Back to Journals » International Journal of Nanomedicine » Volume 17
Nanomaterial-Based Electrically Conductive Hydrogels for Cardiac Tissue Repair
Received 18 August 2022
Accepted for publication 23 November 2022
Published 9 December 2022 Volume 2022:17 Pages 6181—6200
DOI https://doi.org/10.2147/IJN.S386763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Mingyu Lee,1 Min Chul Kim,2 Jae Young Lee1
1School of Materials Science and Engineering, Gwangju Institute of Science and Technology, Gwangju, Republic of Korea; 2Division of Cardiology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Republic of Korea
Correspondence: Jae Young Lee, Tel +82 62 715 2358, Email [email protected]
Abstract: Cardiovascular disease is one of major causes of deaths, and its incidence has gradually increased worldwide. For cardiovascular diseases, several therapeutic approaches, such as drugs, cell-based therapy, and heart transplantation, are currently employed; however, their therapeutic efficacy and/or practical availability are still limited. Recently, biomaterial-based tissue engineering approaches have been recognized as promising for regenerating cardiac function in patients with cardiovascular diseases, including myocardial infarction (MI). In particular, materials mimicking the characteristics of native cardiac tissues can potentially prevent pathological progression and promote cardiac repair of the heart tissues post-MI. The mechanical (softness) and electrical (conductivity) properties of biomaterials as non-biochemical cues can improve the cardiac functions of infarcted hearts by mitigating myocardial cell death and subsequent fibrosis, which often leads to cardiac tissue stiffening and high electrical resistance. Consequently, electrically conductive hydrogels that can provide mechanical strength and augment the electrical activity of the infarcted heart tissue are considered new functional materials capable of mitigating the pathological progression to heart failure and stimulating cardiac regeneration. In this review, we highlight nanomaterial-incorporated hydrogels that can induce cardiac repair after MI. Nanomaterials, including carbon-based nanomaterials and recently discovered two-dimensional nanomaterials, offer great opportunities for developing functional conductive hydrogels owing to their excellent electrical conductivity, large surface area, and ease of modification. We describe recent results using nanomaterial-incorporated conductive hydrogels as cardiac patches and injectable hydrogels for cardiac repair. While further evaluations are required to confirm the therapeutic efficacy and toxicity of these materials, they could potentially be used for the regeneration of other electrically active tissues, such as nerves and muscles.
Keywords: nanomaterials, conductive, hydrogel, cardiac tissue engineering
Graphical Abstract:
Introduction
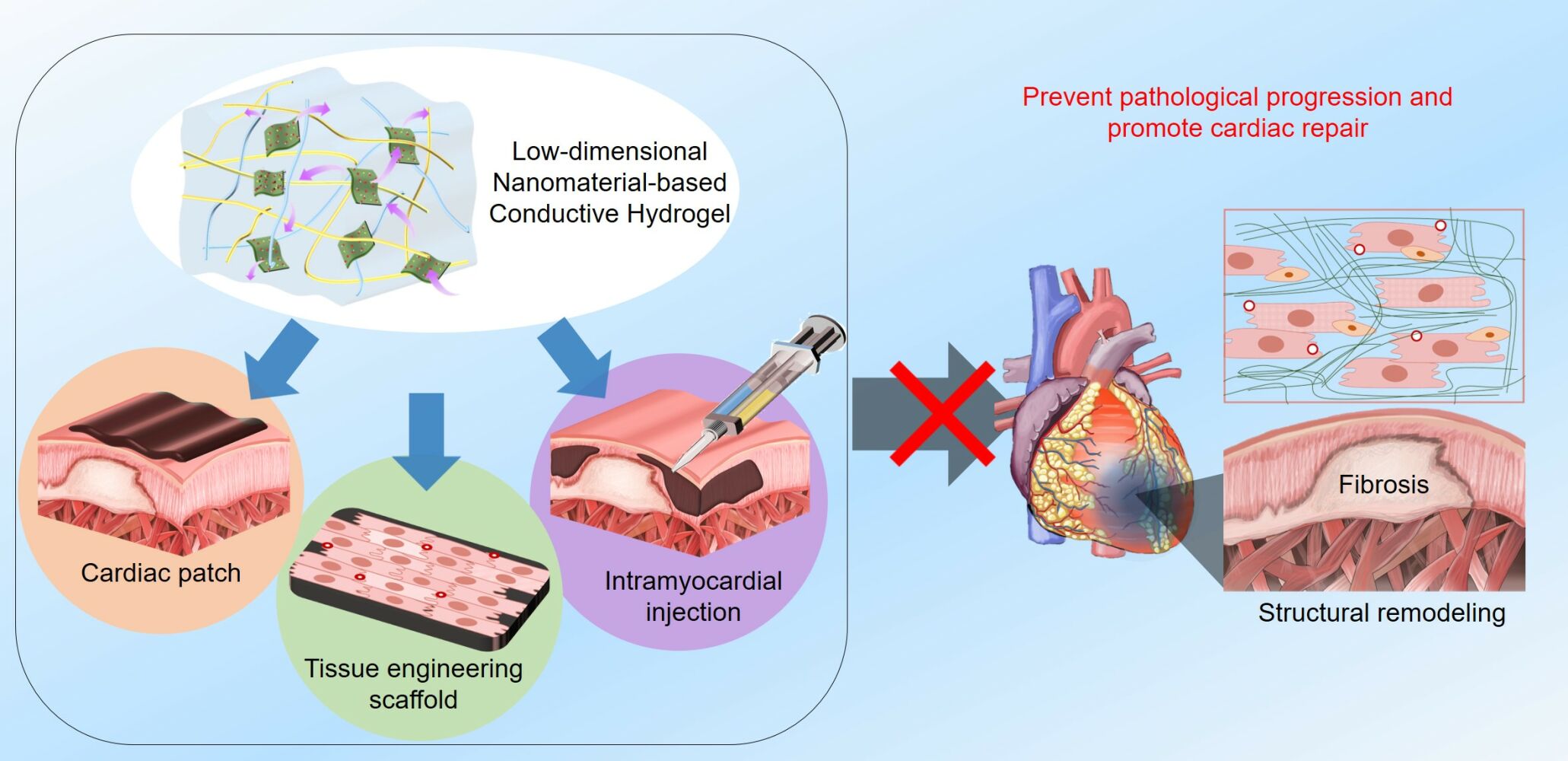
Cardiovascular diseases are fatal, accounting for more than 17.9 million deaths annually worldwide.1,2 Particularly, ischemic heart disease, which ultimately manifest as myocardial infarction (MI), is a major cause of cardiovascular disease-associated deaths.1 For example, MI cases were reportedly 8.8 million (3.1%, age ≥ 20 years) in the United States in 2015–2018 and 104,280 of the patients (all years) died in 2019.1 MI is a consequence of myocardial cell death, mainly driven by myocardial ischemia due to atheromatous plaque rupture and thrombus formation in the coronary artery, which leads to insufficient blood flow and oxygen supply to the heart muscle.3 The infarcted heart tissues undergo ventricular remodeling, which includes necrosis, structural remodeling, ventricular wall thinning, chamber dilatation, and eventually fibrosis.4,5 (Figure 1) This pathological process post-MI (ischemic cardiomyopathy) is associated with development of heart failure. Heart transplantation is currently the most feasible method for heart repair after MI; however, it has several critical issues, such as insufficient number of donors and risks of infection.6 Hence, developing other efficient MI treatments is highly necessary.
 |
Figure 1 Myocardial infarction (MI) and pathological progression of the infarcted heart showing ventricular remodeling and fibrosis. |
After MI, cardiomyocytes (CMs) in the heart muscles undergo severe cell death. The damaged heart tissues suffer the pathological remodeling due to the insufficient self-regeneration capability of the heart tissues, which eventually causes fibrous collagen fiber deposition in the infarcted zone. These fibrotic tissues lead to cardiac tissue wall thinning and stiffening.2 (Figure 1) In addition, CMs in the non-conductive fibrotic area are not electrically interconnected with other cells.13 This electrical disconnection impairs synchronous cardiac cell coupling and cardiac contraction/relaxation. Improper electrical signaling in infarcted areas frequently causes fatal ventricular arrhythmia and sudden cardiac cell death.13 For infarcted heart, mechanical support to the infarcted myocardium can alleviate its pathological remodeling. Hence, biomaterial-based treatment (eg, cardiac patches and hydrogel injection into myocardium) can potentially augment the mechanical functions of the infarcted heart.5,7,8 Concurrently, electrically conductive materials have been found to improve electrophysiological cardiac functions.9–12 Numerous studies have been focused on regenerating infarcted heart function, such as artificial heart transplantation, drugs, cell therapy, and biomaterial implants.14–19 Among these, biomaterials have been actively examined for cardiac repair.20,21 Biomaterial-based approaches can offer several benefits, such as ease in production, versatility in modification, and therapeutic activity. Biomaterials can be designed and engineered to deliver specific mechanical, electrical and biological cues to infarcted heart to enhance its regeneration. In addition, biomaterials can be used together with drugs and cells to further enhance their therapeutic efficacy for regeneration of heart function.
