Back to Journals » International Journal of Nanomedicine » Volume 18
Nanocarrier-Mediated Immunogenic Cell Death for Melanoma Treatment
Authors Wang J, Ma J, Tai Z , Li L, Zhang T, Cheng T, Yu J, Zhu Q, Bao L, Chen Z
Received 5 September 2023
Accepted for publication 20 November 2023
Published 1 December 2023 Volume 2023:18 Pages 7149—7172
DOI https://doi.org/10.2147/IJN.S434582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Jiandong Wang,1– 3,* Jinyuan Ma,1,4,* Zongguang Tai,1,4,* Lisha Li,1,4 Tingrui Zhang,1,4 Tingting Cheng,1– 3 Junxia Yu,1– 3 Quangang Zhu,1,4 Leilei Bao,2 Zhongjian Chen1,4
1Shanghai Skin Disease Hospital, School of Medicine, Tongji University, Shanghai, 200443, People’s Republic of China; 2Department of Pharmacy, Third Affiliated Hospital of Naval Medical University, Shanghai, People’s Republic of China; 3School of Pharmacy, Bengbu Medical College, Bengbu, Anhui, People’s Republic of China; 4Shanghai Engineering Research Center of External Chinese Medicine, Shanghai, 200443, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Leilei Bao; Zhongjian Chen, Department of Pharmacy, Third Affiliated Hospital of Naval Medical University ; Shanghai Skin Disease Hospital, School of Medicine, Tongji University, 1278 Baode Road, Shanghai, 200443, People’s Republic of China, Tel/Fax +86 21 81875571 ; +86 21 36803007, Email [email protected]; [email protected]
Abstract: Melanoma, a highly aggressive skin tumor, exhibits notable features including heterogeneity, a high mutational load, and innate immune escape. Despite advancements in melanoma treatment, current immunotherapies fail to fully exploit the immune system’s maximum potential. Activating immunogenic cell death (ICD) holds promise in enhancing tumor cell immunogenicity, stimulating immune amplification response, improving drug sensitivity, and eliminating tumors. Nanotechnology-enabled ICD has emerged as a compelling therapeutic strategy for augmenting cancer immunotherapy. Nanoparticles possess versatile attributes, such as prolonged blood circulation, stability, and tumor-targeting capabilities, rendering them ideal for drug delivery. In this review, we elucidate the mechanisms underlying ICD induction and associated therapeutic strategies. Additionally, we provide a concise overview of the immune stress response associated with ICD and explore the potential synergistic benefits of combining ICD induction methods with the utilization of nanocarriers.
Keywords: immunogenic cell death, melanoma, immunotherapy, damage associated molecular patterns, nanocarrier
Introduction
Melanoma is one of the most aggressive skin cancers. In the last 50 years, its incidence has risen faster than other malignancies at a much higher rate of 7%-8% per year. Also, from 1990 to 2018, the number of patients with metastatic melanoma has increased by 258%.1 Patients usually have poor prognoses, low survival rates, and limited treatment options. Immune defense, consisting of immune cells, is the main way to maintain the host’s stress response on injury and exogenous substances.2,3 Immune checkpoint inhibitors (ICIs) and Chimeric antigen T cells (CAR-T cells), pericytes, tumor vaccines, and other emerging tumor immunotherapeutic tools utilize immune cell-specific or non-specific immune stress responses for immunotherapeutic purposes have made a significant breakthrough in the field of oncology in recent years without a doubt seen.4–6 However, high costs, complex preparation processes, and a series of therapeutic hazards associated with off-target effects (local inflammation, cytokine release syndrome, and neurotoxicity) have limited their application in clinical work.7 Also, studies on melanoma have shown that immunosuppressive factors in solid tumors diminish the effects of ICIs and cell-based therapy-based immunotherapy.8 Nanoparticles have emerged as promising drug delivery vehicles due to their multifunctional features, including enhanced stability, prolonged blood circulation, and tumor-targeting ability. Moreover, there has been a growing body of research on functionally specific nanoparticles that aim to enhance the specificity of immunogenic cell death (ICD) inducers and improve the efficiency of ICD induction in vivo.
As a new type of immunogenic cell death modality, ICD acts as a “natural tumor vaccine” because of its ability to induce a cycle of tumor cell death. A large number of tumor cell-associated damage associated molecular patterns (DAMPs) generated during the ICD process activates a series of immunogenic responses that reshape the tumor immune microenvironment (TME). Ultimately DAMPs cause an immunocyte effect on tumor cells by increasing the cytotoxic activity of tumor-associated immune effector cells.9 The ICD process involves a wide variety of signaling pathways, receptors and cytokines. Thus, an in-depth analysis of the mechanisms underlying the release of DAMPs and the secretion of related cytokines would contribute to the efficient transition from “cold” to “hot” tumors and to the development of a rational co-induction strategy. This review explains the persistent activating effect of ICD on anti-tumor immune response by introducing the ICD-related immune-stress response, and immune response mechanism (Figure 1). Subsequently, the advantages and potential of nanocarrier-mediated multiple induction of ICD (chemotherapy, radiation therapy, photodynamic therapy, photothermal therapy, magnetic fluid therapy) and combined treatments are described. Finally, we conclude by discussing the progress of ICD-based combination therapy strategies for melanoma treatment, as well as the current status of ICD-related marker content testing in the clinical treatment of melanoma and other skin tumors. The important value of the ICD mechanism in the treatment process of melanoma as well as other skin tumors was analyzed. We would like to promulgate the further application and development of the ICD mechanism in tumor treatment by standardizing ICD-related efficacy reference indexes. Meanwhile, we also hope this review could become a reference for designing more rational, efficient, and sustainable combination treatment strategies based on preclinical research.
