions, Control, and Outcomes (PICO) principles,39 as follows: (P) participant: animals received implantation; (I) intervention: 3D-printed Ti nanoscale surface modification; (C) control group: 3D-printed Ti implants without nanostructured surface modification; (O) outcome: experimental parameters related to osseointegration (ie, histological analysis, micro-CT evaluation, and biomechanical tests).
 |
Figure 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram including study algorithm. |
Study methods followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and were documented in an international prospective register of systematic reviews (PROSPERO) protocol with registration ID: CRD42022334222. To filter studies relevant to the focused question, relevant manuscripts were searched for published up to June 2022 using 4 electronic databases: PubMed, Embase, Cochrane, and Web of Science. A general search was conducted in PubMed using the following terms: (“nano” [All Fields] OR “nano scale” [All Fields] OR “nanopattern” [All Fields] OR “nanomorphology” [All Fields] OR “nanostructures” [MeSH Terms] OR “nanostructures” [All Fields]) AND (“surface” [All Fields] OR “surfacing” [All Fields] OR “surface modification” [All Fields] OR “surface engineering” [All Fields]) AND (“osseointegration” [MeSH Terms] OR “osseointegration” [All Fields] OR “bone integration” [All Fields]) AND (“titanium” [MeSH Terms] OR “titanium” [All Fields] OR “Ti” [All Fields]) AND (“implant” [All Fields] OR “scaffold” [All Fields] OR “prostheses and implants” [MeSH Terms] OR “prostheses and implants” [All Fields]) AND (“3D printing” [All Fields] OR “printing, three dimensional” [MeSH Terms] OR “3d printed” [All Fields] OR “additive manufacturing” [All Fields]). And we use keywords in combination with ‘AND’ or ‘OR’ (Boolean logic operators): ((‘nano’ OR ‘nano scale’ OR ‘nanopattern’ OR ‘nanomorphology’ OR ‘nanostructure’) AND (‘surface’ OR ‘surfacing’ OR ‘surface modification’ OR ‘surface engineering’) AND (‘osseointegration’ OR ‘bone integration’ AND ‘titanium’ OR ‘Ti’) AND (‘implant’ OR ‘scaffold’ OR ‘prothesis’) AND (‘3D printing’ OR ‘3D-printing’ OR ‘3D-printed’ OR ‘3D printed’ OR ‘additive manufacturing’)) to identify the relevant literature in other databases. Two authors (SY and WJ) independently inspected the titles and abstracts of the manuscripts following the eligibility criteria. Then, the full texts of the eligible manuscripts were browsed to select studies appropriate for this systematic review. The reference lists of relevant original and reviews identified in the previous step were further hand-searched. The two authors discussed the selection process until a consensus was reached. The search strategy is shown in Figure 1.
Exclusion and Inclusion Criteria
In vivo peer-reviewed studies evaluating the osseointegration outcomes of the nanostructured surface of 3D-printed Ti implants were included in this systematic review. In vivo was defined as animal studies investigating osseointegration outcomes after treatment with 3D-printed Ti implants. Each animal study was classified according to the Centre for Evidence-Based Medicine’s system for assigning levels of evidence, with all studies in this review being considered basic science studies (level 5). Inclusion criteria for in vivo studies were animal studies of osseointegration outcomes after treatment with nanostructured 3D-printed Ti implants. The exclusion criteria for all studies (in vivo) were as follows: (1) articles not written in English; (2) review and expert opinion articles, conference proceedings, and presentations; (3) ex vivo studies; and (4) studies that did not evaluate the osseointegration capacity of nanopatterns or did not modify Ti surfaces with nanopatterns. Studies were also excluded if the 3D-printed Ti implants were modified only by coating (Table S1).
Risk of Bias and Quality Assessment of the Studies Included
Assessment of the risk of bias of the selected manuscript according to the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE’s tool) with a focus on ten questions to describe selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias.40 The bias for each selected study was evaluated, where a “yes” judgment indicated a low risk of bias, and a “no” judgment indicated a high risk of bias. The judgment was “unclear” if insufficient details had been reported to properly assess the risk of bias. A low risk of bias of the study was considered if at least 7 items were assessed as “low risk” and no item was assessed as “high risk”. Studies were judged as high risk of bias if at least 2 items were judged as “high risk”. A moderate risk of bias was judged in other cases. Assessment of methodological quality for each study followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.41 Assessments were done by two independent reviewers (SY, WJ), and disagreements were resolved after consensus-oriented discussions. If disagreement occurred, the senior author (YS) was consulted.
Results
Identification and Selection of Studies
Electronic database searches identified 75 articles (Figure 1). After screening titles and abstracts for relevance, 56 articles were deemed irrelevant based on the inclusion and exclusion criteria. Out of the 19 full texts of the in vivo animal studies assessed for eligibility, 9 papers were selected and reviewed after applying the criteria. The 10 excluded articles did not evaluate the antibacterial activity of nanopatterns or involve modification of Ti surfaces with a coating. Meta-analysis was not conducted due to the scarcity and heterogeneity of the studies (Figure 1). Figure 2 shows the frequency of publications over the past decade. There has been a substantial increase in publications in recent years, reflecting the growing interest in the field of nanostructured surfaces of 3D-printed Ti implants (Figure 2).
 |
Figure 2 Frequency of studies evaluating osseointegration of the nanostructured surface of 3D-printed Ti implants per five years. |
In vivo Study Characteristics
The general characteristics of the selected in vivo studies are shown in Table 1. Four studies42–45 used New Zealand White rabbits, and five used Sprague Dawley rats,35–37,42,46 while athymic nude rats were used in one study.47 Sample sizes of the included studies ranged from 10 to 48 animals,37,47 with an unknown number used in two studies.45,46 The distal femurs were used as the implant location in seven studies,35–37,44–46 the top of the cranial bone in two studies,42,47 the tibiae in one study, and the (human) mandible in the same study.42 Follow-up periods ranged from 4 to 10 weeks. Micro-CT scan, mechanical tests, and histological analysis were performed to comprehensively assess the osseointegration outcomes. The bone volume (BV), bone volume fraction (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N, 1/mm), and trabecular spacing (Tb.Sp, μm) were analyzed by micro-CT analysis. Histological analysis included bone-to-implant contact (BIC), total osteoid formation, and total bone formation. Biomechanical tests included pull-out tests and modulus values as measures of implant stability. SEM characterizations were performed to show the interfacial bonding of the host tissue and the implants.