Hydrogels are three-dimensional (3D) hydrophilic polymeric networks.22 They are structurally and functionally similar to the natural extracellular matrix (ECM).2 The mechanical properties of hydrogels are similar to those of the cardiac tissue (eg, elastic modulus, toughness, and stretchability), which can provide mechanical support to infarcted hearts and attenuate pathological remodeling.23,24 For cardiac tissue engineering, hydrogels have been produced in the form of a patch and filler which can mechanically support heart tissue functions; furthermore, they have been engineered by controlling their composition and manufacturing processes.8,25 Additionally, hydrogels can be used as delivery vehicles,26–29 and can address the issues associated with directly injecting therapeutic components (drugs and cells) into the cardiac muscle, such as sustained drug release or enhanced cell viability.30,31 The sustained release of therapeutic substances can be achieved by creating specialized interactions between polymeric chains and drug substances. For cell delivery, the 3D microenvironments of hydrogels can enhance the viability and therapeutic functions of the delivered cells.32–35
Recently, electrically conductive hydrogels have garnered significant attention in biomedical fields for bioelectrode and tissue engineering applications.36–39 Because conventional hydrogels are electrically insulating, conductive hydrogels are usually fabricated by formulating conductive materials with hydrophilic polymers.40,41 As a result, conductive hydrogels can be engineered to exhibit mechanical and electrical properties similar to those of normal cardiac tissue.25,42–44 Note that the native myocardium tissue typically shows 1 mS/cm and 20–500 kPa of conductivity and Young’s modulus, respectively.45,46 Electrically conductive biomaterials can promote cardiac contraction/relaxation functions and electrical coupling,42 induce the maturation of CMs in the infarcted heart,43 and prevent its progression to arrhythmia.47 Consequently, conductive hydrogels can be a good option to mechanically support heart tissues and effectively transmit electrical signals within the myocardium for cardiac tissue repair post-MI. Various electrically conductive materials (eg, conductive polymers, metals, and nanomaterials) have been used to produce conductive hydrogels for cardiac tissue engineering. Among them, one-dimensional (1D) and two-dimensional (2D) nanomaterials present unique material characteristics (eg, high electrical conductivity, large surface areas, strong molecular interactions with various [macro]molecules, and versatility in modification), which benefit the development of functional conductive hydrogels capable of effectively modulating cardiac cell behaviors and stimulating cardiac tissue regeneration.48 These unique properties of low-dimensional nanomaterials can be used to develop the high-performance conductive hydrogels presenting desirable biological, electrical, and mechanical characteristics for cardiac repair. Consequently, we focused on the nanomaterial-based conductive hydrogels for cardiac repair in this review. In the first section, we describe the fabrication of conductive hydrogels using different types of low-dimensional nanomaterials. Subsequently, the studies with various conductive hydrogels containing low-dimensional nanomaterials for cardiac repair are described. Next, the applications of conductive hydrogels in cardiac treatments, mainly as patches and injectable hydrogels, are reviewed along with recent results. Finally, conductive hydrogels with newly discovered low-dimensional nanomaterials are described.
Conductive Hydrogels for Cardiac Repair
Fabrication of Conductive Hydrogels
Conductive hydrogels are typically fabricated by incorporating conductive components into hydrogel networks. These composite hydrogels were prepared by the in situ formation of a conductive network within the pre-formed hydrogels or polymerization of a pre-polymer solution containing conductive materials.49 To fabricate conductive hydrogels with nanomaterials, pre-polymer solutions containing conductive 1D or 2D nanomaterials are prepared and subjected to gelation via various polymerization methods (eg, photopolymerization, thermal polymerization, and self-assembly).50–52 Importantly, low-dimensional conductive components should be homogeneously dispersed and stabilized within the polymer matrices to obtain an appropriately conductive network.53–55 Therefore, the molecular interactions between conductive components and polymeric chains should be carefully examined. Conductive components can be covalently conjugated with polymer matrices to enhance the dispersion of conductive components and the stability of the resultant conductive hydrogels.44,56,57 The fabrication methods also greatly influence the characteristics of the prepared conductive hydrogels, such as conductivity, stability, biosafety, and processability. For example, conductive hydrogels can be fabricated simply by blending and cross-linking. However, several issues, such as the non-uniform distribution of the conductive components within a hydrogel and the potential instability and toxicity resulting from the leakage of conductive components, frequently cause difficulties in their biomedical applications.44,58 Alternatively, the chemical conjugation of a conductive component to a hydrogel network can improve conductivity, long-term stability, and safety; however, this process is relatively complicated and requires an additional washing step to remove unreacted chemicals and by-products.59,60 Overall, the performance of conductive hydrogels is affected by various factors, such as the polymer type, crosslinking type and degree, conductive component type, and composition.61 For example, biodegradable conductive hydrogels can be prepared by formulating conductive components and natural biodegradable polymers (eg, gelatin, collagen, chitosan, and alginate) or synthetic hydrolyzable polymers (eg polyesters, polyanhydrides, and polycarbonates).62–67 Furthermore, multifunctional conductive hydrogels (eg mechanical, sensory, anti-freezing, adhesive and self-healing properties) can be designed and fabricated using polymers with specific biological, chemical, and physical properties.68
Various conductive nanomaterials, including carbon-based nanomaterials and MXenes, have been developed (Figure 2). One- and two-dimensional nanomaterials have unique characteristics, such as high electrical conductivity, large surface area, various molecular interactions, and flexibility, which make them promising for developing new functional materials in various fields, such as energy, optics, electronics, and medicine.69–73 Other conductive components, such as conductive polymers and metal nanomaterials, can also be useful for producing conductive hydrogels and have shown utility in various applications, including cardiac tissue engineering.39,74–76 However, several challenges remain concerning material stability, mass production, and effectiveness.77 Therefore, we focused on conductive hydrogels composed of non-metallic low-dimensional nanomaterials with respect to their fabrication and potential uses in cardiac repair. It should be noted that the biosafety or toxicity of most nanomaterials has not been clearly determined because their interpretations differ depending on the type, structure, and functionalization of nanomaterials, and in vitro and in vivo experimental conditions. Accordingly, the clinical feasibility of conductive hydrogels composed of different 1D and 2D nanomaterials should be carefully evaluated in future studies.
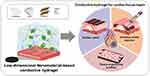 |
Figure 2 Conductive hydrogels containing low-dimensional non-metallic nanomaterials and their various applications in cardiac repair. |
Applications of Conductive Hydrogels in Cardiac Treatment
For cardiac regeneration, conductive hydrogels have been administered via intramyocardial injection, intrapericardial injection, or by attaching to the epicardium of the heart.5,78,79 Administration of a hydrogel by injection mainly supports the mechanical function of the myocardium within the local cardiac tissue. On the other hand, a cardiac patch assists the mechanical function of the heart by preventing excessive dilation. In addition, therapeutic molecules can be loaded into hydrogels for local delivery in the myocardium (hydrogel injection) or from the heart surface (attachment). These conductive hydrogels can also serve as in vitro platforms to study cardiac cell/tissue responses to mechanical and electrical environments and develop functional tissue engineering scaffolds for cardiac repair (Figure 2).
Injectable hydrogels can be administered with minimal invasiveness.80 Various injectable hydrogels have been developed for tissue engineering and drug delivery applications. For example, temperature-responsive polymers, pH-responsive polymers, mild chemical reactions, photopolymerization, and magnetic polymerization have been developed to produce injectable hydrogel systems.81 The conductive hydrogel injected into the myocardium can provide a 3D electrical bypass around the MI site and aid in restoring the electrical communications between cells and/or tissue.82,83 Various conductive materials can be incorporated into injectable hydrogel systems to produce injectable conductive hydrogels. Specifically, injectable conductive hydrogels can be formed after intramyocardial or intrapericardial injection through in situ crosslinking of pre-polymers containing conductive materials based on various reactions, such as photo-crosslinking, click reactions, Schiff base reactions, and Michael reactions.84,85 Wang et al designed an injectable conductive hydrogel via in situ polymerization of tetraaniline-polyethylene glycol (TA-PEG) and thiolated hyaluronic acid (HA-SH) via Michael addition.86 Adipose-derived stem cells (ADSCs) and lipofectamine nanocomplexes containing plasmid DNA-eNOs (endothelial nitric oxide synthase) were loaded into the gels and subsequently injected into the infarcted heart. They demonstrated an increased expression of eNOs in the myocardium with upregulated proangiogenic factors and structural and functional cardiac improvements, as indicated by electrocardiography, cardiography, and histological analysis. (Figure 3A) However, these hydrogels had low conductivity (0.023 S/m) and the conducting polymer (tetraaniline) showed drawbacks, such as lack of biosafety and stability in vivo.87,88 Intrapericardial injection of hydrogels is an attractive treatment option for cardiac repair.89–91 The pericardium is a double-walled sac that plays a key role in protecting cardiac tissues from infection and provides lubrication for heart movements.91–93 The space between the double-walled sac, called the pericardial cavity, is usually filled with pericardial fluid. Hydrogel injection into this pericardial cavity does not require fixation of the injected hydrogel using surgical sutures or adhesives, and supports the mechanical properties of heart tissues. Zhu et al injected a decellularized ECM hydrogel embedded with mesenchymal stem cell-derived exosomes and induced pluripotent stem cell-derived cardiac progenitors into the pericardial cavity.91 Although conductive hydrogels have not yet been tested for pericardial injection, an injectable conductive hydrogel might be beneficial to promote heart regeneration after MI by providing physical support and electrical bypass to the heart.