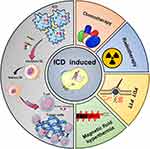 |
Figure 1 Major ICD inducers and the process of ICD immune activation against tumors. |
DAMPs Associated with ICD
ICD is a stress cell death model that mainly acts through apoptosis or necrosis, autophagy, and endoplasmic reticulum (ER) stress response.10 DAMPs are broadly classified into constitutive DAMP (CDAMP) and inducible endogenous DAMP (IDAMP), depending on the release mechanism. CDAMP is an immunogenic endogenous molecule released from its constituent structures prior to cell death; IDAMP is an inducible endogenous DAMP (IDAMP) produced by potential cell death pathways during cell death.11,12 CDAMP mainly consists of HMGB1, ATP, CRT, HSP70, HSP90, ANXA1, while IDAMP mainly consists of IFN, CCL2, CXCL1, CXCL10, etc.13–15 During autophagy, lysosomes secrete large amounts of adenosine triphosphate (ATP), which acts as a chemokine to attract myeloid immune cells, such as DC precursors to the tumor. In the meantime, ER stress induces calreticulin (CRT) translocation, allowing CRT to act as a “eat me” signal.16 CRT then draw DCs to take up tumor-associated antigens (TAA) and cross-present them to cytotoxic T cells before the immune response of cytotoxic T cells is activated.17 In addition to ATP and CRT secretion, ICD also releases other characteristic DAMPs membrane-linked protein 1 (ANXA1), high mobility group protein 1 (HMGB1), and heat-shock protein (HSP) to boost the immune responding effect (Figure 2). Therefore, the detection of relevant DAMPs is of great value for the identification of ICD-inducing drugs and the detection of relevant therapeutic prognosis.18
 |
Figure 2 Release of DAMPs and binding of related receptors during ICD. DAMPs: CRT, ATP, ANXA1, HMGB1; Receptors: LRP1, P2RY2/P2RX7, FPR1, TLR2/ TLR4, LOX1. |
CRT
CRT is a calcium-binding chaperone protein on the endoplasmic reticulum lumen, which mainly participates in maintaining intracellular Ca2+ stability and affects the protein folding process in the endoplasmic reticulum.19 During the ICD process, CRT migrates, clusters, and is exposed by the ER to the damaged cell membrane surface. The process involves ER stress, a fusion of ER with the cytoplasmic membrane, translocation of ER-resident protein (CNX), apolipoprotein on the cytoplasmic membrane; upregulation of eIF2α phosphorylation, unfolded protein response (UPR), and activation of cysteinyl aspartate specific proteinase 8 (Caspase 8).20,21 The translocated CRT acts as a “eat me” signal, attracting APCs to uptake and process tumor cell remnants, and activates the migration and antigen presentation of antigen presenting cells (APCs) by binding to the surface receptor CD91/LRP1, ultimately stimulating the immune response of cytotoxic T cells (CTL).
HMGB1
HMGB1 is a class of non-histone chromatin-binding proteins responsible for DNA transcription, replication, and repair. It mainly functions in the late stages of the cell death process. HMGB1 leaks into the extracellular compartment during ICD and acts as a “danger” signal accompanying the permeabilization of the nuclear and plasma membranes.22 HMGB1 mainly binds to Toll-like receptor 4 (TLR4) on the surface of DCs after releasing from tumor cells, stimulating myeloid differentiation pro-response gene 88 (MYD88) cascade signaling, facilitating DC processing of TAA and presentation to CTL.23 HMGB1 also triggers an inflammatory response following binding to TLR2/ TLR4 receptors and receptors for advanced glycosylation end products (RAGE) receptors.24 For example, when HMGB1 binds to RAGE, the secretion of type I interferon (IFN) and pro-inflammatory cytokines is stimulated, which induces monocyte-macrophage polarization toward the inflammatory phenotype.25 Studies have also shown a tight association between HMGB1 and ROS secretion.26
HSP
In the past, HSPs have been considered highly conserved emergency proteins that have an important role in protein synthesis and the folding of stress-responsive protein synthesis. However, recent studies have shown that HSPs are secreted extracellularly when ICD occurs and engage in organismal immune regulation.27 HSPs can be classified into six major classes according to their molecular weight: HSP10 (HSPE), HSP20 (HSPB), HSP40 (DNAJA, DNAJB, DNAJC), HSP60 (HSPD), HSP70 (HSPA), and HSP90 (HSPC).28 high-molecular-weight-HSP (HMW-HSP) consisting of HSP60, HSP70, and HSP90, is the most ardently expressed during cellular stress.29 Most HSPs are expressed in the cytoplasm or organelles during ICD, while HSP70 and HSP90 can also translocate to the plasma membrane and participate in the recruitment of immune cells during this process.30 HSP70 and HSP90, exposed on the cell membrane surface, interact with the APC surface receptors CD91, LOX1, and CD40 to promote TAA-dependent cross-presentation of MHC-I molecules and ultimately activate the immune response in CTL.31,32
ATP
ATP is the most plentiful intracellular small molecule metabolite, and its release in healthy cells is mainly through lysosomal cytokinesis. Although the detailed mechanism of active ATP secretion by tumor cells during ICD is not well defined, studies have discovered a close correlation between ATP secretion and autophagy.33 It has been shown that the membrane-linked protein pannexin 1 (PANX1) is involved in the release of cellular ATP. Cysteinyl aspartate specific proteinase 3 (Caspase-3), which performs apoptosis, is associated with the C-terminal auto-inhibitory domain of pannexin 1; caspase-3 could cut PANX1 to a protein fragment (tPANX1). Then, tPANX1 constitutes an active channel to facilitate the extracellular release of ATP.34,35 According to studies related to ICD inducer mitoxantrone and oxaliplatin treatment, complex crosstalk of signaling pathways, such as cytoprotective signaling pathways, ER stress, CRT exposure, apoptotic protein caspase enzyme cascade reaction, PANX1 activation, autophagy, transshipment and release of ATP vesicles, lysosomal cytokinesis, LAMP1, VAMP1 activation, membrane blebbing membrane exudate actin (ROCK1, myosin II) expression are the main determinants of ATP release. The immune effectiveness caused by the release of ATP during ICD is reflected in the recruitment of APC by ATP, the activation of inflammatory vesicles and the stimulation of monocyte polarization.18,36
ANXA1
ANXA1 is one of the essential DAMPs in the event of ICD. Unfortunately, the exact mechanism of ANXA1 release during ICD is not completely established.37 ANXA1 is an important homing factor that can bind to the G protein-coupled receptor formyl peptide receptor (FPR1) expressed by myeloid-derived immune cells, including DCs. After receptor binding reactions, ANXA1 turns into a chemotactic factor, which drives immature DCs to migrate toward dying tumor cells, and, in turn, phagocytose and tackle tumor cells.38 ANAX1 secretion also affects the extracellular exposure process of CRT, leading to improved infiltration of myeloid-derived DC and CTL at tumor sites.39
Immune Cell “Normalization” Based on ICD
The “immunosuppressive tumor microenvironment” (ITM) in melanoma treatment assists the immune escape of tumor cells and reduces the immune response. For example, associated fibroblasts (CAFs) in ITM promote matrix metalloproteinase (MMP) secretion and cause downregulation of NKG2D ligands MICA and MICB expression, thereby reducing the NK cell-mediated killing effect.40–42 Reversing ITM within melanoma, reducing tumor cell escape, and gaining immune cell boosting “leverage” are important guidelines for ICD combination therapy strategies.