 |
Table 1 General Characteristics of the Selected Studies |
The implant-related characteristics of the selected studies are presented in Table 2. The number of implants applied per study ranged from 10 to 48. LENS™ had been utilized to develop the implants in two studies,36,46 SLS had been used in five studies,37,42–44,47 LENS™ had been used in three studies,36,45,46 and EBM was used in one study to prepare Ti-6Al-4V implants.35 The implant shape was rod-shaped in six studies,35–37,44–46 cuboidal in one study,47 disk-shaped in two studies,42,43 and one study42 created custom wrap implants for rabbit models and patients. The diameter and length of the implants ranged from 2.3 to 6.0 mm and 3.0 to 10.0 mm, respectively. The porosity of the 3D-printed implants ranged from 25% to 68%, with four studies did not mention the porosity.35,37,42,43 Three studies used anodic oxidation to generate implants with porosity based on micro-/nano-surface roughness,36,45,46 four studies produced nanoscale surface morphology via grit blasting and acid etching,42–44,47 one study introduced an ultrasonic-assisted acid etching process,35 and one study developed an alkali-heat treatment to fabricate nano-topography.37 Four studies formed nanotubes with a diameter ranging from 40 to 105 nm on the surface of 3D-printed Ti implants,35,36,45,46 four studies42–44,47 constructed nano-texture on the surface and samples exhibited nodular nanostructures in one study.37
 |
Table 2 Implant-Related Characteristics of the Selected Studies |
Risk of Bias and Quality Assessment of the Studies Included
The included studies presented heterogeneous levels of risk of bias, which are presented in Figure 3. Three studies reported that animals were randomly allocated to different groups during the experiment.44,45,47 Three included studies were evaluated as having unclear baseline characteristics.42,45,46 One study reported the labeled cages and it was unclear how the cages were labeled and how the allocation sequence was concealed in the other studies.46 Three studies36,44,47 clearly explained the random animal housing, while others were unclear. The risk of detection bias was low in one study.42 One study reported that the outcome assessors were blinded, while other studies had a moderate risk of blinding detection bias.42 All the selected studies were assessed as having a moderate risk of blinding performance bias and a low risk of reporting and other biases. Attrition bias was described as low risk in six studies36,37,42,44–46 and high in three studies.35,43,47 All of the studies had a moderate probability of RoB (n = 9). The risk of bias was low in 41.1% of items, unclear in 53.3%, and high in 5.6%. Assessment of methodological quality for each study was summarized in Table S2. The quality of the selected studies (ARRIVE [Animal Research: Reporting of In Vivo Experiments] guidelines) achieved a mean score of 17.67 and only two studies mentioned that they followed the ARRIVE guidelines.44,47
Discussion
Modern manufacturing technology constantly evolves in biomedical implant design, aiming to control what happens at the bone-implant interface. Altering surface morphology, plasma-sprayed coatings, and the construction of a porous structure through various manufacturing methods have been shown to influence the bone-implant interface in animal, preclinical and clinical studies.48,49 3D printing technology represents an alternative method of implant production where anatomical shapes can be produced that would be considered too complex or costly to achieve through traditional methods.21 In terms of microstructure, 3D printing technology can precisely control the Young’s modulus of the implants to match natural bone tissues by designing pores that can effectively reduce the stress-shielding effect of the implants and reduce the incidence of peri-implant osteolysis.50 In terms of macrostructure, 3D printing technology can efficiently print the implant in a complex shape according to the patient’s personalized anatomical characteristics and clinical needs through CT images to adapt the mechanical properties and custom shapes of the implants to the natural bone tissue.6 Compared with traditional Ti implants, microscale porous structure can promote the early vascularization of 3D-printed Ti implants and improve the osteointegration.51,52 The 3D printing technology has shown remarkable advantages in terms of free design, single-piece customization, and high process efficiency. These features allow coping with the urgent biomedical field needs, thus have attracted the interests of researchers and significantly stimulated the clinical applications of 3D printing in recent years.5,35 Ti is currently the most popular material for 3D printing of orthopedic implants because of its advantages of excellent mechanical strength, biocompatibility and corrosion resistance in vivo.53 However, untreated titanium alloy implants always possess a bioinert surface that fails to simulate the structure of cortical bone and cancellous bone in real bone tissue and prevents reactions between the organism and implants, so surface modification technologies on 3D-printed Ti implants are essential for further osteointegration.50
The surface topographies of traditional Ti implants have been modified on the micro-nano scale and nanoscale, which play a positive role in osteointegration.18,54–56 Over the past six decades, the development of surface modification of orthopedic Ti implants can be classified into four main stages. The surface technologies of implants have progressed from bio-inert surfaces to biocompatible Ti surfaces, to bioactive surfaces, to the most recent and probable future generation of biomimetic engineered, nanoscale surfaces such as Ti surface treated with Ti oxide (TiO2) nanotube arrays. Now latest studies are approaching the stage of the fourth generation with recent remarkable advancements in cell biology, nanomedicine, material science, nanotechnology, and additive manufacturing. A broad range of surface modification techniques such as acid etching, anodic oxidation, sandblasting, and heat treatments have been explored with demonstrated ability to fabricate different forms of nanomorphology such as nanotubes, nanogrooves, nanopits, nanopillars, nanocolloids, and nanofibers. Numerous studies have confirmed greater bone-implant contact area and new bone formation on nanotubes on the surface of Ti implants.57 Nanogrooved patterns of 50μm in parallel or radial arrangements on Ti implants have been proved to enhance the migration and proliferation of osteoblasts58 and demonstrated superior bone regeneration than in the nonpatterned implants in vivo studies.59 Nanopits of 30nm exhibited a more branched cell morphology and promoted early osteoblastic differentiation on Ti surfaces.60 Sjöström et al found that cells on nanopillars of 15nm on the Ti surface showed a trend toward more large and super-mature osteogenic focal adhesions than those on the polished Ti surface and cells on the nanoscale surface had longer adhesions and produced larger osteocalcin deposits.61 Ning et al suggested that growing nanorods with length and diameter of 100 and 20nm and a medium density on the surface of traditional Ti implants was an efficient method for promoting bone formation and osteointegration in rabbit tibia.62 Recent in vitro and in vivo studies have focused on the osteointegration outcomes of 3D-printed Ti implants with nanomorphology like nanotubes, nanodots and nanotexture, and future preclinical studies should pay attention to other nanomorphology such as nanopits, nanogrooves, nanorods, and nanofibers. To summarize, nanostructured surface topographies modified at the nanoscale positively contribute to the early osteoblastic differentiation on the surface of Ti in vitro, providing a novel way to enhance osteointegration of the 3D-printed Ti implants.