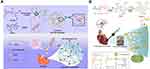 |
Figure 3 Conductive hydrogels for cardiac repair. (A) Intramyocardial injection of a conductive hydrogel consisting of TA-PEG and HA-SH loaded with ADSCs and Lipofectamine/plasmid-eNOs. (B) Conductive cardiac patch composed of HPAE, gelatin, and PPy nanospheres. The composite was paintable and in situ formed as a conductive and adhesive cardiac patch on the epicardium of infarcted heart. Notes: (A) Reprinted with permission from Wang W, Tan B, Chen J et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials. 2018;160:69–81.86 Copyright (2018) Elsevier. (B) Reprinted with permission from Liang S, Zhang Y, Wang H et al. Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv Mater. 2018;30(23):1,704,235.44 ©Wiley 2018. |
Conductive cardiac patches are another promising application of conductive hydrogels for inducing cardiac tissue regeneration.47,94 Unlike injection into the myocardium, a cardiac patch does not require invasion of the myocardium or pericardium. Conductive hydrogel patches can provide a 2D electrical bypass surrounding the epicardium.47 Cardiac patches, including conductive hydrogels, are usually fixed onto the epicardium using conventional surgical sutures, staples, or light irradiation,94,95 which can lead to bleeding, blockage of blood supply, or further inflammatory responses. Therefore, the adhesive property of conductive hydrogels is desired to stably immobilize conductive patches onto the epicardium. The adhesiveness of hydrogels can be achieved through physical or chemical interactions between hydrogel components and cardiac tissue.7,44 For example, Tang et al prepared an adhesive cardiac patch using commercial fibrin glue.96 However, commercial bioadhesives (eg, cyanoacrylates and fibrin glue) often exhibit cytotoxicity, high stiffness, insufficient adhesion, and structural instability.95,97–99 Recently, several adhesive hydrogels were developed in the forms of paintable gels,44 sprayable gels,100 and lyophilized tapes101,102 to improve adhesion strengths at cardiac tissue interfaces. Liang et al developed paintable and rapidly bondable conductive hydrogels via Fe3+-triggered polymerization of pyrrole-conjugated hyperbranched poly(amino ester) with gelatin.44 This conductive and adhesive hydrogel could be painted onto the epicardium, and it bonded strongly to the beating heart, remained intact for four weeks, and significantly restored cardiac function and revascularization of the infarcted myocardium. (Figure 3B) However, in view of cytotoxicity, the residual pyrrole monomers in the in situ-formed paintable hydrogel may be a potential concern. In addition, the resultant conductive hydrogel had very low conductivity (0.0651 S/m).44 Few studies have reported on adhesive and conductive cardiac patches. Nevertheless, adhesive and conductive hydrogel patches composed of low-dimensional nanomaterials (eg, carbon nanotube (CNT), graphene, and MXene) are expected to be promising for efficient cardiac repair.
Nanomaterials-Incorporated Conductive Hydrogels for Cardiac Repair
Carbon-Based Nanomaterials
Carbon-based nanomaterials have diverse structures and chemical properties. Such diverse structures of carbon nanomaterials are attributed to various hybridization modes (sp, sp2, and sp3) between the carbon atoms.103 The ratios of each hybridization determine the structures of the carbon nanomaterials as 0D, 1D, and 2D, which correspond to quantum dots, carbon nanotubes, and graphene, respectively.104 Allotropes, with their unique advantages, can be extensively used in a variety of applications, including the fabrication of conductive hydrogels. The electrical properties of a carbon nanostructure increase as the anisotropy and degree of replication increase. For example, typical CNT and graphene exhibit excellent electrical conductivities of 104–108 S/m and 104–105 S/m, respectively.105–107 Carbon nanomaterials are very small and have high surface-area-to-mass ratios. For example, graphene is an atomically thin 2D material with lateral sizes ranging from tens to thousands of nanometers. These unique structures enable efficient conductive network formation, intimate interactions with biological components (eg, proteins), and effective electrical transmission with cells and tissues in relatively small amounts. Additional functionalization of the carbon nanomaterials can improve the performance of the prepared conductive hydrogels by modulating their interactions with other components (eg, solvents, nanomaterials, matrices, and biological molecules) and/or themselves. In this section, we focus on conductive hydrogels composed of various carbon-based nanomaterials for cardiac tissue applications.
CNTs
CNTs are 1D cylindrical nanostructured carbon materials with high anisotropy and extremely high aspect ratio.108 Based on the number of cylindrical graphene layers, CNTs are classified into single-walled (SWCNTs; 0.4–2 nm in diameter) or multi-walled (MWCNTs; 2–100 nm in diameter) CNTs.109 CNTs typically exhibit high electrical conductivities, flexibilities, and mechanical strengths.104 CNTs have been used in a wide range of biomedical applications, including in drug delivery and tissue engineering.110–112 For fabricating CNT-incorporated conductive hydrogels, CNTs must be well dispersed in a hydrophilic polymer matrix and aqueous solution, owing to the inherent hydrophobicity of CNT.113,114 To this end, CNTs are commonly functionalized with substances containing polar functional groups, such as carboxyl acid groups, to be hybridized with various hydrophilic polymers to form a conductive hydrogel.115
CNT-based conductive hydrogels have been produced using various polymers for cardiac tissue applications.52,116 Shin et al prepared CNT–gelatin methacrylate (GelMA) hydrogels and reported their excellent mechanical integrity, electrophysiological functions, and potential to be used as cardiac patches.46 Myocardial tissues cultured on the 50 μm thick CNT-GelMA showed higher synchronous beating rates and lower excitation thresholds than those cultured on CNT-free hydrogel controls. Li et al chemically conjugated SWCNTs with temperature-responsive poly(N-isopropylacrylamide) (PNIPAAm) and intramyocyadially injected this composite hydrogel with brown adipose stem cells (BASCs) into infarcted hearts.52 They found that myocardial injection of PNIPAAm/SWCNT hydrogels promoted the engraftment of encapsulated BASCs in the myocardium and improved cardiac tissue organization and echocardiographic functions. Various types of cells, such as neonatal CMs, BASCs, human-induced pluripotent stem cell (hiPSC)-derived CMs, and human coronary artery endothelial cells (HCAECs), have been encapsulated in CNT-based conductive hydrogels and studied as engineered cardiac tissues.52,117–119 CMs in or on CNT-based conductive hydrogels showed enhanced cell-cell electrical coupling, synchronous beating, and CM maturation (eg, high expression of sarcomeric α-actinin and gap junction protein). Furthermore, CNTs have been functionalized with various moieties to improve their miscibility with polymer chains and/or to provide additional cues to promote cardiac regeneration. For example, conductive hydrogels composed of hydrazide-functionalized CNTs and the pericardial matrix enhanced the maturation of hiPSC-derived cardiomyocytes. Hydrogels with positively charged hydrazide-functionalized CNTs increased cellular alignment, Connexin-43 expression, and sarcomere organization of hiPSC-derived CMs, which was speculated to be due to enhanced interactions between the positively charged CNTs and negatively charged cellular membrane.118 (Figure 4A–C) In another study, Izadifar et al fabricated a cell-laden hydrogel cardiac patch consisting of HCAECs, carboxyl-functionalized CNTs, and alginate.119 The incorporation of carboxyl-functionalized CNTs significantly improved the viscoelastic behavior and electrical conductivity of the composite hydrogels. Furthermore, CNTs aligned within hydrogel matrices can induce cellular orientation and differentiation. Ahadian et al fabricated CNT-aligned GelMA hydrogels via dielectrophoresis and found that these hydrogels showed higher conductivity and promoted cardiac differentiation (eg, expression of cardiac markers and beating) of embryonic bodies compared to pure GelMA and randomly aligned CNT/GelMA hydrogels.120
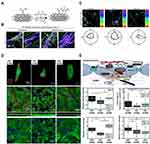 |
Figure 4 CNT-based hydrogels for cardiac repair. (A) Schematic illustration of the synthesis of hydrazide-functionalized MWCNTs. (B) Confocal images of of hiPSC-derived CM-laden tissue constructs stained for Troponin I and Sarcomeric α-Actinin. hiPSC-derived CMs were cultured pm pericardial tissue (PM-tissues) tissues and conductive hydrogels composed of hydrazide-functionalized CNTs and pericardial matrix (PMCNT-tissues) were cultured for 27 and stained. (C) Representative color-coded immunofluorescence images and radar graphs indicating orientation distribution in individual images on day 7. The CMs in the conductive hydrogels showed promoted cellular alignment, Connexin 43 expression, and sarcomere organization. (D) Immunostaining of CMs cultured on various carbon nanomaterials-based conductive hydrogels (CNT-, GO-, and rGO-GelMA). CMs cultured on different conductive hydrogels were stained for cardiac specific markers (ie, Vinculin, Sarcomeric α-actinin, Connexin 43 and Troponin (I) on day 5. (E) Schematic illustration of the proteins for CM maturation on the conductive hydrogels. And the relative intensities of the proteins (ie, Vinculin, Sarcomeric α-actinin, Cx43, and Troponin (I) for CMs cultured on various samples for 5 days (*P < 0.05, **P < 0.005, ***P < 0.005). Notes: (A–C) Reprinted with permission from Roshanbinfar K, Mohammadi Z, Mesgar ASM et al. Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering. Biomater Sci. 2019;7(9):3906–3917.118 ©The Royal Society of Chemistry 2019. (D and E) Reprinted with permission from Lee J, Manoharan V, Cheung L et al. Nanoparticle-Based Hybrid Scaffolds for Deciphering the Role of Multimodal Cues in Cardiac Tissue Engineering. ACS Nano. 2019;13(11):12,525–12,539.123 Copyright (2019) American Chemical Society. |
Although the mechanisms by which CNTs influence the behavior of CMs have not been clearly elucidated, several mechanisms have been proposed. Sun et al found that neonatal CMs in SWCNT-incorporated collagen hydrogels exhibited enhanced cell adhesion, intercalated disc (IDs)-related protein expression, ID assembly, and functionality.121,122 In particular, the β1-integrin-mediated FAK and RhoA signaling pathways were suggested to be responsible for upregulating the electrical and mechanical junction proteins of CMs in the hydrogels, respectively. Lee et al prepared three different types of carbon-nanomaterial-based hydrogels (CNT-, graphene oxide (GO)-, and reduced GO (rGO)-GelMA) and explored CM maturation on individual hydrogels.123 They suggested that hydrogels with higher electrical conductivity (CNT- and rGO-GelMA) enhanced CM maturation compared with the less conductive hydrogel (GO-GelMA). Interestingly, the CM phenotypes were different on these conductive hydrogels. For example, CMs on CNT-GelMA, GO-GelMA, and rGO-GelMA showed ventricular-like, atrial-like, and ventricular/atrial mixed phenotypes, respectively. Also, cardiac tissues produced on CNT-GelMA showed expression levels of maturation markers that were similar to those of native cardiac tissues, as examined by gene expression analyses, and showed increased functionality through integrin-mediated mechanotransduction via YAP/TAZ. (Figure 4D and E) In vivo studies on CNT-based hydrogels for cardiac patch and intramyocardial injection are still insufficient. Most studies have focused on the effects of CNT-based hydrogels on in vitro feasibility, such as cytotoxicity. (Table 1) Particularly, the safety and performance of CNT-based conductive hydrogels in cardiac repair remain unclear. In the future, in-depth studies on CNT-based conductive hydrogels for in vivo cardiac applications and clinical translation are necessary.