Tumor-associated immune cells are widely distributed and have essential roles in tumor immunotherapy.43–46 ICD induction strategy differs from the immune checkpoint and immune cell therapy in that means of ICD induction, such as chemotherapy, radiotherapy, and thermotherapy have powerful penetration ability and can trigger strong immune-stress effects locally in the tumor. In detail, ICD is characterized by the release of damage-associated molecular patterns (DAMPs) such as CRT, ATP, and HMGB1 from dying cells. This process promotes the maturation of dendritic cells (DCs) and differentiation of macrophages, ultimately leading to the activation of effector T cells and their proliferation in response to immune stress. (Figure 3).
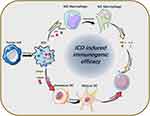 |
Figure 3 Release of ICD-associated DAMPs from tumor cells and their catalytic “functionalization” to immune cells. |
TAMs
Macrophages are mononuclear immune cells with phagocytic ability, mostly distributed in the lungs, liver, skin, and bone marrow.47 They are sensitive to the onset of diseases, such as cancer and skin damage, and are the “forerunners” of the body’s immune response.48 Tumor-associated macrophages (TAMs) are macrophage species that accumulate at tumor sites and regulate TME by releasing basic fibroblast growth factor (bFGF), angiogenic factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor beta (TGFβ), angiopoietin (Ang1, Ang2), interleukins (IL-1, IL-8, IL-6), tumor necrosis factor-alpha (TNF-α), and tumor necrosis factor (TGF, TGFβ), angiopoietin (Ang1, Ang2), thymidine phosphorylase (TP), matrix metalloproteinases (MMP-9, MMP-2), nitric oxide (NO) and other endogenous factors to interfere with the process of tumor cell genesis and development.49,50
TAMs are mainly categorized into two phenotypes, TAM1 and TAM2, in accordance with the levels of expression of specific receptors on its surface. TAM2, which lacks the expression of specific receptors hemoglobin scavenger receptor (CD163) and high expression of mannose receptor (CD206), can assist tumor escape from immune system surveillance.51 In ICD-based combination therapy, the DAMPs bind to TLR2 or TLR4 on the surface of TAM to induce inflammatory phenotypic polarization of TAM. In addition, the combination of the immune adjuvants imiquimod (R837) and R848, which can activate TLR7 receptors and stimulate the NF-κB signaling pathway, enhance TAM remodeling, the release of inflammatory immune factor and immune reactions of DC, CTL.52,53
DCs
Dendritic cells (DCs) are the main “monitors” in TME, mainly engaged in antigen uptake and presentation during the ICD process.54 The numerous DAMPs during ICD bind to PPR, Toll-like receptors on the DCs surfaces, fostering the maturation of DCs and the active processing presentation function of TAAs.9 Mature DCs express high levels of MHC-I/II molecules, CCR7, and have the capacity to actively migrate to draining lymph nodes.55 Ultimately, DCs co-present tumor-associated antigens and their plasma membrane expressing MHC-I/II molecules to T cells, activating CD8+ T cells.56,57
The immature phenotype of DCs in melanoma, which expresses high levels of the co-suppressor molecules PD-L1, PD-L2 and B7S1, secretes indoleamine 2,3-dioxygenase (IDO), IL-10, and IL-6. This phenomenon suppresses the antigen-specific T cell immune response, leading to a proliferation of Treg cells in tumors and lymph nodes.58 Considering the high correlation of DC maturation to increased T cell activation in TME,59 heme, hydrogen peroxide oxidase, glucose oxidase (Gox) and carbonic anhydrase IX (CA IX) were used to Stimulate DC maturation by improving hypoxia and reduce lactic acid accumulation in TME.60–62
T Cells
T cells, as the immune immune-functional killer cells of the body, are the “tumor terminators” in tumor immunotherapy.63 T cells in lymph node tissue are normally present as naive (TN) but rapidly proliferate and differentiate into CTL after being stimulated by TAAs.64 The secretion of DAMPs (including HMGB1, ATP, and ANAX1) during ICD can accelerate the proliferation and differentiation of T cells, with an enhancement of CTL infiltration at the tumor site and activation of a broad anti-tumor immune response.65,66
Molecular mechanisms related to the T cell immune response have demonstrated that the immune checkpoint molecule CTL-associated antigen 4 (CTLA-4), the programmed cell death 1 (PD-1) receptor and its ligand PD-L1/PD-L2, are key mediators in activating the T cell immune response and blocking the escape of tumor cells.67 When the T cell receptor (TCR) and CD28 binding occurs, CTLA-4 translocases to the cell membrane and participates in the competition of ligands on the surface of APC to inhibit the proliferation and activation of T cells.68 The PD-1/PD-L1/PD-L2 signaling pathway affects T cell cytokine secretion and tumor permeability, inhibits TCR signaling and messaging between T cells and APCs, and weakens the anti-tumor immune response of T cells. Therefore, the application of ICBs has made considerable progress in tumor immunotherapy research. Yet, mutations caused by PD-L1 overexpression in melanoma cells could contribute to the ineffectiveness of PD-(L) 1 blockade in the therapeutic studies of immune checkpoint blockers (ICIs).69 Since the predictive factors of antigenic response primarily utilized for immune checkpoint inhibitor treatment response are limited to individuals, not all somatic mutations could lead to the emergence of immunogenic neoantigens, which may be addressed by the broad anti-tumor immune effect elicited by ICD.