Numerous engineering technologies of surface nanopatterns on Ti implants have been developed during the past decade, which can be divided into physical surface modification, chemical surface modification and biological surface modification (Figure 4). Physical surface modification technologies depend on lasers, ultrasonics, high-energy particles, and magnetic fields to modify Ti materials’ surface morphology and ultrastructure. These methods, such as laser surface engineering (LSE), arc ion plating (AIP), sandblasting, and ultrasonic nanotechnology, are commonly used to establish various surface topographies on the surface of implants and improve their resistance to wear, oxidation, and corrosion. The main advantage of LSE entails the capability of hierarchically controlling the chemical composition and the surface texture (pits, grooves, ablation tracks, ripples, pillars, columns) using different laser-based approaches and techniques such as: laser ablation, laser induced periodic surface structures (LIPSSs), laser melting, laser cladding, laser engineered net shaping (LENS™), direct laser interference patterning (DLIP), and matrix assisted pulsed evaporation (MAPLE).63–65 However, physical surface modification requires more advanced equipment than chemical modification, and the resulting bonding force is weaker compared to chemical bonds. In contrast to physical methods, chemical surface modification technologies alter the chemical properties of implants by exposing them to a chemical solution or gas that bonds with bioactive substances. Chemical surface modification technologies include anodic oxidation, micro-arc oxidation (MAO), electrophoretic deposition, chemical vapor deposition, acid/alkali etching, and atomic layer deposition. In addition, implants with complex shapes always adopt chemical surface modification technologies and have excellent prospects of application in bone regeneration on the implant surface (contact osteogenesis) of 3D-printed implants. After processing Ti structural materials into the required implant design, surface modification is carried out by combining sandblasting and acid etching to form sandblasted, large-grit, and acid-etched surfaces, which commercially use the terminology SLA. Abrasive particles (110–250 μm in size) such as aluminum oxide (Al2O3), silicon oxide (SiO2) or TiO2 are used in sandblasting.66,67 Hydrochloric acid (HCl), hydrofluoric acid (HF), nitric acid (HNO3), and sulfuric acid (H2SO4) are the common acidic substances for acid etching, which can remove entrapped abrasive particles on the air-braided Ti surfaces followed by the dissolution of the Ti oxide film and micro-scale bulk layer in the Ti subsurface.34 Biological surface modification is a novel technology that constructs biofunctional coatings to fabricate bio-functionalized Ti-based implants, including biopolymers, growth factors, extracellular matrix proteins, peptides, and drugs on the surface of implants, which could enhance and maintain osseointegration and bioactivity of Ti alloy implants.68–71
 |
Figure 4 3D-printed Ti surface modification technologies: physical, chemical, and biological surface modification technologies. Reprinted from J Mech Behav Biomed Mater, Vol 105, Li J, Cui X, Hooper et al, Rational Design, Bio-functionalization and Biological Performance of Hybrid Additive Manufactured Titanium Implants for Orthopaedic Applications: A review, 103,671, Copyright © 2020, with permission from Elsevier;.72 Reprinted from Applied Surface Science, Vol 480, Surmeneva M, Lapanje A, Chudinova et al, Decreased Bacterial Colonization of Additively Manufactured Ti6Al4V Metallic Scaffolds with Immobilized Silver and Calcium Phosphate Nanoparticles, 822–829, Copyright © 2019, with permission from Elsevier.73 Reprinted from Sheng X, Wang A, Wang Z, Liu H, Wang J, Li C. Advanced Surface Modification for 3D-Printed Titanium Alloy Implant Interface Functionalization. Front Bioeng Biotechnol. 2022;10:850,110. Creative Commons;50 Reprinted from Soyama H, Takeo F. Effect of Various Peening Methods on the Fatigue Properties of Titanium Alloy Ti6Al4V Manufactured by Direct Metal Laser Sintering and Electron Beam Melting. Materials (Basel). 2020;13(10). Creative Commons;74 Reprinted from Mieszkowska A, Beaumont H, Martocq et al. Phenolic-Enriched Collagen Fibrillar Coatings on Titanium Alloy to Promote Osteogenic Differentiation and Reduce Inflammation. Int J Mol Sci. 2020;21(17). Creative Commons.75 |
3D-printed Ti implants constructed with nano-surface roughness significantly promote cell adhesion, matrix mineralization, and osteogenic differentiation, building a suitable microenvironment for cell growth.76 The protein adsorption and cell adhesion experiments confirmed that the micro-/nano-topography notably enhanced porous Ti’s protein binding capacity and promoted MSCs adhesion on the surface.43,77 More importantly, cell differentiation experiments have shown that MSCs on the 3D-printed Ti implants with nanostructured surfaces elevated the osteoblastic gene expressions like BMP-2, BMP-4, RUNX2, osteocalcin, and vascular endothelial growth factor (VEGF), proving that nanoscale surfaces could enhance growth factor production and osteoblastic differentiation of MSCs.43,76,78 The rapid recruitment of osteoblasts is a necessary prerequisite for the effective repair of bone defects.79 Nanoscale roughness on the surface of 3D-printed Ti implants could improve the differentiation and maturation of osteoblasts and promote matrix mineralization, which suggested the enhanced performance of these surfaces for increasing osseointegration. However, studies on the surface modification of 3D-printed Ti implants and the effects of surface characteristics on osteogenesis in vivo are still needed for the next generation of Ti implants with improved biological performances.
Porosity in 3D-printed Ti implants helps in inducing surface roughness which results in higher surface area and promotes osteogenesis by the new bone formation and vascular ingrowth between the implant surface and the living tissue, while better interfacial bonding was seen in surface-modified porous samples with nanostructures.71,78,80 Five of the selected studies reported that better interfacial bonding and more bone ingrowth were seen in surface-modified nanomorphology samples with porous structures.36,44–47 Three studies reported that nanoscale design elements at the surface of porous Ti and its alloy implants could also be expected to enhance interfacial shear modulus, migration of cells, adhesion, and influence overall bone remodeling.36,44,46 Bandyopadhyay et al36 compared to the dense implants, 3D-printed porous samples showed better bone ingrowth, bonding of porous implants with the tissue, and new osteoid formation in the high-resolution CT scan images and histological evaluation after 4 weeks (Figure 5A). They observed almost complete bone regeneration with no gaps or incomplete bonding after 10 weeks in porous implants, which confirmed enhanced early-stage osseointegration. Cohen et al44 suggested that 3D-printed Ti-6Al-4V implants with trabecular bone-inspired porosity and nanorough showed more bone ingrowth and deposition in the apical portions of implants in both groups, as observed via Micro-CT and histology, suggesting that mechanical strength is enhanced in porous areas. Modifying the porous implant surface topography at a nanoscale level could further facilitate the process of early-stage osseointegration. Specifically, in the primary outcomes of the six included studies,35–37,42,45,46 the histological analysis, micro-CT evaluation, or biomechanical tests results of the nanostructured surfaces of 3D-printed Ti implants were significantly higher than the smooth surfaces, meaning better biocompatibility and stronger osteoconductive and osseointegration capabilities of the Ti nanostructured surface (Figure 5B). 3D-printed Ti implants with periodic surface nanopatterns dominated by ordered nanotubes were shown to enhance the early stage osseointegration and improve mechanical interlocking between the host bone and the implant compared to dense and pure porous Ti implants.35,36,45,46 In the qualitative histology and Micro-CT scan, Ren et al35 and Mitra et al45 proved that nanotube samples had a higher value at all time domains in trabecular formation, and Bose et al46 found that porous Ti rods with nanotube film had higher total osteoid formation and total bone formation than pure porous Ti rods (Figure 5C). Bandyopadhyay et al36 and Bose et al46 obtained the SEM images of the stained samples and found that the gaps reduced as they moved from dense Ti samples towards the porous samples and were significantly reduced for porous Ti implants with nanotubes, suggesting a significant improvement of osseointegration across the bone-implant interface (Figure 6). 3D-printed Ti implants with through-pores and non-periodic micro/nanoscale surface texture showed enhanced early-stage osseointegration in rat and rabbit models, as evidenced by 3D analysis of osseointegration and new bone infiltration into the pores and increased pull-out values.37,42–44,47 All evidence suggests that irregular nano-texture, nanodots and nanotubes with a diameter of 40–105nm on the surface of porous/solid 3D-printed Ti implants result in better osseointegration and vertical bone ingrowth compared to the untreated/polished ones.