 |
Table 1 Summary of Studies Reviewed That Used CNT-Based Hydrogels for Cardiac Repair |
Graphene and Its Derivatives
Graphene is a two-dimensional (2D) carbon sheet with a hexagonal aromatic structure composed of sp2-hybridized carbon atoms.124,125 Graphene displays excellent electrical conductivity and mechanical strength, with a Young’s modulus of 1.0 TPa.126 Graphene and its derivatives (eg, GO and rGO) have been widely used to produce electrically conductive materials.127 Large graphene sheets are usually produced by chemical vapor deposition, which is mostly used in the electronics industry.128 For biomedical applications, graphene derivatives are commonly obtained from graphite via exfoliation under strong acidic and oxidizing conditions.127 The oxidized product was called GO. However, this oxidative process leads to defects in graphene structures with the formation of oxygenated functional groups (eg, carboxylic, hydroxyl, and epoxy groups) at the edges and planes.129 Although GO is well dispersed in aqueous solutions or polar solvents, it has poor electrical conductivity due to the defects in the sp2 carbons.130 The sp2-carbons in GO can be partially restored to rGO by various reduction methods (eg, chemical and thermal reduction).131,132 The resulting rGO exhibits enhanced electrical conductivity and hydrophobicity. To prepare conductive hydrogels using GO or rGO, it is important to appropriately select the GO or rGO considering both dispersion within hydrophilic polymer chains and electroactivity.
Atomically thin 2D structures of graphene derivatives offer effective interactions with various biomolecules and biopolymers, improving the capacity to load drug molecules and strengthening their mechanical properties.133,134 In particular, the hydrogels formulated with graphene derivatives have been shown to be beneficial for drug delivery, tissue engineering, and other biomedical applications.50,135,136 The characteristics of graphene-based hydrogels can be further tailored to improve their functions (eg, mechanical, electrical, chemical, and biological) by adjusting the graphene content and/or controlling interactions between the hydrogel network and graphene derivatives. For example, the functionalization of GO (or rGO) and/or the reduction degree of GO substantially influences the uniformity and electroactivity of composite hydrogels, resulting in dramatic changes in the mechanical and electrical characteristics. Hydrogels prepared by blending GO or rGO with pre-gel solution typically increase elastic modulus (1.1~10 times) and electrical conductivity (2.3~1000 times) compared to GO-free pristine gel. (Table 2) In the case of rGO-based hydrogels, hydrophobic rGO readily aggregates in hydrogels, frequently resulting in a low electrical conductance.137 Although GO is well mixed with hydrophilic polymers, its intrinsically poor electrical properties hinder the production of conductive hydrogels. To overcome these issues, sequential gelation and mild chemical reduction can be used to produce conductive, uniform hydrogels. For instance, our group previously developed a simple and effective strategy to produce conductive rGO-containing hydrogels; a GO-based hydrogel was first prepared by gelation of a pregel solution containing GO and subsequently reduced.43,138 This process substantially increased the electrical conductivity while maintaining the rGO distribution within the matrix without severe aggregation. Various rGO-containing hydrogels could be prepared via subsequent gelation and reduction procedures for muscular and neural tissue engineering.43,138,139
 |
Table 2 Summary of Studies Reviewed That Used Graphene-Based Hydrogels for Cardiac Repair |
Graphene hydrogels can be fabricated to display mechanical and electrochemical characteristics similar to myocardial tissue; such biomimetic graphene-hydrogels can be utilized as cardiac patches and intramyocardial implants to regenerate an infarcted heart. In addition to electrical properties, other beneficial properties of graphene derivatives, including large surface area and various molecular interactions, have enabled the production of hydrogels presenting various interesting characteristics, such as self-healing, anti-oxidant, and antibacterial effects, and delivery of therapeutic agents, for cardiac repair.140–143 By taking these advantages from graphene derivatives, various graphene-based hydrogels have been developed and utilized for cardiac regeneration.137,140,144–147 Jing et al prepared chitosan/GO hydrogels and further reduced them using dopamine.140 The resultant hydrogels (chitosan/rGO/polydomapine (PDA)) showed excellent electrical conductivity (0.122 S/m) and elasticity (0.75 kPa), and promoted the cell viability and proliferation of human embryonic stem cell-derived fibroblasts (HEF1) and CMs. They observed that the spontaneous beating rate was faster in the chitosan/GO/PDA group than that in the control group (GO-free hydrogel), implying the favorable effects of electrical conductance on cardiac functional maturation. Moreover, a micro-patterned graphene-based hydrogel has been created to additionally provide topographical cues.138,146 Zhang et al patterned microstructures on gelatin/GO hydrogels using a microcontact printing technique.146 They found that the patterned gelatin/GO hydrogel significantly promoted the alignment and maturation of CMs. Interestingly, introduction of GO and surface features synergistically enhanced contraction amplitude, synchronization, and cardiac gene expression of CMs.
Graphene derivatives have been frequently functionalized and used for formulating conductive hydrogels.142,145 Functionalized graphene derivatives can participate in physical or chemical bonding with the hydrogel network and/or interact with various therapeutic agents. For example, Mousavi et al formulated 3-(2-aminoethyl amino) propyltrimethoxysilane (APTMS)-functionalized rGO with oxidized alginate (OA) and decellularized extracellular matrix (dECM).145 Amine groups in the rGO formed covalent bonds with the aldehyde group of OA through Schiff-base reaction, which significantly improved the physical and electrochemical properties of the composite hydrogels. The conductivity of the APTMS-rGO/OA/dECM hydrogel was 110 times higher compared to that of the APTMS-free hydrogel control. (Table 2) Functionalized GO in hydrogel can act as a carrier of specific therapeutics for MI treatment. Paul et al produced polyethylenimine (PEI)-functionalized GO/GelMA hydrogels as a gene delivery vehicle for MI treatment.142 They incorporated DNA-loaded PEI-GO in the low-modulus GelMA hydrogels. Intramyocardial injection of the PEG-GO/DNA/GelMA hydrogel to the peri-infarct regions of the infarcted heart of a rat substantially induced neovascularization, reduced scar area, and improved cardiac functions.
Graphene based-conductive hydrogels have been examined for in vivo intramyocardial injection to the heart post-MI.82,83,142,148 Zhu et al fabricated injectable hydrogels composed of GelMA, oxidized dextran, and rGO. Injection of a mixture of the precursor solutions led to spontaneous gelation in the body through Schiff base and UV crosslinking.83(Figure 5A) They encapsulated umbilical cord mesenchymal stem cells (UCMSCs) with GelMA/oxidized dextran/rGO gels and injected them into infarcted myocardium of a rat. They observed significant improvement of the ejection fraction (EF), reduction of myocardial infarct areas, and enhanced expression levels of cardiac markers (cardiac Troponin I and connexin 43) in the USCMSs with GelMA/oxidized dextran/rGO group compared to the control groups (rGO free hydrogels). (Figure 5B and C) However, studies on graphene-based cardiac patches are few. While graphene-based-hydrogels have been employed,137,143 most studies mainly demonstrated the possibility based on in vitro results as proof-of-concept studies, with minimal investigation of therapeutic effectiveness for cardiac treatment in vivo. (Table 2).