Multimodal Treatment Strategy Based on ICD
ICD induction strategies, including common chemotherapeutic agents, photothermal therapy, photodynamic therapy, RT therapy, etc. can trigger a “vaccine-like” function at the tumor site and activate adaptive immune stress. In recent years, the development and application of novel nanocarriers driven by nanotechnology have also accelerated the progress of melanoma research on ICD-induced multimodal therapeutic strategies. Diverse nanocarriers, including biomimetic cell membranes like macrophage membranes, erythrocyte membranes, exosomes, protein carriers like lactoferrin, albumin, structurally stable polymeric nano micelles like liposomes, chitosan, as well as inorganic nanocarriers carrying electric charges, have been developed.70,71 Polymeric nanomedicines supported by nanocarriers have become the main research direction to enhance the induction efficiency of ICD and improve the effect of tumor treatment.72
Chemotherapy
Chemotherapy has been used as one of the classical anti-tumor treatments. Clinical chemotherapeutic agents that have also been identified as ICD inducers include cyclophosphamide, methotrexate, doxorubicin, oxaliplatin, and other platinum derivatives.38,73 For instance, DOX has an induction impact on ICD-related mechanisms such as autophagy, CRT externalization, and HMGB1 emission from B16F10 cells.74 Nanocarrier-mediated induction of chemotherapy using PH-sensitive boronic acid ester phenylboronic acid complexes to structurally modify platinum metal nanoparticles can target thioredoxin reductase (TrxR) or other thiol-rich proteins and enzymes to stimulate the occurrence of the ICD cascade.75 Besides simple structural modifications, polymeric nanomaterials coupled with chemotherapeutic drugs, the investigators coupled 5-fluorouracil with oxaliplatin to form the nano-delivery system Nano-Folox, which exhibit stronger ICD induction and therapeutic effects than single drugs;76 the coupling of 1st generation N-(2-hydroxypropyl) methacrylamide (HPMA) with epirubicin (EPI) solved the problem of poor biocompatibility and metabolism of EPI, prolonged the circulation time of EPI, and thus enhanced the ability of EPI to perform ICD-inducing functions in tumor sites.77 Moreover, high-density lipoprotein (sHDL) consisting of apolipoprotein A1 (ApoA1) mimetic peptide and phospholipid encapsulated with DOX nanoparticles and polyethyleneimine-lithospermic acid conjugate (PEI-LCA) encapsulated with paclitaxel, both improved the drug self-targeting and ICD induction efficiency (Figure 4).78,79
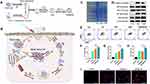 |
Figure 4 Schematic illustration of the DOX@LINV for tumor inhibition based on immunogenic tumor nanovesicles (TNVs) synergistic with the DOX-induced ICD effect. (A) Preparation of DOX@LINV by the confusion of TNVs with artificial liposomes. (B) Mechanism of immunochemotherapy based on the DOX@LINV for tumor suppression. (C) SDS–PAGE of B16F10 cells, B16F10-derived nanovesicles, and LINVs. (D) Western blotting of specific antigen preservation on TNVs and LINVs. (E) Representation flow cytometry plots of mature DCs after a 24 h treatment. (F) ATP, (G) HMGB-1, and (H) CRT release from B16F10 cells analyzed with the ELISA kit after 24 h of incubation (n = 3, *p < 0.05; **p < 0.01). (I) CRT expression after treatment evaluated by CLSM. Scale bar: 50 μm. Reprinted from Hu M, Zhang J, Kong L, et al . Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer ImmunochemotherapyImmunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. Acs Nano. 2021;15(2):3123–3138. Copyright © 2021, American Chemical Society.80 |
Radiotherapy
Radiotherapy(RT), as one of the traditional tumor treatments, is used to treat tumor by causing damage to double-stranded DNA (dsDNA) by ionizing radiation.81,82 RT-induced ICD causes an increase in the level of ROS secretion and ER and mitochondrial stress responses, resulting in the accelerated release of DAMPs from tumor cells.83–85 The accumulation of cDNA activates the cGAS/STING pathway, which stimulates IFN-I secretion and the recruitment of DC to reach the tumor site. ICD-associated DAMPs present TAAs to CD8+ T cells and exert systemic anti-tumor immune effects through lymphatic circulation.86
The main limitations faced by RT treatment include endogenous antioxidant resistance factors and irradiation absorption capacity. Hence, addressing the lack of OH production and the imbalance of oxygen supply and demand in solid tumors during RT treatment, accelerating the secretion of ROS and H2O2, and depleting endogenous antioxidant substances (catalase, superoxide dismutase and glutathione), are the main mechanisms to reshape the immune microenvironment for enhancing the effect of ICD. In recent years, the metal nanoparticles, such as platinum (Pt), ruthenium (Ru), iridium (Ir), copper (Cu), gold (Au), iron (Fe), and calcium (Ca), which provoke the Fenton catalytic effect and have the role of catalytic H2O2 decomposition and GSH depletion, have shown excellent oxidative stress amplification ability to become ideal drugs that deserve further research and development for RT-ICD treatment.87,88 In addition to reversing the endogenous tumor suppressive environment, utilizing different irradiation types, irradiation time, dose, and frequency to improve the intensity of irradiation uptake is also an effective mean to enhance the ICD-inducing effect of RT.89 The effect of different forms of irradiation mediated by protons, photons, and carbon ions on the secretion levels of ICD-DAMPs (CRT, HSP70, and HMGB1) in common human-derived cancer cell lines (CNE-2, A549, U251, and Tca8113) at different durations were analyzed by a previous correlational study.90 The effects of various radiation doses on the expression of apoptosis-related receptor-interacting serine/threonine-protein kinase 1 (RIPK1), RIPK3 and mixed lineage kinase domain-like protein (MLKL) in tumor cells were also observed.91 For example, low-dose radiation therapy (HFRT) has shown significant activation of MLKL, which has been investigated in related tumor treatment studies.92 Therefore, a reasonable format of RT irradiation, with optimal dose and duration, could effectively increase the efficiency of ICD-DAMPs production and reduce the risk of immune resistance elements arising during RT treatment.
In studies that focused on reversing TME and sensitizing ICD-inducing ability in RT therapy, researchers have constructed supramolecular self-assembled nanoplatforms of gadolinium (Gd3+) and 5′-guanosine monophosphate (5′-GMP) to homogeneously integrate heme (PANHEMATIN) and peroxidase into Gd3+/5′-GMP NCPs (Gd-NCPs), finally forming the novel radiosensitizer nanoplatform Hemin@ Gd3+/5′-GMP NCP (H@Gd-NCP) (Figure 5).93 Some researchers have designed Cu-NCPs self-assembled with Cu2+ and 5′-guanosine monophosphate (5’-GMP), where Cu-based nanoparticles possess a wider pH range and Fenton affect catalytic activity.94 Moreover, for more stable CaCO3 nanoparticles, researchers have designed curcumin (CUR)-doped CaCO3 nanoparticles (PEGCaCUR), modified with PHM-sensitive polyethylene glycol (PEG), to obtain PEGCaCUR nanoparticles.95
 |
Figure 5 Schematic illustration of the nano-ligand polymer for radio sensitization by amplifying intracellular oxidative stress. (A) Schematic diagram of the preparation of nano-ligand polymer. (B) H@Gd-NCP H@Gd-NCPs enhance the mechanism of checkpoint blockade immunotherapy. (C) Immunofluorescence of CRT antibody. (D) Western blot of HMGB1. (E) Detection of HMGB1 release by ELISA kit (n = 3, ***p = 0.0001, ***p = 0.0001, **p = 0.0014). (F) Detection of ATP secretion by luciferin-based ATP assay kit (n = 3, ***p = 0.0001, ***p = 0.0003). (G) Flow cytometry analysis of DCs maturation in tumor-draining lymph nodes (TDLNs) after radiotherapy (0 or 6 Gy × 1, n = 6, **p = 0.0063). N.S. represented non-significance, and **p < 0.01, ***p < 0.001. Reprinted from Huang Z, Wang Y, Yao D, Wu J, Hu Y, Yuan A. Nanoscale coordination polymers induce immunogenic cell death by amplifying radiation therapy mediated oxidative stress. Nat Commun. 2021;12(1):145. Creative Commons.93 |
In summary, the heme-enhanced peroxidase overexpression products H2O2 and Cu2+ and Ca2+ own Fenton effect were utilized to disrupt the antioxidant barrier to accelerate oxidative stress, enhancing the ICD-inducing ability of RT. Secondly, the optical-acoustic/fluorescent dual-mode imaging advantage of metal kinase grains provides unlimited potential for the application of RT diagnosis and treatment combination as well as further development.