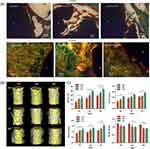 |
Figure 5 Evidence of 3D implants with nanoscale surface promoting osseointegration. (A) Histology images after 4 weeks (a, b, c) and 10 weeks (d, e, f) where signs of osteoid like new bone formation could be seen in Orange/ red color; Reprinted from Ann Biomed Eng, Vol 45(1), Bandyopadhyay A, Shivaram A, Tarafder et al, In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants, 249–260, Copyright © 2017, with permission from SNCSC.36(B) Micro-CT reconstructed 3D models at 2, 4, and 8 weeks; (C) Quantitative analysis of Micro-CT: bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb. Sp) (*p < 5%, **p < 1%). Reprinted from Mater Sci Eng C, Vol 118, Ren B, Wan Y, Liu et al, Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study, 111,505, Copyright © 2021, with permission from Elsevier.35 |
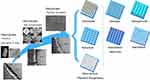 |
Figure 6 Nanotubes, nano-texture (random roughness), nanodots constructed on nanoscale surface of 3D-printed Ti implants promote osseointegration. Reprinted from Mater Today, Volume 45, Mitra I, Bose S, Dernell WS, et al 3D Printing in alloy design to improve biocompatibility in metallic implants. 20–34, Copyright 2021, with permission from Elsevier.45 Ann Biomed Eng, Vol 45(1), Bandyopadhyay A, Shivaram A, Tarafder et al, In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants, 249–260, Copyright © 2017, reprinted with permission from SNCSC.36 |
In addition, methodological and statistical heterogeneity also exists in designing the trial protocol and response evaluation, which may eventually affect the accuracy of the analysis result.81 No studies qualitatively compared the characteristics of different additive manufacturing technologies. Bone remodeling involves the removal of mineralized bone by osteoclasts followed by the formation of the bone matrix through the osteoblasts that subsequently become mineralized, which starts as early as 3 weeks after implantation and continues at a substantial level up to 12 weeks.82 It is worth noting that the nanostructured surface engineering to 3D-printed Ti implant surfaces appeared to accelerate early-stage osseointegration in 10 weeks, while it has to be clarified in studies that include more extended observational periods if this observation is of clinical relevance.83 Furthermore, small animal models like rats and rabbits were used to study the osseointegration of nanostructured 3D-printed Ti implants in all of the selected studies, which possess significant differences in bone structure and remodeling compared to humans. Although the rabbit femur models better simulate the clinical placement of orthopedic implants through both cortical and trabecular bone than the cranial bone onlay models in rats, it is important to validate efficacy with higher-order animal models before transitioning to clinical trials to confirm the optimal surface design and long-term function. The methods of analysis were similar among the included studies and could contribute to the evaluation of osseointegration, but the shape and position of 3D-printed Ti implants were non-negligible variables that affected the osseointegration.
Recently, the clinical implant research community has gained an interest in 3D printing technology and claimed it as a “game changer” in the field, which is a viable method for producing orthopedic implants leading to enhanced biological response, even when compared to a traditionally manufactured, currently used commercial implant.84,85 In the selected study, laser sintering was used with surface treatments to produce novel Ti-6Al-4V implant surfaces and implants with hierarchical micro- and nano-roughness and hydrophilicity that increased osteoblast response in vitro and demonstrated a significant improvement in cortical bone-implant in rabbit models, when compared to a traditional computer numerical control (CNC).43 In the last five years, the composite treatment of the nanoscale surface of 3D-printed Ti implants by other physical, chemical, and biological modification methods played a synergistic role in enhancing osseointegration and could be a potential application in the orthopedic field.37,42,45,86–88 Cohen et al42 found that the use of the demineralized bone matrix putty (DBX) had an osteoinductive effect, enhancing osseointegration of the roughened surface in the rat calvaria. Bose et al46 found that 3D-printed porous Ti implants with TiO2 nanotubes induced early-stage osteogenesis, while the addition of calcium phosphate coating further enhanced defect healing and mechanical interlocking at the interface. However, Cheng et al47 reported that adding DBX did not significantly enhance mechanical pull-out testing force or total vertical bone volume growth into 3D-printed porous Ti implants, which contradicted those related to the osseointegration effects in other studies. Thus, longer-term and larger animal studies should be performed to confirm the synergistic effects on osseointegration achieved by composite treatment of the nanoscale surface with other modification methods.
Osseointegration is a dynamic and complex biological process that achieves stable anchorage of an implant by direct bone-to-implant contact, including osteoconduction, de novo bone formation and slow process of bone remodeling.89,90 Reactions between the surfaces of Ti materials and living tissues are the initial events that occur when Ti implants are placed in vivo. When Ti implants contact the blood of the surgical site, calcium and phosphorus are incorporated into the oxide layer, which promote osseointegration.91 3D-printed porous Ti implants are deliberately designed and provide room for new blood vessels and facilitate interlocking between host bone and implants because of their suitable mechanical properties and abundant interconnected macroporous structure. Moreover, the interconnected macroporous structure benefits body fluid circulation, nutrients, oxygen transport, vascularization, and the growth factor accumulation and signaling between osteoblasts and mesenchymal stem cells near the implant site induce bone ingrowth into the implant pores.16,70 Physicochemical methods have been developed to produce surface roughness and chemical modification of Ti surfaces to increase the adsorption of blood proteins, ions, and molecules, regulate gene expression, and to stimulate blood platelet activation and osteogenic cell migration.92–94 The physicochemical property of the Ti surface is the crucial factor affecting cellular behaviors and osseointegration, and physical morphology and surface chemistry are not isolated for the modifying of surface morphology will inevitably involve the change in chemical composition at the same time.24 For example, the improvement of the hydrophilicity of the nanotube surface is not only due to the “capillary effect” of the tube structures but also related to the hydroxyl groups generated after surface modification.35 The existence of nanotubes has been proven to change the location and spacing of transmembrane integrins, thus affecting the cytoskeleton tension in the actin filaments of the adhered cells.95 Cell surface integrins, which act as the physical scaffolds between the cytoskeleton and extracellular matrix and sense stimulation of the external environment and regulate cells, have been proven to mediate cell response to biomaterials.96,97 Studies have shown that integrin α2β1 plays a critical role in osteoblast and mesenchymal response to microscale and nanoscale surface structure and surface energy of Ti substrates.98,99 Surface modification at the nanoscale surface further enhances biocompatibility of the 3D-printed Ti porous implant surface, thereby promoting better osteoconductive properties, and has been one of the most commonly used methods to promote early-stage osseointegration.100,101 It helps increase the surface roughness and results in a higher surface area making the contact angle of the surface significantly lower and the surface more hydrophilic, creating a perfect environment for osteoblast cell attachment, maturation, differentiation, and local factor production.36,102–104 As a result of surface modification, the apatite formation on the surface improves, making it more reactive and osteoconductive, while the nanotopography also promotes osteocyte dendrite formation and maturation which further enhances the 3D formation of osteocyte networks.105 Furthermore, improved cell adhesion leads to better cell growth and stimulates its differentiation at both protein and gene levels, resulting in strong bonding between the surface and the tissue.106,107 Studies have reported an up-regulation of pro-osteogenic cell signaling pathways on nanostructured surface including TGF-β/BMP, PGE2, Wnt and Notch, and increased differentiation markers such as alkaline phosphatase (ALP), bone sialoprotein (BSP), and osteocalcin when the hydrophily of nanostructured Ti surfaces improving.108,109
This systematic review study has limitations. Although a systematic literature search was performed and the registration of this systematic review was completed before the data extraction was finished, the limited number of studies and heterogeneity in reporting and experimental designs may influence outcomes and hinder result comparison. Due to the above reasons, the optimal surface nanomorphology for promoting the osteointegration of 3D-printed metal implants in vivo cannot be determined.