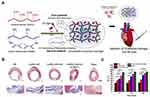 |
Figure 5 Graphene-based conductive hydrogels for cardiac repair. (A) An injectable, conductive hydrogel composed of GelMA, oxidized dextran, and rGO. The gel could be formed through in situ Schiff base and UV crosslinking post injection. UCMSCs were encapsulated in the GelMA/oxidized dextran/rGO gels and injected into MI area. (B) Histological micrographs of heart tissues treated with various samples and controls. (C) Ejection fraction (EF) of various groups treated with various samples. (*P < 0.05, **P < 0.01, ***P < 0.001). Notes: Reprinted with permission from Zhu S, Yu C, Liu N et al. Injectable conductive gelatin methacrylate/oxidized dextran hydrogel encapsulating umbilical cord mesenchymal stem cells for myocardial infarction treatment. Bioact Mater. 2022;13:119–134.83 © 2021 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd. Under a Creative Commons Licence. |
MXene
Two-dimensional transition metal carbide/nitride (MXene) has been highlighted as a new 2D nanomaterial since Gogotsi et al proposed it in 2011.149 MAX is a group of ternary carbides and nitrides in a layered structure, where “M” stands for the early transition metal layer (eg, Ti, Nb, Cr, and Mo), “A” stands for A-group (mostly group IIIA and IVA elements of the periodic table) metal layer, and “X” stands for C or N elements.150,151 MXene is prepared by etching an “A” metal layer in the MAX phase.152 During the etching process, 2D nano-layered MXenes with surface functional groups (eg, –F, –OH, –O, and–Cl) are obtained.153 MXene exhibits excellent properties such as metallic conductivity,154 optical155 and mechanical156 properties, hydrophilicity, chemical stability, and large surface area.157
MXenes and MXene-based materials have been actively studied for biomedical applications such as tissue engineering.158 MXene has outstanding hydrophilicity, unlike other 1D and 2D nanomaterials, such as CNTs and graphene, and is well dispersed in hydrogels. MXene-based conductive hydrogels show excellent electrical, chemical, and mechanical properties. Hence, MXene-based hydrogels can provide an electrical microenvironment and mechanical support to the myocardium for cardiac tissue regeneration. Basara et al fabricated patterned Ti3C2Tx MXene-PEG hydrogels by printing MXene onto PEG hydrogels to mimic the ordered structure of the native ECM and electrical conductivity of the myocardium.51 CMs cultured on the 3D-printed MXene hydrogels exhibited significantly increased expression of cardiac-related genes (ie, MYH7, SERCA2, and TNNT2) and improved synchronous beating. Ye et al produced a CM-seeded MXene cryogel cardiac patch by formulating PEG, GelMA, and Ti2C MXene.159 The MXene-incorporated cryogels exhibited higher mechanical strength compared to MXene-free cryogels. The cryogel with a moderate MXene proportion (0.8%) was found to have mechanical properties and conductivity similar to the natural myocardium. (Table 3) The MXene cryogel showed no cytotoxicity and promoted the functional maturation of CMs, including beating. In vivo transplantation of MI rats revealed that the MXene cryogel cardiac patch significantly suppressed the inflammatory reaction and improved cardiac function. (Figure 6) In addition, Ti3C2 MXene quantum dots (MQD) in hydrogels can provide additional functions, such as anti-inflammatory properties and promotion of cellular activity. Rafieerad et al fabricated MQD-containing chitosan hydrogels as injectable shape memory hydrogels for stem cell delivery.160 They reported that MQD-containing hydrogels are promising for tissue repair and treatment of inflammatory and degenerative diseases, including for cardiac applications.
 |
Table 3 Summary of Studies Reviewed That Used MXene Based Hydrogels for Cardiac Repair |
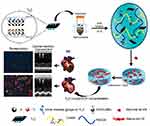 |
Figure 6 MXene-based hydrogels for cardiac repair. MXene Ti2C-cryogels were produced as cardiac patches using PEG, GelMA and Ti2C MXene, seeded with CMs, and implanted on the surface of infarcted heart. These hydrogels improve cardiac functions and revascularization. Notes: Reprinted with permission from Ye G, Wen Z, Wen F et al. Mussel-inspired conductive Ti2C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction. Theranostics. 2020;10(5):2047–2066.159 Copyright © The author(s) Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). Ivyspring International Publisher. |
Although MXene-based hydrogels display excellent physicochemical, conductive, and immunosuppressive properties that are appropriate for cardiac tissue repair, their in vivo and in vitro studies are insufficient compared with carbon nanomaterials (eg, CNTs and graphene). Therefore, the fabrication of various MXene-containing hydrogels and their efficacy tests are of great interest for cardiac repair after MI.
Emerging Nanomaterials
Recently, various inorganic nanomaterials such as black phosphorus (BP) and molybdenum disulfide (MoS2) have been discovered and studied for biomedical applications.63,161–163 These materials have rarely been used for cardiac tissue engineering; however, these new nanomaterials are expected to contribute to the development of new functional biomaterials for cardiac repair, including conductive hydrogels. BP nanosheets are a type of 2D nanomaterial that was discovered in 2014 after graphene.164 BP nanosheets exhibit several beneficial properties, such as high electrical conductance and mechanical strength, for tissue regeneration, drug delivery, photothermal therapy, and photodynamic therapy.163,165–169 Xu et al prepared BP-containing hydrogels as a conductive platform for neural tissue engineering.63 BP-GelMA hydrogel had 4-fold higher conductivity (0.2 S/m) than BP-free GelMA hydrogels. They suggested that conductive BPs within the composite hydrogel may be useful for improving the regeneration of other electroactive tissues, such as cardiac and skeletal muscle tissues, as well as neural tissues. However, studies on BP-based hydrogels for cardiac applications have not yet been reported.
MoS2 is a new-generation nanosheet with a large surface area, high electrical conductivity, optical properties, and biocompatibility.170 MoS2 has been employed in several biomedical applications including photothermal therapy, drug delivery, and biosensors. Several scaffolds with MoS2 have been studied for bone and neural tissue engineering, as MoS2 was found to induce osteogenic and neural differentiation of MSCs.161,162 Nazari et al fabricated nylon6 electrospun nanofibers incorporated with MoS2 nanosheets and observed enhanced mechanical properties and electrical conductivity.171 They found that mouse embryonic cardiac cells (mECCs) on the MoS2-scaffolds showed improved maturation and upregulation of cardiac functional genes (eg, GATA-4, c-TnT, Nkx 2.5, and α-MHC) compared to those on pristine nylon scaffolds. These MoS2-reinforced scaffolds have been suggested as promising materials for cardiac tissue engineering. However, MoS2-based hydrogels have not yet been developed for cardiac tissue engineering and repair. Additional trials and toxicity and performance evaluations are required to use these 2D nanomaterial (BP and MoS2)-incorporated hydrogels for cardiac repair.
Conclusion
Development of novel functional biomaterials that can facilitate cardiac tissue repair after cardiovascular diseases is highly required due to the lack of current effective treatment. Electrically conductive hydrogels can mimic the characteristics of native cardiac tissues and induce cardiac regeneration by providing mechanical and electrical cues to infarcted heart tissue. Low-dimensional inorganic nanomaterials (CNT, graphene derivatives, and MXenes) have been used to fabricate conductive hydrogels for cardiac applications (injection into the myocardium or pericardial cavity, and attachment on the heart). Conductive hydrogels composed of individual nanomaterials and various polymers exhibit unique characteristics such as excellent electrical conductivity, mechanical strength, and biological activities. Nanomaterial-containing conductive hydrogels have been proven to be promising materials for cardiac tissue repair. The newly discovered 2D nanomaterials (BP and MoS2) are expected to offer potentials to facilitate the fabrication of functional conductive hydrogels for cardiac repair. Biocompatibility of low-dimensional nanomaterials has not been clearly determined yet although numerous materials containing various nanomaterials have successfully demonstrated their utilities for various biomedical applications. In particular, nanomaterial-based conductive hydrogels for cardiac tissue repair are emerging and even their animal studies are not sufficient for their clinical translation. Overall, future studies on further material engineering, therapeutic efficacy, and biocompatibility of nanomaterial-based conductive hydrogels are necessary. In addition, these conductive hydrogels can be used for the regeneration of other electrically active tissues such as nerves and muscles.
Abbreviations
MI, myocardial infarction; CMs, cardiomyocytes; ECM, extracellular matrix; TA-PEG, tetraaniline-polyethylene glycol; HA-SH, thiolated hyaluronic acid; ADSCs, adipose-derived stem cells; CNT, carbon nanotube; SWCNTs, single-walled CNTs; MWCNTs, multi-walled CNTs; GelMA, gelatin methacrylate; PNIPAAm, poly(N-isopropylacrylamide); BASCs, brown adipose stem cells; hiPSCs, human-induced pluripotent stem cells; HCAECs, human coronary artery endothelial cells; IDs, intercalated discs; GO, graphene oxide; rGO, reduced GO; R, resistance; Z, impedance; σ, conductivity; EB, embryoid body; HEF1, human embryonic stem cell-derived fibroblasts; APTMS, 3-(2-aminoethyl amino) propyltrimethoxysilane; OA, oxidized alginate; dECM, decellularized extracellular matrix; PEI, polyethylenimine; UCMSCs, umbilical cord mesenchymal stem cells; EF, ejection fraction; PDA, polydopamine; ODEX, oxidized dextran; MQDs, MXene quantum dots; BP, black phosphorus; MoS2, molybdenum disulfide; mECCs, mouse embryonic cardiac cells.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2021R1A4A3025206).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639.