Photodynamic Therapy
Photodynamic therapy(PDT) mainly relies on the conversion of photosensitizer from a single-linear ground state to an excited state under an appropriate wavelength of light, which eventually generates single-linear oxygen and directly damages proteins and lipid molecules in tumor cells to trigger cytotoxic effects, eventually leading to tumor cell death.96 In addition to the direct or indirect killing effect on tumor cells, the strong oxidative stress induced by the existing photosensitizers pheophorbide A (PPa), temoporfin, chlorin e6 (Ce6), hypericin, could lead to the appearance of ICD, thus encouraging the further development of PDT.97,98
The planar structure of conventional photosensitizers could be disrupted in aqueous/cellular environments due to π-π stacking of intermolecular interactions, which could significantly reduce their ROS generation efficiency. Therefore, researchers have developed novel photosensitizers with aggregation-induced emission (AIE) properties, AIE photosensitizers with peripheral intramolecular moving units (eg, benzene rings as rotors) and three-dimensional molecular structures.98 The stable steric structure of the AIE photosensitizer allows the irradiation energy to be concentrated as much as possible to stimulate fluorescence emission and ROS production from the selected target site, such as the AIE photosensitizers (TPE-DPA-TCyP, TPA-DCR) exhibit superior ROS generation efficiency, and ICD induction ability than Ce6.99
Nanocarrier-mediated coupling of the AIE photosensitizer TPEBTP with up conversion nanoparticles (UCNPs) increases lymph node drainage and T-cell activation. The oxygen-rich carrier HPOC nanoparticles made by protein cross-hybridization can be encapsulated with Ce6, increasing the effect of photosensitizer cell incorporation and stimulation efficiency.100–102 Based on the development of ICD-based PDT combinatorial tools, the investigators used a novel oxidized reduced liposome (RAL) self-assembled from phospholipid-porphyrin conjugates encapsulated with a checkpoint blocker IDO inhibitor (NLG-8189) that depletes glutathione (GSH) and enhances the ICD-inducing activity of RAL to boost the local immune response of tumors.103 Self-assembled smart nanovesicles (pRNVs) containing photosensitizer (HPPH) and IDO inhibitor IND designed using pH-responsive block copolymer polyethylene glycol-b-cationic polypeptide (PEG-b-cPPT) to stimulate HPPH by exploiting the ability of pRNVs to release drugs in response to an acidic environment, ultimately achieving enhanced induction of ICD and tumor immune response of CD8+ T cells (Figure 6).99,104
 |
Figure 6 Preparation and mechanism of pRNVs/HPPH/IND. (A) Construction of pH-Responsive Nanovesicles (pRNVs/HPPH/IND) via Co-assembly of HPPH, IND, and pH-Responsive Polypeptide. (B) Single low dose i.v. Injection of pRNVs/HPPH/IND to promote host immunity and induce tumor cell death. CRT release from B16F10 cells after 24 h incubation analyzed with CLSM (C) and flow cytometry (D) Scale bar: 40 µm. (E) Apoptosis in B16F10 cells induced by different formulation via flow cytometry Symbol (+) denotes laser irradiation at 671 nm (100 mW/cm2, 1 min). Reprinted from Yang W, Zhang F, Deng H, et al. Smart Nanovesicle-Mediated Immunogenic Cell Death through Tumor Microenvironment Modulation for Effective Photodynamic Immunotherapy. ACS Nano. 2020;14(1):620–631. Copyright © 2020, American Chemical Society.104 |
Photothermal Therapy
Photothermal therapy (PTT) mainly harnesses photosensitive materials’ huge thermal conversion effect after near-infrared (NIR) irradiation to ablate tumors. The ablation of tumors by PTT is also accompanied by the emergence of ICD.66 So far, common photothermal conversion nanomaterials include gold nanoparticles gold nanoshells (GNShs), gold nanorods (GNRs), gold nanocages (GNCs), gold nanostars (GNSs)) carbon nanomaterials (Sin-Gle wall carbon nanotubes (SWCNTs), graphene), semiconductor nanoparticles (copper sulfide (CuS), molybdenum disulfide (MoS2)), and organic near-infrared dyes (indocyanine green (IGC), IR780, IR820), all of which exhibit excellent photothermal transformation efficiency.105–107
Considering the high photothermal effect of PTT and the efficiency of ICD induction, the release of endogenous markers (CRT, HMGB1, ATP) of ICD at different excitation temperatures (thermal dose) was examined. For example, the ICD induction efficiency of PBNP-PTT at various thermal windows and using self-assembled gold nanoparticle liposomes at diverse IR intensities (NIR(I) and NIR(II) bio windows) were analyzed in relation to the excitation temperature.108,109 Designing the optimal strategy of photothermal conversion and ICD induction is expected to provide more safety and security for the clinical application of PTT.