Conclusion
According to current preclinical studies, irregular nano-texture, nanodots and nanotubes with a diameter of 40–105nm constructed on the surface of porous/solid 3D-printed Ti implants result in better osseointegration and vertical bone ingrowth compared to the untreated/polished ones by significantly promoting cell adhesion, matrix mineralization, and osteogenic differentiation through increased integrin expression. However, the limited number and heterogeneity of current studies influence the determination of the optimal surface nanomorphology of 3D-printed Ti implants. Although the application of surface morphology modification, coating, and drug or cell loading has brought new opportunities to orthopedic implant design and application, future work need to be focus on the synergistic effects of composite treatment of the nanoscale surface with other modification methods, evaluation of mechanical properties, fatigue and anti-corrosion properties, the durability of the fabricated nanostructures, and consideration of the different roles of physical morphology and chemistry. More standardized and thorough reporting of the nanoscale surface features is also needed to improve our understanding of the surface factors influencing osseointegration in 3D-printed Ti implant applications.
Abbreviations
Ti, Titanium; 3D, Three dimensional; CT, Computed tomography; SLM, Selective laser melting; SLS, Selective laser sintering; EBM, Electron beam melting; LENS, Laser engineered net shaping; DMLS, Direct metal laser sintering; cp Ti, Commercially pure titanium; Ti-6Al-4V, Titanium hexaaluminum tetravanadium; GPa, Gigapascal; ECM, Extracellular matrix; BV, Bone volume; BV/TV, Bone volume fraction; Tb.Th, Trabecular thickness; Tb.N, Trabecular number; Tb.Sp, Trabecular spacing; BIC, Bone to implant contact; SEM, Scanning electron microscopy; LENS™, Laser engineered net shaping; SLS, Selective laser sintering; LSE, Laser surface engineering; AIP, arc ion plating; MAO, Micro-arc oxidation; TiO2, Titanium dioxide; MSCs, Mesenchymal stem cells; BMP-2, Bone morphogenetic protein 2; BMP-4, Bone morphogenetic protein 4; RUNX2, Runt-related transcription factor 2; VEGF, Vascular endothelial growth factor; CNC, Computer numerical control; DBX, Demineralized bone matrix putty; Ta, Tantalum; OS/BS, Osteoid surface/bone surface.
Acknowledgments
The authors would like to acknowledge Can He for her help with graphic design.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Department of Finance of Jilin Province, P.R. China [Grant Number 3D5197435429 and 3D5204944429]; the Education Department of Jilin Province, P.R. China [Grant Number JJKH20211157KJ].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salmi M. Additive manufacturing processes in medical applications. Materials. 2021;14(1). doi:10.3390/ma14010191
2. Wang X, Xu S, Zhou S, et al. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials. 2016;83:127–141. doi:10.1016/j.biomaterials.2016.01.012
3. Guzzi EA, Tibbitt MW. Additive manufacturing of precision biomaterials. Adv Mater. 2020;32(13):e1901994. doi:10.1002/adma.201901994
4. Serrano-Aroca Á, Cano-Vicent A, Sabater ISR, et al. Scaffolds in the microbial resistant era: fabrication, materials, properties and tissue engineering applications. Mater Today Bio. 2022;16:100412. doi:10.1016/j.mtbio.2022.100412
5. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230–11. doi:10.5435/JAAOSGlobal-D-20-00230
6. Wong KC. 3D-printed patient-specific applications in orthopedics. Orthop Res Rev. 2016;8:57–66. doi:10.2147/orr.S99614
7. Ni J, Ling H, Zhang S, et al. Three-dimensional printing of metals for biomedical applications. Mater Today Bio. 2019;3:100024. doi:10.1016/j.mtbio.2019.100024
8. Cheng L, Suresh KS, He H, et al. 3D printing of micro- and nanoscale bone substitutes: a review on technical and translational perspectives. Int J Nanomedicine. 2021;16:4289–4319. doi:10.2147/ijn.S311001
9. Goldmann WH. Biosensitive and antibacterial coatings on metallic material for medical applications. Cell Biol Int. 2021;45(8):1624–1632. doi:10.1002/cbin.11604
10. Lu M, Chen H, Yuan B, et al. The morphological effect of nanostructured hydroxyapatite coatings on the osteoinduction and osteogenic capacity of porous titanium. Nanoscale. 2020;12(47):24085–24099. doi:10.1039/d0nr06306a
11. Day SJ, Riley SP. Utilising three-dimensional printing techniques when providing unique assistive devices: a case report. Prosthet Orthot Int. 2018;42(1):45–49. doi:10.1177/0309364617741776
12. Luan HQ, Wang LT, Ren WY, et al. The effect of pore size and porosity of Ti6Al4V scaffolds on MC3T3-E1 cells and tissue in rabbits. Sci China. 2019;62(7):1160–1168. doi:10.1007/s11431-018-9352-8