2. Zheng Z, Tan Y, Li Y, et al. Biotherapeutic-loaded injectable hydrogels as a synergistic strategy to support myocardial repair after myocardial infarction. J Controlled Release. 2021;335:216–236.
3. Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72(3):865–867.
4. Zhang Y, Zhu D, Wei Y, et al. A collagen hydrogel loaded with HDAC7-derived peptide promotes the regeneration of infarcted myocardium with functional improvement in a rodent model. Acta Biomater. 2019;86:223–234.
5. Wu T, Cui C, Huang Y, et al. Coadministration of an adhesive conductive hydrogel patch and an injectable hydrogel to treat myocardial infarction. ACS Appl Mater Interfaces. 2020;12(2):2039–2048.
6. Pecha S, Eschenhagen T, Reichenspurner H. Myocardial tissue engineering for cardiac repair. J Heart Lung Transplant. 2016;35(3):294–298.
7. Lin X, Liu Y, Bai A, et al. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat Biomed Eng. 2019;3(8):632–643.
8. Hasan A, Khattab A, Islam MA, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci. 2015;2(11):1500122.
9. Li Y, Wei L, Lan L, et al. Conductive biomaterials for cardiac repair: a review. Acta Biomater. 2022;139:157–178.
10. Gokce C, Gurcan C, Delogu LG, Yilmazer A. 2D materials for cardiac tissue repair and regeneration. Front Cardiovasc Med. 2022;9:802551.
11. Aragón Á, Cebro-Márquez M, Perez E, et al. Bioelectronics-on-a-chip for cardio myoblast proliferation enhancement using electric field stimulation. Biomater Res. 2020;24:1–10.
12. Moon SH, Cho YW, Shim HE, et al. Electrically stimulable indium tin oxide plate for long-term in vitro cardiomyocyte culture. Biomater Res. 2020;24(1):10.
13. Menasché P, Hagège AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41(7):1078–1083.
14. Minicucci MF, Azevedo PS, Polegato BF, Paiva SAR, Zornoff LAM. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol. 2011;34(7):410–414.
15. Pedron S, van Lierop S, Horstman P, Penterman R, Broer DJ, Peeters E. Stimuli responsive delivery vehicles for cardiac microtissue transplantation. Adv Funct Mater. 2011;21(9):1624–1630.
16. Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction: an overview of results from randomized controlled trials. JAMA. 1993;270(13):1589–1595.
17. Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28(2):208–216.
18. Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48(5):907–913.
19. Zhao T, Wu W, Sui L, et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47–72.
20. Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface. 2012;9(66):1–19.
21. Higuchi A, Ku NJ, Tseng YC, et al. Stem cell therapies for myocardial infarction in clinical trials: bioengineering and biomaterial aspects. Lab Invest. 2017;97(10):1167–1179.
22. Kopeček J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28(34):5185–5192.
23. Zhu Y, Matsumura Y, Wagner WR. Ventricular wall biomaterial injection therapy after myocardial infarction: advances in material design, mechanistic insight and early clinical experiences. Biomaterials. 2017;129:37–53.
24. Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium. Circulation. 2006;114(24):2627–2635.
25. Wang L, Liu Y, Ye G, et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat Biomed Eng. 2021;5(10):1157–1173.
26. Choe G, Lee M, Oh S, et al. Three-dimensional bioprinting of mesenchymal stem cells using an osteoinductive bioink containing alginate and BMP-2-loaded PLGA nanoparticles for bone tissue engineering. Biomater Adv. 2022;136:212789.
27. Cheng YH, Lin FH, Wang CY, et al. Recovery of oxidative stress-induced damage in Cisd2-deficient cardiomyocytes by sustained release of ferulic acid from injectable hydrogel. Biomaterials. 2016;103:207–218.
28. Mackiewicz M, Romanski J, Drabczyk K, Waleka E, Stojek Z, Karbarz M. Degradable, thermo-, pH- and redox-sensitive hydrogel microcapsules for burst and sustained release of drugs. Int J Pharm. 2019;569:118589.
29. Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–170.
30. Liang W, Chen J, Li L, et al. Conductive hydrogen sulfide-releasing hydrogel encapsulating adscs for myocardial infarction treatment. ACS Appl Mater Interfaces. 2019;11(16):14619–14629.
31. Sy JC, Seshadri G, Yang SC, et al. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat Mater. 2008;7(11):863–868.
32. Hao T, Li J, Yao F, et al. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano. 2017;11(6):5474–5488.
33. Jongpaiboonkit L, King WJ, Lyons GE, et al. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29(23):3346–3356.
34. Li Z, Guo X, Guan J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials. 2012;33(25):5914–5923.
35. Kook YM, Hwang S, Kim H, Rhee KJ, Lee K, Koh WG. Cardiovascular tissue regeneration system based on multiscale scaffolds comprising double-layered hydrogels and fibers. Sci Rep. 2020;10(1):20321.
36. Gong HY, Park J, Kim W, Kim J, Lee JY, Koh WG. A novel conductive and micropatterned PEG-based hydrogel enabling the topographical and electrical stimulation of myoblasts. ACS Appl Mater Interfaces. 2019;11(51):47695–47706.
37. Wang Q, Pan X, Lin C, et al. Modified Ti3C2TX (MXene) nanosheet-catalyzed self-assembled, anti-aggregated, ultra-stretchable, conductive hydrogels for wearable bioelectronics. Chem Eng J. 2020;401:126129.
38. Han L, Yan L, Wang M, et al. Transparent, adhesive, and conductive hydrogel for soft bioelectronics based on light-transmitting polydopamine-doped polypyrrole nanofibrils. Chem Mater. 2018;30(16):5561–5572.
39. Mawad D, Stewart E, Officer DL, et al. A single component conducting polymer hydrogel as a scaffold for tissue engineering. Adv Funct Mater. 2012;22(13):2692–2699.
40. Athukorala SS, Tran TS, Balu R, et al. 3D printable electrically conductive hydrogel scaffolds for biomedical applications: a review. Polymers. 2021;13(3):474.
41. Min JH, Patel M, Koh WG. Incorporation of conductive materials into hydrogels for tissue engineering applications. Polymers. 2018;10(10):1078.
42. Luo Y, Fan L, Liu C, et al. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact Mater. 2022;7:98–111.
43. Park J, Jeon J, Kim B, et al. Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv Funct Mater. 2020;30(39):2003759.
44. Liang S, Zhang Y, Wang H, et al. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv Mater. 2018;30(23):1704235.
45. Reis LA, Chiu LLY, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med. 2016;10(1):11–28.
46. Shin SR, Jung SM, Zalabany M, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7(3):2369–2380.
47. Chen S, Hsieh MH, Li SH, et al. A conductive cell-delivery construct as a bioengineered patch that can improve electrical propagation and synchronize cardiomyocyte contraction for heart repair. J Controlled Release. 2020;320:73–82.
48. Morsink M, Severino P, Luna-Ceron E, Hussain MA, Sobahi N, Shin SR. Effects of electrically conductive nano-biomaterials on regulating cardiomyocyte behavior for cardiac repair and regeneration. Acta Biomater. 2022;139:141–156.
49. Han X, Xiao G, Wang Y, et al. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J Mater Chem A. 2020;8(44):23059–23095.
50. Yi J, Choe G, Park J, Lee JY. Graphene oxide-incorporated hydrogels for biomedical applications. Polym J. 2020;52(8):823–837.
51. Basara G, Saeidi-Javash M, Ren X, et al. Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2022;139:179–189.
52. Li X, Zhou J, Liu Z, et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials. 2014;35(22):5679–5688.
53. Alam A, Meng Q, Shi G, et al. Electrically conductive, mechanically robust, pH-sensitive graphene/polymer composite hydrogels. Compos Sci Technol. 2016;127:119–126.
54. Qiu L, Liu D, Wang Y, et al. Mechanically robust, electrically conductive and stimuli-responsive binary network hydrogels enabled by superelastic graphene aerogels. Adv Mater. 2014;26(20):3333–3337.
55. Mottet L, Cornec DL, Noël JM, et al. A conductive hydrogel based on alginate and carbon nanotubes for probing microbial electroactivity. Soft Matter. 2018;14(8):1434–1441.
56. Luan VH, Chung JS, Kim EJ, Hur SH. The molecular level control of three-dimensional graphene oxide hydrogel structure by using various diamines. Chem Eng J. 2014;246:64–70.
57. Han L, Lu X, Wang M, et al. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small. 2017;13(2):1601916.
58. Wang L, He J, Zhu L, et al. Assembly of pi-functionalized quaternary ammonium compounds with graphene hydrogel for efficient water disinfection. J Colloid Interface Sci. 2019;535:149–158.