In an ICD-based PTT conjoint treatment strategy, some investigators designed super-molecular cationic gold nanorods equipped with a heat-inducible promoter (HSP) CRISPR/Cas9 plasmid to enhance the activation of the immune response effect of ICD under PTT induction by inducing Cas9 plasmid targeting PD-L1 guide RNA9 (sgRNA) and activating the reprogramming of PD-L1 gene by sgRNA transcription in NIR-II near-infrared optical window (NIR-II, 1000–1350 nm). Other investigators designed the co-encapsulate ferroptosis inducer and exosome inhibitor GW4869 in semiconductor polymer PFG MPNs with excellent photothermal conversion performance to enhance the vaccination effect on B16F10 cells by exploiting the ICD-inducing ability of PFG MPNs under NIR-II irradiation in coordination with the Fenton effect and PD-L1 blocking effect of GW4869.110 The NIR-II light-modifiable polymeric nano-agonist APNA was also employed, which consists of a NIR-II light-absorbing semiconductor polymeric backbone as a photothermal transducer that couples a thermally unstable cleavable junction (7,8′-azobis[7-(8-imidazolin-848-yl)propane]:VA-2) to conjugate with a potent toll-like receptor type 7 and 8 (TLR7/8) agonist (Resiquimod: R848). RO44 can promote the maturation and polarization of APCs and enhance the anti-tumor immune effect of APNA after the photothermal effect triggering ICD (Figure 7).111 The discovery of a robust link between photothermal conversion and immune response in related studies provides a reference for further development of ICD-based combination therapy strategies.
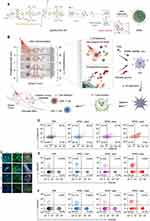 |
Figure 7 Preparation and mechanism of APNA. (A) Chemical structure of pBODO-PEG-VR and preparation of APNA. (B) Mechanism of anti-tumor immune response by APNA-mediated NIR-II photothermal immunotherapy. (C) Immunofluorescent images of Cas-3 (green), HMGB1 (green), and CD80/CD86 (Orange) in tumor sections at different photothermal depths after different treatments. Nuclei staining indicated by DAPI (blue). Scale bar: 20 µm. (D) DC maturation (gated on CD11c+ DCs) in tumor-draining lymph nodes from mice after different treatments. (E) Representative flow cytometry plots of CD8+ T cells and CD4+ T cells in tumor-infiltrating CD45+ lymphocytes in primary tumors from mice after various treatments. (F) Representative flow cytometry plots of CD8+ T cells and CD4+ T cells in tumor-infiltrating CD45+ lymphocytes in distant tumors from mice after various treatments. Reprinted from Jiang Y, Huang J, Xu C, Pu K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat Commun. 2021;12(1):742. Creative Commons.111 |
Magnetic Fluid Hyperthermia
Magnetic fluid hyperthermia (MFH) is a treatment that produces thermal energy by putting the rapid oscillation of magnetic nanoparticles (MNPs) in an alternating magnetic field (AMF).112 With the application of highly specific magnetic nanomaterials, the capability of local heating and excellent tumor permeability of MFH make it a potential treatment for tumor. The approval by European regulatory authorities (CE mark of conformity) for the clinical treatment of glioblastoma multiforme and the Phase II clinical study of prostate cancer may propel MFH into an essential next-generation tumor therapy.113–115
The elimination of tumor cells by magnetothermal therapy is mainly attributed to the thermally invasive ability of MFH on tumor cells. In recent years, researchers have discovered the immunogenicity of tumor cell death induced by MFH.116 The heat-induced ICD occurs in the controlled temperature range (>43°C) of MFH, causing HSP secretion, which stimulates immune cell responses, including DCs and macrophages, resulting in the activation of the immune response capacity of CD8+ T cells.117
In therapeutic studies of magnetic thermotherapy, the AC induction heating power of magnetic thermotherapy at AC magnetic fields in the biosafety range (Happl-fappl < 3.0–5.0×109 A m-1 s-1) is a standard measure of MNPs materials and their structures.118 The investigators expected to control the cation concentration and occupancy by doping (Fe2+, Fe3+ and M2+ (M=Mn, Zn, Co, Mg or Ni)) in Fe3O4 SPNPs and changing the MNPs particle size, dimensions, and shapes to improve magnetic susceptibility, magnetic exchange energy and enhance the AC induction heating effect.119 Some researchers have designed Mg0.13-γFe2O3 MNPs with a hundred times higher magnetothermal conversion efficiency than commercial Fe3O4.120 In ICD-based combination therapeutic strategies, some investigators have used Ferrimagnetic vortex-domain iron oxide nanorings (FVIOs) modified with iron oxide nanoparticles (IONPs) in combination with PD-L1 checkpoint blockade to enhance ICD-induced tumor-immune response and therapeutic efficacy (Figure 8).116 Other researchers investigated using MNPs synthesized by FVIOs-GO and PEGylated FVIOs, combined with checkpoint blockers, to amplify the immune activating effect of magnetothermal therapy on tumor sites.121
 |
Figure 8 (A) Mechanism of the FVIO-mediated mild magnetic hyperthermia can activate the host immune systems and efficiently cooperate with PD-L1 blockade to inhibit the potential metastatic spreading as well as the growth of distant tumors. (B and C) Quantification of CRT exposure on the surface after treatment by flow cytometry (***p < 0.001). (D) Quantification of CRT exposure on the surface by RT-PCR analysis (*p < 0.05, **p < 0.01, ***p < 0.001). (E) Representative multispectral fluorescence images of distant tumors after treatment. Scale bar: 100 μm. Reprinted from Liu X, Zheng J, Sun W, et al. Ferrimagnetic Vortex Nanoring-Mediated Mild Magnetic Hyperthermia Imparts Potent Immunological Effect for Treating Cancer Metastasis. ACS Nano. 2019;13(8):8811–8825. Copyright © 2019, American Chemical Society.116 |
Preclinical Study on ICD-Based Therapy in Melanoma
This review summarized data from the Web of Science core collection of 222 articles on ICD-based melanoma treatment in the last five years, retrieving the following characteristics: article title, journal title, year of publication, and author and institution of interest. The obtained data were charted in a bibliometric network using the VOSviewer software tool (Research Centre for Science and Technology, Leiden University, The Netherlands); each induction mean was matched to a frame on the network diagram, and the width of the reciprocal lines between each frame indicated the correlation, blue clusters were PTT-based ICD induction means, red clusters were DOX-based chemotherapy induction means, and green clusters were PDT-based ICD induction means. According to data analysis about ICD-induced melanoma treatment from the Web of Science core collection over the last five years, the current combination treatments (PDT, PTT, and chemotherapy) based on ICD are important treatment strategies (Figure 9). In addition, the relevant DAMPs markers for ICD detection in melanoma (CRT, HSP70, HMGB1, and ATP) summarized in this paper are important evaluation criteria for basic research. While traditional melanoma treatments face many bottlenecks, such as recurrence, metastasis and drug resistance, combining ICD induction and checkpoint blockade inhibitors to remodel ITM and enhance immune response has become a prospective melanoma treatment strategy. As shown in (Table 1), ICD induction means (PDT, PPT, and chemotherapy) are mostly combined with immunotherapy means such as immune checkpoint inhibitors to improve immune cell responses such as DC and CTL to maintain a broad immune activation effect at the tumor site and throughout the body.