13. Zhang XY, Fang G, Zhou J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: a review. Materials. 2017;10(1):1.
14. Wang H, Su K, Su L, Liang P, Ji P, Wang C. The effect of 3D-printed Ti(6)Al(4)V scaffolds with various macropore structures on osteointegration and osteogenesis: a biomechanical evaluation. J Mech Behav Biomed Mater. 2018;88:488–496. doi:10.1016/j.jmbbm.2018.08.049
15. Yu T, Gao H, Liu T, Huang Y, Wang C. Effects of immediately static loading on osteointegration and osteogenesis around 3D-printed porous implant: a histological and biomechanical study. Mater Sci Eng C Mater Biol Appl. 2020;108:110406. doi:10.1016/j.msec.2019.110406
16. Ran Q, Yang W, Hu Y, et al. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J Mech Behav Biomed Mater. 2018;84:1–11. doi:10.1016/j.jmbbm.2018.04.010
17. Han TX, Chang B, Ding X, et al. Improved bone formation and ingrowth for additively manufactured porous Ti6Al4V bone implants with strontium laden nanotube array coating. RSC Adv. 2016;6(17):13686–13697. doi:10.1039/c5ra20370h
18. Hou C, An J, Zhao D, et al. Surface modification techniques to produce micro/nano-scale topographies on Ti-based implant surfaces for improved osseointegration. Front Bioeng Biotechnol. 2022;10:835008. doi:10.3389/fbioe.2022.835008
19. Chen W, Shao Y, Li X, Zhao G, Fu J. Nanotopographical surfaces for stem cell fate control: engineering mechanobiology from the bottom. Nano Today. 2014;9(6):759–784. doi:10.1016/j.nantod.2014.12.002
20. van Hengel IAJ, Gelderman FSA, Athanasiadis S, et al. Functionality-packed additively manufactured porous titanium implants. Mater Today Bio. 2020;7:100060. doi:10.1016/j.mtbio.2020.100060
21. Mobbs RJ, Parr WCH, Choy WJ, McEvoy A, Walsh WR, Phan K. Anterior lumbar interbody fusion using a personalized approach: is custom the future of implants for anterior lumbar interbody fusion surgery? World Neurosurg. 2019. doi:10.1016/j.wneu.2018.12.144
22. Mendonça G, Mendonça DB, Aragão FJ, Cooper LF. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials. 2008;29(28):3822–3835. doi:10.1016/j.biomaterials.2008.05.012
23. Rani VV, Vinoth-Kumar L, Anitha VC, Manzoor K, Deepthy M, Shantikumar VN. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012;8(5):1976–1989. doi:10.1016/j.actbio.2012.01.021
24. Ueno T, Tsukimura N, Yamada M, Ogawa T. Enhanced bone-integration capability of alkali- and heat-treated nanopolymorphic titanium in micro-to-nanoscale hierarchy. Biomaterials. 2011;32(30):7297–7308. doi:10.1016/j.biomaterials.2011.06.033
25. Gittens RA, McLachlan T, Olivares-Navarrete R, et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32(13):3395–3403. doi:10.1016/j.biomaterials.2011.01.029
26. Salou L, Hoornaert A, Louarn G, Layrolle P. Enhanced osseointegration of titanium implants with nanostructured surfaces: an experimental study in rabbits. Acta Biomater. 2015;11:494–502. doi:10.1016/j.actbio.2014.10.017
27. Yu X, Xu R, Zhang Z, et al. Different cell and tissue behavior of micro-/nano-tubes and micro-/nano-nets topographies on selective laser melting titanium to enhance osseointegration. Int J Nanomedicine. 2021;16:3329–3342. doi:10.2147/ijn.S303770
28. Karazisis D, Petronis S, Agheli H, et al. The influence of controlled surface nanotopography on the early biological events of osseointegration. Acta Biomater. 2017;53:559–571. doi:10.1016/j.actbio.2017.02.026
29. Wang Q, Huang YX, Qian ZY. Nanostructured surface modification to bone implants for bone regeneration. J Biomed Nanotechnol. 2018;14(4):628–648. doi:10.1166/jbn.2018.2516
30. Su EP, Justin DE, Pratt CR, et al. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Joint J. 2018;100B(1):9–16. doi:10.1302/0301-620x.100b1.Bjj-2017-0551.R1
31. Wang Q, Zhou P, Liu S, et al. Multi-scale surface treatments of titanium implants for rapid osseointegration: a review. Nanomaterials. 2020;10(6). doi:10.3390/nano10061244
32. Shan L, Kadhum AAH, Al-Furjan MSH, et al. In situ controlled surface microstructure of 3D printed ti alloy to promote its osteointegration. Materials. 2019;12(5):815. doi:10.3390/ma12050815
33. van Noort R. The future of dental devices is digital. Dent Mater. 2012;28(1):3–12. doi:10.1016/j.dental.2011.10.014
34. Souza JCM, Sordi MB, Kanazawa M, et al. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019;94:112–131. doi:10.1016/j.actbio.2019.05.045
35. Ren B, Wan Y, Liu C, et al. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: an in vitro and in vivo study. Mater Sci Eng C Mater Biol Appl. 2021;118:111505. doi:10.1016/j.msec.2020.111505
36. Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, Bose S. In vivo response of laser processed porous titanium implants for load-bearing implants. Ann Biomed Eng. 2017;45(1):249–260. doi:10.1007/s10439-016-1673-8
37. Wang H, Zhang X, Wang H, et al. Enhancing the osteogenic differentiation and rapid osseointegration of 3D printed Ti6Al4V Implants via nano-topographic modification. J Biomed Nanotechnol. 2018;14(4):707–715. doi:10.1166/jbn.2018.2551
38. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535
39. van Loveren C, Aartman IH. De PICO-vraag [The PICO (patient-intervention-comparison-outcome) question]. Ned Tijdschr Tandheelkd. 2007;114(4):172–178. Dutch.
40. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi:10.1186/1471-2288-14-43
41. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1577–1579. doi:10.1111/j.1476-5381.2010.00872.x
42. Cohen DJ, Cheng A, Kahn A, et al. Novel osteogenic Ti-6Al-4V Device for restoration of dental function in patients with large bone deficiencies: design, development and implementation. Sci Rep. 2016;6:20493. doi:10.1038/srep20493
43. Hyzy SL, Cheng A, Cohen DJ, et al. Novel hydrophilic nanostructured microtexture on direct metal laser sintered Ti-6Al-4V surfaces enhances osteoblast response in vitro and osseointegration in a rabbit model. J Biomed Mater Res A. 2016;104(8):2086–2098. doi:10.1002/jbm.a.35739
44. Cohen DJ, Cheng A, Sahingur K, et al. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed Mater. 2017;12(2):25021. doi:10.1088/1748-605X/aa6810
45. Mitra I, Bose S, Dernell WS, et al. 3D Printing in alloy design to improve biocompatibility in metallic implants. Mater Today. 2021;45:20–34. doi:10.1016/j.mattod.2020.11.021
46. Bose S, Banerjee D, Shivaram A, Tarafder S, Bandyopadhyay A. Calcium phosphate coated 3D printed porous titanium with nanoscale surface modification for orthopedic and dental applications. Mater Des. 2018;151:102–112. doi:10.1016/j.matdes.2018.04.049
47. Cheng A, Cohen DJ, Kahn A, et al. Laser sintered porous Ti-6Al-4V implants stimulate vertical bone growth. Ann Biomed Eng. 2017;45(8):2025–2035. doi:10.1007/s10439-017-1831-7
48. Mobbs RJ, Phan K, Assem Y, Pelletier M, Walsh WR. Combination Ti/PEEK ALIF cage for anterior lumbar interbody fusion: early clinical and radiological results. J Clin Neurosci. 2016;34:94–99. doi:10.1016/j.jocn.2016.05.028
49. Wang Y, Gao M, Wang D, Sun L, Webster TJ. Nanoscale 3D bioprinting for osseous tissue manufacturing. Int J Nanomedicine. 2020;15:215–226. doi:10.2147/ijn.S172916
50. Sheng X, Wang A, Wang Z, Liu H, Wang J, Li C. Advanced surface modification for 3D-printed titanium alloy implant interface functionalization. Front Bioeng Biotechnol. 2022;10:850110. doi:10.3389/fbioe.2022.850110
51. Wang H, Su K, Su L, Liang P, Ji P, Wang C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater Sci Eng C Mater Biol Appl. 2019;104:109908. doi:10.1016/j.msec.2019.109908
52. Xiu P, Jia Z, Lv J, et al. Tailored surface treatment of 3D printed porous Ti6Al4V by microarc oxidation for enhanced osseointegration via optimized bone in-growth patterns and interlocked bone/implant interface. ACS Appl Mater Interfaces. 2016;8(28):17964–17975. doi:10.1021/acsami.6b05893
53. Jing Z, Zhang T, Xiu P, et al. Functionalization of 3D-printed titanium alloy orthopedic implants: a literature review. Biomed Mater. 2020;15(5):052003. doi:10.1088/1748-605X/ab9078
54. Bai L, Liu Y, Du Z, et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018;76:344–358. doi:10.1016/j.actbio.2018.06.023
55. Wang C, Hu H, Li Z, et al. Enhanced osseointegration of titanium alloy implants with laser microgrooved surfaces and graphene oxide coating. ACS Appl Mater Interfaces. 2019;11(43):39470–39483. doi:10.1021/acsami.9b12733
56. Sinha A, Simnani FZ, Singh D, et al. The translational paradigm of nanobiomaterials: biological chemistry to modern applications. Mater Today Bio. 2022;17:100463. doi:10.1016/j.mtbio.2022.100463
57. Alves-Rezende MCR, Capalbo LC, De Oliveira Limírio JPJ, Capalbo BC, Limírio P, Rosa JL. The role of TiO(2) nanotube surface on osseointegration of titanium implants: biomechanical and histological study in rats. Microsc Res Tech. 2020;83(7):817–823. doi:10.1002/jemt.23473
58. Chen S, Shi X, Chinnathambi S, Wu H, Hanagata N. Generation of microgrooved silica nanotube membranes with sustained drug delivery and cell contact guidance ability by using a Teflon microfluidic chip. Sci Technol Adv Mater. 2013;14(1):015005. doi:10.1088/1468-6996/14/1/015005
59. Yoon JK, Kim HN, Bhang SH, et al. Enhanced bone repair by guided osteoblast recruitment using topographically defined implant. Tissue Eng Part A. 2016;22(7–8):654–664. doi:10.1089/ten.TEA.2015.0417
60. Lavenus S, Berreur M, Trichet V, Pilet P, Louarn G, Layrolle P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur Cell Mater. 2011;22:84–96. doi:10.22203/ecm.v022a07
61. Sjöström T, Dalby MJ, Hart A, Tare R, Oreffo RO, Su B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009;5(5):1433–1441. doi:10.1016/j.actbio.2009.01.007
62. Ning C, Wang S, Zhu Y, et al. Ti nanorod arrays with a medium density significantly promote osteogenesis and osteointegration. Sci Rep. 2016;6:19047. doi:10.1038/srep19047
63. Faeda RS, Tavares HS, Sartori R, Guastaldi AC, Marcantonio E. Biological performance of chemical hydroxyapatite coating associated with implant surface modification by laser beam: biomechanical study in rabbit tibias. J Oral Maxillofac Surg. 2009;67(8):1706–1715. doi:10.1016/j.joms.2009.03.046
64. Wu PK, Ringeisen BR, Callahan J, et al. The deposition, structure, pattern deposition, and activity of biomaterial thin-films by matrix-assisted pulsed-laser evaporation (MAPLE) and MAPLE direct write. Thin Solid Films. 2001;398–399:607–614. doi:10.1016/S0040-6090(01)01347-5
65. Cunha A, Serro AP, Oliveira V, Almeida A, Vilar R, Durrieu M-C. Wetting behaviour of femtosecond laser textured Ti–6Al–4V surfaces. Appl Surf Sci. 2013;265:688–696. doi:10.1016/j.apsusc.2012.11.085
66. Gehrke SA, Taschieri S, Del Fabbro M, Coelho PG. Positive biomechanical effects of titanium oxide for sandblasting implant surface as an alternative to aluminium oxide. J Oral Implantol. 2015;41(5):515–522. doi:10.1563/aaid-joi-d-13-00019
67. Medvedev AE, Ng HP, Lapovok R, Estrin Y, Lowe TC, Anumalasetty VN. Effect of bulk microstructure of commercially pure titanium on surface characteristics and fatigue properties after surface modification by sand blasting and acid-etching. J Mech Behav Biomed Mater. 2016;57:55–68. doi:10.1016/j.jmbbm.2015.11.035
68. Geng Z, Li X, Ji L, et al. A novel snail-inspired bionic design of titanium with strontium-substituted hydroxyapatite coating for promoting osseointegration. J Mater Sci Technol. 2021;79:35–45. doi:10.1016/j.jmst.2020.11.041
69. Geng Z, Li Z, Cui Z, Wang J, Yang X, Liu C. Novel bionic topography with MiR-21 coating for improving bone-implant integration through regulating cell adhesion and angiogenesis. Nano Lett. 2020;20(10):7716–7721. doi:10.1021/acs.nanolett.0c03240
70. Shuai C, Yang W, Feng P, Peng S, Pan H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact Mater. 2021;6(2):490–502. doi:10.1016/j.bioactmat.2020.09.001
71. Feng P, Zhao R, Tang W, et al. Structural and functional adaptive artificial bone: materials, fabrications, and properties. Adv Funct Mater. 2023;33(23):2214726. doi:10.1002/adfm.202214726