59. Distler T, Polley C, Shi F, et al. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole: pSSHydrogels for tissue engineering. Adv Healthc Mater. 2021;10(9):2001876.
60. Distler T, Boccaccini AR. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors – a review. Acta Biomater. 2020;101:1–13.
61. Xu J, Tsai YL. Design strategies of conductive hydrogel for biomedical applications. Molecules. 2020;25(22):5296.
62. Kaith BS, Sharma R, Kalia S. Guar gum based biodegradable, antibacterial and electrically conductive hydrogels. Int J Biol Macromol. 2015;75:266–275.
63. Xu C, Xu Y, Yang M, et al. Black-phosphorus-incorporated hydrogel as a conductive and biodegradable platform for enhancement of the neural differentiation of mesenchymal stem cells. Adv Funct Mater. 2020;30(39):2000177.
64. Deng Z, Wang H, Ma X. P, Guo B. Self-healing conductive hydrogels: preparation, properties and applications. Nanoscale. 2020;12(3):1224–1246.
65. Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G. Electroconductive natural polymer-based hydrogels. Biomaterials. 2016;111:40–54.
66. Guo B, Finne-Wistrand A, Albertsson AC. Facile synthesis of degradable and electrically conductive polysaccharide hydrogels. Biomacromolecules. 2011;12(7):2601–2609.
67. Gunatillake P, Mayadunne R, Adhikari R. Recent developments in biodegradable synthetic polymers. In: El-Gewely MR, editor. Biotechnology Annual Review. Vol. 12. Elsevier; 2006:301–347.
68. Chen Z, Chen Y, Hedenqvist S. M, et al. Multifunctional conductive hydrogels and their applications as smart wearable devices. J Mater Chem B. 2021;9(11):2561–2583.
69. Chimene D, Alge DL, Gaharwar AK. Two-dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv Mater. 2015;27(45):7261–7284.
70. Erol O, Uyan I, Hatip M, Yilmaz C, Tekinay AB, Guler MO. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomedicine Nanotechnol Biol Med. 2018;14(7):2433–2454.
71. Li Y, Li Z, Chi C, Shan H, Zheng L, Fang Z. Plasmonics of 2D nanomaterials: properties and applications. Adv Sci. 2017;4(8):1600430.
72. Tan C, Cao X, Wu XJ, et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem Rev. 2017;117(9):6225–6331.
73. Huang X, Tan C, Yin Z, Zhang H. 25th anniversary article: hybrid nanostructures based on two-dimensional nanomaterials. Adv Mater. 2014;26(14):2185–2204.
74. Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015;5(47):37553–37567.
75. Rong Q, Lei W, Liu M. Conductive hydrogels as smart materials for flexible electronic devices. Chem Eur J. 2018;24(64):16930–16943.
76. Wang Z, Cong Y, Fu J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J Mater Chem B. 2020;8(16):3437–3459.
77. Sun X, Yao F, Li J. Nanocomposite hydrogel-based strain and pressure sensors: a review. J Mater Chem A. 2020;8(36):18605–18623.
78. Dong R, Zhao X, Guo B, Ma PX. Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl Mater Interfaces. 2016;8(27):17138–17150.
79. Song X, Wang X, Zhang J, et al. A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials. 2021;273:120811.
80. Ifkovits JL, Tous E, Minakawa M, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci. 2010;107(25):11507–11512.
81. Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–1481.
82. Bao R, Tan B, Liang S, Zhang N, Wang W, Liu W. A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials. 2017;122:63–71.
83. Zhu S, Yu C, Liu N, et al. Injectable conductive gelatin methacrylate/oxidized dextran hydrogel encapsulating umbilical cord mesenchymal stem cells for myocardial infarction treatment. Bioact Mater. 2022;13:119–134.
84. Nguyen QV, Huynh DP, Park JH, Lee DS. Injectable polymeric hydrogels for the delivery of therapeutic agents: a review. Eur Polym J. 2015;72:602–619.
85. Zhang J, Wang S, Zhao Z, et al. An In situ forming hydrogel based on photo-induced hydrogen bonding. Macromol Res. 2020;28(12):1127–1133.
86. Wang W, Tan B, Chen J, et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials. 2018;160:69–81.
87. Rivers TJ, Hudson TW, Schmidt CE. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv Funct Mater. 2002;12(1):33–37.
88. Guo Y, Li M, Mylonakis A, et al. Electroactive oligoaniline-containing self-assembled monolayers for tissue engineering applications. Biomacromolecules. 2007;8(10):3025–3034.
89. Li Z, Zhu D, Hui Q, et al. Injection of ROS-responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv Funct Mater. 2021;31(15):2004377.
90. Garcia JR, Campbell PF, Kumar G, et al. A minimally invasive, translational method to deliver hydrogels to the heart through the pericardial space. JACC Basic Transl Sci. 2017;2(5):601–609.
91. Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun. 2021;12(1):1412.
92. Ramer SA, Sapp JL. Percutaneous intrapericardial injection of triamcinolone in a patient with incessant pericarditis: a novel technique. Can J Cardiol. 2013;29(6):751.e1–751.e2.
93. Laham RJ, Rezaee M, Post M, Xu X, Sellke FW. Intrapericardial administration of basic fibroblast growth factor: myocardial and tissue distribution and comparison with intracoronary and intravenous administration. Catheter Cardiovasc Interv. 2003;58(3):375–381.
94. Wang L, Jiang J, Hua W, et al. Mussel-inspired conductive cryogel as cardiac tissue patch to repair myocardial infarction by migration of conductive nanoparticles. Adv Funct Mater. 2016;26(24):4293–4305.
95. Walker BW, Lara RP, Yu CH, et al. Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials. 2019;207:89–101.
96. Tang J, Wang J, Huang K, et al. Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci Adv. 2018;4(11):eaat9365.
97. Suh M, Proctor D, Chappell G, et al. A review of the genotoxic, mutagenic, and carcinogenic potentials of several lower acrylates. Toxicology. 2018;402:50–67.
98. Assmann A, Vegh A, Ghasemi-Rad M, et al. A highly adhesive and naturally derived sealant. Biomaterials. 2017;140:115–127.
99. Annabi N, Zhang YN, Assmann A, et al. Engineering a highly elastic human protein–based sealant for surgical applications. Sci Transl Med. 2017;9(410):eaai7466.
100. Tang J, Vandergriff A, Wang Z, et al. A regenerative cardiac patch formed by spray painting of biomaterials onto the heart. Tissue Eng Part C Methods. 2017;23(3):146–155.
101. Shin J, Choi S, Kim JH, et al. Tissue tapes—phenolic hyaluronic acid hydrogel patches for off-the-shelf therapy. Adv Funct Mater. 2019;29(49):1903863.
102. Yuk H, Varela CE, Nabzdyk CS, et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature. 2019;575(7781):169–174.
103. Wang Y, Yang P, Zheng L, Shi X, Zheng H. Carbon nanomaterials with sp2 or/and sp hybridization in energy conversion and storage applications: a review. Energy Storage Mater. 2020;26:349–370.
104. Loh KP, Ho D, Chiu GNC, Leong DT, Pastorin G, Chow EKH. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv Mater. 2018;30(47):1802368.
105. Earp B, Dunn D, Phillips J, et al. Enhancement of electrical conductivity of carbon nanotube sheets through copper addition using reduction expansion synthesis. Mater Res Bull. 2020;131:110969.
106. Fang C, Zhang J, Chen X, Weng GJ. Calculating the electrical conductivity of graphene nanoplatelet polymer composites by a monte carlo method. Nanomaterials. 2020;10(6):1129.
107. Stankovich S, Dikin DA, Dommett GHB, et al. Graphene-based composite materials. Nature. 2006;442(7100):282–286.
108. Shin SR, Farzad R, Tamayol A, et al. A bioactive carbon nanotube-based ink for printing 2D and 3D flexible electronics. Adv Mater. 2016;28(17):3280–3289.
109. Madani SY, Mandel A, Seifalian AM. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013;4(1):21521.
110. Sinha N, Yeow JTW. Carbon nanotubes for biomedical applications. IEEE Trans NanoBioscience. 2005;4(2):180–195.
111. Beg S, Rizwan M, Sheikh AM, Hasnain MS, Anwer K, Kohli K. Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol. 2011;63(2):141–163.
112. Aslnejad S, Nasiri M, Abbasi F, Abdipour H. Preparation of MWCNT/PDMS conductive micro-patterned nanocomposites. Macromol Res. 2020;28(8):733–738.
113. Iglesias D, Bosi S, Melchionna M, Ros TD, Marchesan S. The glitter of carbon nanostructures in hybrid/composite hydrogels for medicinal use. Curr Top Med Chem. 2016;16(18):1976–1989.
114. Adewunmi AA, Ismail S, Sultan AS. Carbon nanotubes (CNTs) nanocomposite hydrogels developed for various applications: a critical review. J Inorg Organomet Polym Mater. 2016;26(4):717–737.
115. Ma PC, Siddiqui NA, Marom G, Kim JK. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: a review. Compos Part Appl Sci Manuf. 2010;41(10):1345–1367.
116. Peña B, Bosi S, Aguado BA, et al. Injectable carbon nanotube-functionalized reverse thermal gel promotes cardiomyocytes survival and maturation. ACS Appl Mater Interfaces. 2017;9(37):31645–31656.