 |
Table 1 Preclinical Studies of Nanocarrier-Mediated ICD for Melanoma Treatment |
 |
Figure 9 Overview of the literature visualization analysis on ICD induced therapies on melanoma in the last five years from the Web of Science. (A) Network visualization. (B) Density visualization. |
ICD-Based Clinical Treatment Progress
The viability of induction of ICD by chemotherapy, radiotherapy, and cryoablation was confirmed. The application of the ICD in combination with carboplatin plus paclitaxel (CP) standard-of-care chemotherapy regimen, a combination of the chemotherapeutic agent melphalan, stereotactic radiotherapy (SBRT) with the PD-L1/PD-1 blockers nivolumab and CTLA-4 checkpoint blockers have been already approved for the treatment of solid tumors such as melanoma. Besides the evaluation of the treatment effect by monitoring the absolute lymphatic count of Foxp3+ Treg cells, Th1/Th2/Th17, and plasmacytoid dendritic cells, myeloid-derived suppressor cells and their IDO expression have also been well investigated (Table 2). Yet, the lack of clarity in clinical studies regarding the means of monitoring ICD-associated factors has hindered further investigation into the significance and value of ICD around combined immunotherapy therapies for tumors. Standardization of ICD efficacy criteria are top priority for relevant clinical studies, referring to the Detection of Circulating Biomarkers of Immunogenic Cell Death (ICD) clinical study. ICD biomarkers may involve tumor-associated pro-tumor factors like IL1A, IL10, IL6, TGF-B, VEGFA, VEFGC, IDO enzyme, CXCL12, IL8; immune cell-associated pro-tumor factors like IL10, IDO enzyme, TGF-B, IL4, IL5, IL13, TNF, M-CSF, GM-CSF, and IL8, Chemokines like IFN-a, IFN-b, CXCL9, CXCL10, CXCL1, CCL2; immune cell-associated anti-tumor cytokines or chemokines like IL1B, IL12p70, IL15, IFNG, IL22, IL23, IL17A, IL2, CCL4, CCL5, CXCL13, CCL8, CCL19, CXCL11, CCL12 CXCL11, CCL12, CCL17, CCL23, CCL22, CCL13, CCL24, CCL1, CCL26, CXCL2, CXCL16, etc. (NCT02921854, Complete). Therefore, the further studies should identify key components. Furthermore, the vigorous translation of ICD induction tools from basic research may enrich the treatment of clinical melanoma and other tumors.
 |
Table 2 Clinical Studies of Nanocarrier-Mediated ICD for Melanoma Treatment |
Conclusion and Future Directions
Nanocarrier-enabled ICD, considered as one of the promising strategies for cancer immunotherapy, has demonstrated significant potential in tumor therapy, including the treatment of melanoma as mentioned previously. However, the stability, efficiency of ICD induction, and toxicity are all potential factors need to be considered for the effective application of nanomedicines in vivo. Therefore, the search for rational and stable nanocarriers is an important progress of step in the development of ICD-induced therapeutic strategies. We believe that the development of a single multifunctional nano-platform as an ideal vehicle for multiple ICD-inducing agents is a promising direction. We were delighted to find some researchers have achieved successful results in this area, such as copper kinase particles, which not only exhibit a photothermal effect but also possess their own catalytic Fenton effect, thereby accelerating the destruction of the local antioxidant immunosuppressive environment of the tumor.132 Additionally, MnO2 nanoparticles have found to induce ICD and activate the cGAS-STING pathway, therapy promoting DC maturation.130 Furthermore, UCNPs have been shown to promote the secretion of ROS and possess imaging potential, converting NIR light to visible light.100,156 New agents, such as gaseous molecules, including nitric oxide (NO), hydrogen (H2), carbon monoxide (CO), hydrogen sulfide (H2S), and sulfur dioxide (SO2), were found to be potent ICD inducers. These groundbreaking discoveries have significantly contributed to the advancement of ICD-based combination therapies.
Enhancing the efficacy of tumor immunotherapy is a pressing concern in clinical studies, particularly in the context of monoclonal antibody-based blocker immunotherapy. Objectively, the translation of nanocarrier-mediated induction in the clinic has been hindered by the inadequacy of ICD evaluation metrics, such as the assessment of the release of relevant immune substances, and the limitations of ICD induction strategies, such as safety concerns. Additionally, there are still numerous challenges and limitations for ICD from bench to bedside. Currently, there are only a few clinically approved drugs that can effectively stimulate a strong immune response, and the complete dynamics of these drugs in relation to ICD-guided immunotherapy remain unclear. Moreover, the impact of immune checkpoint blockade (ICB) therapy on the auto-tumor immune response can exhibit considerable inter-patient variability.157,158
Personalized design for patients may be necessary to address the heterogeneity of tumors. Additionally, it is important to clarify the role of each step in immune responses and the interplay among pathways. Consequently, there is an urgent need to explore sustainable and efficient strategies for activating immune responses. The clinical application of ICD combination therapy requires the establishment of rational, accurate, and reliable evaluation criteria to assess its effectiveness.
In this comprehensive review, we discuss various classical research tools based on ICD therapy, aiming to provide valuable insights for researchers working in this field and foster the continued advancement of ICD-based research in the treatment of melanoma.