72. Li J, Cui X, Hooper GJ, Lim KS, Woodfield TBF. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: a review. J Mech Behav Biomed Mater. 2020;105:103671. doi:10.1016/j.jmbbm.2020.103671
73. Surmeneva M, Lapanje A, Chudinova E, et al. Decreased bacterial colonization of additively manufactured Ti6Al4V metallic scaffolds with immobilized silver and calcium phosphate nanoparticles. Appl Surf Sci. 2019;480:822–829. doi:10.1016/j.apsusc.2019.03.003
74. Soyama H, Takeo F. Effect of various peening methods on the fatigue properties of titanium alloy Ti6Al4V manufactured by direct metal laser sintering and electron beam melting. Materials. 2020;13(10). doi:10.3390/ma13102216
75. Mieszkowska A, Beaumont H, Martocq L, et al. Phenolic-enriched collagen fibrillar coatings on titanium alloy to promote osteogenic differentiation and reduce inflammation. Int J Mol Sci. 2020;21(17). doi:10.3390/ijms21176406
76. Cheng A, Cohen DJ, Boyan BD, Schwartz Z. Laser-sintered constructs with bio-inspired porosity and surface micro/nano-roughness enhance mesenchymal stem cell differentiation and matrix mineralization in vitro. Calcif Tissue Int. 2016;99(6):625–637. doi:10.1007/s00223-016-0184-9
77. Qasim M, Chae DS, Lee NY. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 2019;14:4333–4351. doi:10.2147/ijn.S209431
78. Cheng A, Humayun A, Cohen DJ, Boyan BD, Schwartz Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication. 2014;6(4):045007. doi:10.1088/1758-5082/6/4/045007
79. Li S, Huan Y, Zhu B, et al. Research progress on the biological modifications of implant materials in 3D printed intervertebral fusion cages. J Mater Sci Mater Med. 2021;33(1):2. doi:10.1007/s10856-021-06609-4
80. Cornell CN, Lane JM. Current understanding of osteoconduction in bone regeneration. Clin Orthop Relat Res. 1998;355:S267–73. doi:10.1097/00003086-199810001-00027
81. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–129. doi:10.1111/1469-0691.12494
82. Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi:10.1196/annals.1365.035
83. Zhou W, Kuderer S, Liu Z, Ulm C, Rausch-Fan X, Tangl S. Peri-implant bone remodeling at the interface of three different implant types: a histomorphometric study in mini-pigs. Clin Oral Implants Res. 2017;28(11):1443–1449. doi:10.1111/clr.13009
84. McGuire MK, Wilson TG
85. Harawaza K, Cousins B, Roach P, Fernandez A. Modification of the surface nanotopography of implant devices: a translational perspective. Mater Today Bio. 2021;12:100152. doi:10.1016/j.mtbio.2021.100152
86. Xu J, Zhang J, Shi Y, et al. Surface modification of biomedical Ti and Ti alloys: a review on current advances. Materials. 2022;15(5). doi:10.3390/ma15051749
87. Skjöldebrand C, Tipper JL, Hatto P, Bryant M, Hall RM, Persson C. Current status and future potential of wear-resistant coatings and articulating surfaces for Hip and knee implants. Mater Today Bio. 2022;15:100270. doi:10.1016/j.mtbio.2022.100270
88. Feng P, Shen S, Yang L, Kong Y, Yang S, Shuai C. Vertical and uniform growth of MoS2 nanosheets on GO nanosheets for efficient mechanical reinforcement in polymer scaffold. Virtual Phys Prototyp. 2023;18(1):e2115384. doi:10.1080/17452759.2022.2115384
89. Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(Suppl 2):S96–101. doi:10.1007/s005860100282
90. Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67(8):932–949.
91. Hanawa T. A comprehensive review of techniques for biofunctionalization of titanium. J Periodontal Implant Sci. 2011;41(6):263–272.
92. Brett PM, Harle J, Salih V, et al. Roughness response genes in osteoblasts. Bone. 2004;35(1):124–133. doi:10.1016/j.bone.2004.03.009
93. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–854. doi:10.1016/j.dental.2006.06.025
94. Shuai C, Peng B, Feng P, Yu L, Lai R, Min A. In situ synthesis of hydroxyapatite nanorods on graphene oxide nanosheets and their reinforcement in biopolymer scaffold. J Adv Res. 2022;35:13–24. doi:10.1016/j.jare.2021.03.009
95. Brammer KS, Frandsen CJ, Jin S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012;30(6):315–322. doi:10.1016/j.tibtech.2012.02.005
96. Chakraborty S, Banerjee S, Raina M, Haldar S. Force-Directed “Mechanointeractome” of Talin-Integrin. Biochemistry. 2019;58(47):4677–4695. doi:10.1021/acs.biochem.9b00442
97. LaFlamme SE, Mathew-Steiner S, Singh N, Colello-Borges D, Nieves B. Integrin and microtubule crosstalk in the regulation of cellular processes. Cell Mol Life Sci. 2018;75(22):4177–4185. doi:10.1007/s00018-018-2913-x
98. Olivares-Navarrete R, Hyzy SL, Berg ME, et al. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann Biomed Eng. 2014;42(12):2551–2561. doi:10.1007/s10439-014-1108-3
99. Olivares-Navarrete R, Hyzy SL, Hutton DL, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31(10):2728–2735. doi:10.1016/j.biomaterials.2009.12.029
100. Xue W, Krishna BV, Bandyopadhyay A, Bose S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007;3(6):1007–1018. doi:10.1016/j.actbio.2007.05.009
101. Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92(3):1218–1224. doi:10.1002/jbm.a.32463
102. Gittens RA, Olivares-Navarrete R, Cheng A, et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013;9(4):6268–6277. doi:10.1016/j.actbio.2012.12.002
103. Gittens RA, Olivares-Navarrete R, McLachlan T, et al. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium–aluminum–vanadium alloy surfaces. Biomaterials. 2012;33(35):8986–8994. doi:10.1016/j.biomaterials.2012.08.059
104. Wennerberg A, Jimbo R, Stübinger S, Obrecht M, Dard M, Berner S. Nanostructures and hydrophilicity influence osseointegration: a biomechanical study in the rabbit tibia. Clin Oral Implants Res. 2014;25(9):1041–1050. doi:10.1111/clr.12213
105. He X, Yamada M, Watanabe J, et al. Titanium nanotopography induces osteocyte lacunar-canalicular networks to strengthen osseointegration. Acta Biomater. 2022;151:613–627. doi:10.1016/j.actbio.2022.08.023
106. Das K, Bose S, Bandyopadhyay A. TiO 2 nanotubes on Ti: influence of nanoscale morphology on bone cell-materials interaction. J Biomed Mater Res A. 2009;90A(1):225–237. doi:10.1002/jbm.a.32088
107. Zreiqat H, Valenzuela SM, Nissan BB, et al. The effect of surface chemistry modification of titanium alloy on signalling pathways in human osteoblasts. Biomaterials. 2005;26(36):7579–7586. doi:10.1016/j.biomaterials.2005.05.024
108. Hyzy SL, Olivares-Navarrete R, Ortman S, Boyan BD, Schwartz Z. Bone morphogenetic protein 2 alters osteogenesis and anti-inflammatory profiles of mesenchymal stem cells induced by microtextured titanium in vitro. Tissue Eng Part A. 2017;23(19–20):1132–1141. doi:10.1089/ten.tea.2017.0003
109. Nagasawa M, Cooper LF, Ogino Y, et al. Topography influences adherent cell regulation of osteoclastogenesis. J Dent Res. 2016;95(3):319–326. doi:10.1177/0022034515616760
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