117. Yu H, Zhao H, Huang C, Du Y. Mechanically and and electrically enhanced CNT–collagen hydrogels as potential scaffolds for engineered cardiac constructs. ACS Biomater Sci Eng. 2017;3(11):3017–3021.
118. Roshanbinfar K, Mohammadi Z, Mesgar ASM, et al. Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering. Biomater Sci. 2019;7(9):3906–3917.
119. Izadifar M, Chapman D, Babyn P, Chen X, Kelly ME. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods. 2018;24(2):74–88.
120. Ahadian S, Yamada S, Ramón-Azcón J, et al. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016;31:134–143.
121. Sun H, Lü S, Jiang XX, et al. Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the β1-integrin-mediated signaling pathway. Biomaterials. 2015;55:84–95.
122. Sun H, Tang J, Mou Y, et al. Carbon nanotube-composite hydrogels promote intercalated disc assembly in engineered cardiac tissues through β1-integrin mediated FAK and RhoA pathway. Acta Biomater. 2017;48:88–99.
123. Lee J, Manoharan V, Cheung L, et al. Nanoparticle-based hybrid scaffolds for deciphering the role of multimodal cues in cardiac tissue engineering. ACS Nano. 2019;13(11):12525–12539.
124. Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6(3):183–191.
125. Huang X, Yin Z, Wu S, et al. Graphene-based materials: synthesis, characterization, properties, and applications. Small. 2011;7(14):1876–1902.
126. Lee C, Wei X, Kysar JW, Hone J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science. 2008;321(5887):385–388.
127. Smith AT, LaChance AM, Zeng S, Liu B, Sun L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater Sci. 2019;1(1):31–47.
128. Wang XY, Narita A, Müllen K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat Rev Chem. 2017;2(1):1–10.
129. Chen J, Zhang Y, Zhang M, et al. Water-enhanced oxidation of graphite to graphene oxide with controlled species of oxygenated groups. Chem Sci. 2016;7(3):1874–1881.
130. Yoo BM, Shin HJ, Yoon HW, Park HB. Graphene and graphene oxide and their uses in barrier polymers. J Appl Polym Sci. 2014;131:1.
131. Gao X, Jang J, Hydrazine NS. Thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J Phys Chem C. 2010;114(2):832–842.
132. Moon IK, Lee J, Ruoff RS, Lee H. Reduced graphene oxide by chemical graphitization. Nat Commun. 2010;1(1):73.
133. Li D, Zhang W, Yu X, Wang Z, Su Z, Wei G. When biomolecules meet graphene: from molecular level interactions to material design and applications. Nanoscale. 2016;8(47):19491–19509.
134. Guo C, Lu R, Wang X. Graphene oxide-modified Polyetheretherketone with excellent antibacterial properties and biocompatibility for implant abutment. Macromol Res. 2021;29(5):351–359.
135. Ghawanmeh AA, Ali GAM, Algarni H, Sarkar SM, Chong KF. Graphene oxide-based hydrogels as a nanocarrier for anticancer drug delivery. Nano Res. 2019;12(5):973–990.
136. Shin SR, Li YC, Jang HL, et al. Graphene-based materials for tissue engineering. Adv Drug Deliv Rev. 2016;105:255–274.
137. Shin SR, Zihlmann C, Akbari M, et al. Reduced graphene oxide-gelma hybrid hydrogels as scaffolds for cardiac tissue engineering. Small. 2016;12(27):3677–3689.
138. Park J, Choi JH, Kim S, Jang I, Jeong S, Lee JY. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019;97:141–153.
139. Jo H, Sim M, Kim S, et al. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017;48:100–109.
140. Jing X, Mi HY, Napiwocki BN, Peng XF, Turng LS. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon. 2017;125:557–570.
141. Choe G, Kim SW, Park J, et al. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials. 2019;225:119513.
142. Paul A, Hasan A, Kindi HA, et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8(8):8050–8062.
143. Norahan MH, Amroon M, Ghahremanzadeh R, Mahmoodi M, Baheiraei N. Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering. J Biomed Mater Res A. 2019;107(1):204–219.
144. Jiang L, Chen D, Wang Z, et al. Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl Biochem Biotechnol. 2019;188(4):952–964.
145. Mousavi A, Mashayekhan S, Baheiraei N, Pourjavadi A. Biohybrid oxidized alginate/myocardial extracellular matrix injectable hydrogels with improved electromechanical properties for cardiac tissue engineering. Int J Biol Macromol. 2021;180:692–708.
146. Zhang F, Zhang N, Meng HX, et al. Easy applied gelatin-based hydrogel system for long-term functional cardiomyocyte culture and myocardium formation. ACS Biomater Sci Eng. 2019;5(6):3022–3031.
147. Li XP, Qu KY, Zhou B, et al. Electrical stimulation of neonatal rat cardiomyocytes using conductive polydopamine-reduced graphene oxide-hybrid hydrogels for constructing cardiac microtissues. Colloids Surf B Biointerfaces. 2021;205:111844.
148. Zhou J, Yang X, Liu W, et al. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics. 2018;8(12):3317–3330.
149. Anasori B, Lukatskaya MR, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater. 2017;2(2):1–17.
150. Naguib M, Mashtalir O, Carle J, et al. Two-dimensional transition metal carbides. ACS Nano. 2012;6(2):1322–1331.
151. Zhou C, Zhao X, Xiong Y, et al. A review of etching methods of MXene and applications of MXene conductive hydrogels. Eur Polym J. 2022;167:111063.
152. Sokol M, Natu V, Kota S, Barsoum MW. On the chemical diversity of the MAX phases. Trends Chem. 2019;1(2):210–223.
153. Kamysbayev V, Filatov AS, Hu H, et al. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science. 2020;369(6506):979–983.
154. Zhang J, Kong N, Uzun S, et al. Scalable manufacturing of free-standing, strong Ti3C2Tx MXene films with outstanding conductivity. Adv Mater. 2020;32(23):2001093.
155. Xu H, Ren A, Wu J, Wang Z. Recent advances in 2D mxenes for photodetection. Adv Funct Mater. 2020;30(24):2000907.
156. Guo Y, Zhou X, Wang D, Xu X, Xu Q. Nanomechanical properties of Ti3C2 mxene. Langmuir. 2019;35(45):14481–14485.
157. Iravani S, Varma S. R. MXenes and MXene-based materials for tissue engineering and regenerative medicine: recent advances. Mater Adv. 2021;2(9):2906–2917.
158. Zhang J, Fu Y, Mo A. Multilayered titanium carbide mxene film for guided bone regeneration. Int J Nanomedicine. 2019;14:10091–10103.
159. Ye G, Wen Z, Wen F, et al. Mussel-inspired conductive Ti2C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction. Theranostics. 2020;10(5):2047–2066.
160. Rafieerad A, Yan W, Sequiera GL, et al. Application of Ti3C2 mxene quantum dots for immunomodulation and regenerative medicine. Adv Healthc Mater. 2019;8(16):1900569.
161. Zhang X, Nie J, Yang X, et al. Nanostructured molybdenum disulfide biointerface for adhesion and osteogenic differentiation of mesenchymal stem cells. Appl Mater Today. 2018;10:164–172.
162. Wang S, Qiu J, Guo W, et al. A nanostructured molybdenum disulfide film for promoting neural stem cell neuronal differentiation: toward a nerve tissue-engineered 3D scaffold. Adv Biosyst. 2017;1(5):1600042.
163. Xing C, Chen S, Qiu M, et al. Black phosphorus hydrogels: conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy (adv. healthcare mater. Adv Healthc Mater. 2018;7(7):1870030.
164. Li L, Yu Y, Ye GJ, et al. Black phosphorus field-effect transistors. Nat Nanotechnol. 2014;9(5):372–377.
165. Yang B, Yin J, Chen Y, et al. 2D-black-phosphorus-reinforced 3d-printed scaffolds: a stepwise countermeasure for osteosarcoma. Adv Mater. 2018;30(10):1705611.
166. Wang Z, Zhao J, Tang W, et al. Multifunctional nanoengineered hydrogels consisting of black phosphorus nanosheets upregulate bone formation. Small. 2019;15(41):1901560.
167. Qiu M, Wang D, Liang W, et al. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc Natl Acad Sci. 2018;115(3):501–506.
168. Chen W, Ouyang J, Liu H, et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29(5):1603864.
169. Zhao Y, Tong L, Li Z, et al. Stable and multifunctional dye-modified black phosphorus nanosheets for near-infrared imaging-guided photothermal therapy. Chem Mater. 2017;29(17):7131–7139.
170. Teo WZ, Chng ELK, Sofer Z, Pumera M. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem Eur J. 2014;20(31):9627–9632.
171. Nazari H, Heirani-Tabasi A, Alavijeh MS, et al. Nanofibrous composites reinforced by MoS2 nanosheets as a conductive scaffold for cardiac tissue engineering. ChemistrySelect. 2019;4(39):11557–11563.
172. Ahadian S, Davenport Huyer L, Estili M, et al. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017;52:81–91.
173. Pok S, Vitale F, Eichmann SL, Benavides OM, Pasquali M, Jacot JG. Biocompatible carbon nanotube–chitosan scaffold matching the electrical conductivity of the heart. ACS Nano. 2014;8(10):9822–9832.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