Funding
This review was financially supported by the National Natural Science Foundation of China (No. 82073385, 82172706 and 82003295), Natural Science Foundation of Shanghai (No. 23ZR1478100).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gallicchio L, Devasia TP, Tonorezos E, Mollica MA, Mariotto A. Estimation of the Number of Individuals Living With Metastatic Cancer in the United States. J Natl Cancer Inst. 2022;114(11):1476–1483. doi:10.1093/jnci/djac158
2. Diep YN, Kim TJ, Cho H, Lee LP. Nanomedicine for advanced cancer immunotherapy. J Control Release. 2022;351:1017–1037. doi:10.1016/j.jconrel.2022.10.004
3. Guo ZS. The 2018 Nobel Prize in medicine goes to cancer immunotherapy (editorial for BMC cancer). BMC Cancer. 2018;18(1):1086. doi:10.1186/s12885-018-5020-3
4. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398(10304):1002–1014. doi:10.1016/S0140-6736(21)01206-X
5. Milone MC, Xu J, Chen SJ, et al. Author Correction: engineering-enhanced CAR T cells for improved cancer therapy. Nat Cancer. 2021;2(10):1113. doi:10.1038/s43018-021-00277-7
6. Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168(4):724–740. doi:10.1016/j.cell.2017.01.016
7. Huang K, Liu X, Han G, Zhou Y. Nano-optogenetic immunotherapy. Clin Transl Med. 2022;12(9):e1020. doi:10.1002/ctm2.1020
8. Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. doi:10.1016/j.coi.2015.10.009
9. Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500. doi:10.1038/s41590-022-01132-2
10. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi:10.1038/nm1523
11. Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17(4):262–275. doi:10.1038/nri.2017.9
12. Hayashi K, Nikolos F, Lee YC, et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat Commun. 2020;11(1):6299. doi:10.1038/s41467-020-19970-9
13. Jin MZ, Wang XP. Immunogenic Cell Death-Based Cancer Vaccines. Front Immunol. 2021;12:697964. doi:10.3389/fimmu.2021.697964
14. Bonaventura P, Shekarian T, Alcazer V, et al. Cold Tumors: a Therapeutic Challenge for Immunotherapy. Front Immunol. 2019;10:168. doi:10.3389/fimmu.2019.00168
15. Kim DY, Pyo A, Yun M, et al. Imaging Calreticulin for Early Detection of Immunogenic Cell Death During Anticancer Treatment. J Nucl Med. 2021;62(7):956–960. doi:10.2967/jnumed.120.245290
16. Michaud M, Martins I, Sukkurwala AQ, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. doi:10.1126/science.1208347
17. Senovilla L, Vitale I, Martins I, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337(6102):1678–1684. doi:10.1126/science.1224922
18. Ma Y, Adjemian S, Mattarollo SR, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741. doi:10.1016/j.immuni.2013.03.003
19. Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11(3):122–129. doi:10.1016/s0962-8924(01)01926-2
20. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006. doi:10.1002/1878-0261.12851
21. Bezu L, Sauvat A, Humeau J, et al. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018;25(8):1375–1393. doi:10.1038/s41418-017-0044-9
22. Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–1143. doi:10.1084/jem.20042614
23. Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi:10.1097/01.shk.0000225404.51320.82
24. Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805(1):53–71. doi:10.1016/j.bbcan.2009.08.003
25. Son M, Porat A, He M, et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood. 2016;128(18):2218–2228. doi:10.1182/blood-2016-05-719757
26. Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280(1):74–82. doi:10.1111/imr.12601
27. Li DY, Liang S, Wen JH, Tang JX, Deng SL, Liu YX. Extracellular HSPs: the Potential Target for Human Disease Therapy. Molecules. 2022;27(7):2361. doi:10.3390/molecules27072361
28. Zhang Z, Jing J, Ye Y, et al. Characterization of the dual functional effects of heat shock proteins (HSPs) in cancer hallmarks to aid development of HSP inhibitors. Genome Med. 2020;12(1):101. doi:10.1186/s13073-020-00795-6
29. Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12(3):743–761. doi:10.1111/j.1582-4934.2008.00273.x
30. Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15(1):3–12. doi:10.1038/sj.cdd.4402269
31. Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109(11):4839–4845. doi:10.1182/blood-2006-10-054221
32. Blaschke T, Burnett C, Pekkarinen A. Image Segmentation Methods for Object-based Analysis and Classification. In: Jong SMD, Meer F, editors. Remote Sensing Image Analysis: Including the Spatial Domain. Springer Netherlands; 2004:211–236.
33. Xia H, Green DR, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021;21(5):281–297. doi:10.1038/s41568-021-00344-2
34. Martins I, Wang Y, Michaud M, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91. doi:10.1038/cdd.2013.75
35. Wang Y, Martins I, Ma Y, Kepp O, Galluzzi L, Kroemer G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy. 2013;9(10):1624–1625. doi:10.4161/auto.25873
36. Muller T, Robaye B, Vieira RP, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553. doi:10.1111/j.1398-9995.2010.02426.x
37. Zhuang Y, Liu H, Edward Zhou X, et al. Structure of formylpeptide receptor 2-G(i) complex reveals insights into ligand recognition and signaling. Nat Commun. 2020;11(1):885. doi:10.1038/s41467-020-14728-9
38. Vacchelli E, Ma Y, Baracco EE, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–978. doi:10.1126/science.aad0779
39. Baracco EE, Stoll G, Van Endert P, Zitvogel L, Vacchelli E, Kroemer G. Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology. 2019;8(11):e1647760. doi:10.1080/2162402X.2019.1647760
40. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. doi:10.1038/s41573-018-0004-1
41. Liu T, Han C, Wang S, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86. doi:10.1186/s13045-019-0770-1
42. Vandyck HH, Hillen LM, Bosisio FM, van den Oord J, Zur Hausen A, Winnepenninckx V. Rethinking the biology of metastatic melanoma: a holistic approach. Cancer Metastasis Rev. 2021;40(2):603–624. doi:10.1007/s10555-021-09960-8
43. Masaoutis C, Kokkali S, Theocharis S. Immunotherapy in uveal melanoma: novel strategies and opportunities for personalized treatment. Expert Opin Investig Drugs. 2021;30(5):555–569. doi:10.1080/13543784.2021.1898587
44. Cockram TOJ, Dundee JM, Popescu AS, Brown GC. The Phagocytic Code Regulating Phagocytosis of Mammalian Cells. Front Immunol. 2021;12:629979. doi:10.3389/fimmu.2021.629979
45. Nguyen T, Kocovski N, Macdonald S, Yeang HXA, Wang M, Neeson PJ. Multiplex Immunohistochemistry Analysis of Melanoma Tumor-Infiltrating Lymphocytes. Methods Mol Biol. 2021;2265:557–572. doi:10.1007/978-1-0716-1205-7_39
46. Bernal-Estevez DA, Ortiz Barbosa MA, Ortiz-Montero P, Cifuentes C, Sanchez R, Parra-Lopez CA. Autologous Dendritic Cells in Combination With Chemotherapy Restore Responsiveness of T Cells in Breast Cancer Patients: a Single-Arm Phase I/II Trial. Front Immunol. 2021;12:669965. doi:10.3389/fimmu.2021.669965
47. Zhao J, Huang H, Zhao J, et al. A hybrid bacterium with tumor-associated macrophage polarization for enhanced photothermal-immunotherapy. Acta Pharm Sin B. 2022;12(6):2683–2694. doi:10.1016/j.apsb.2021.10.019
48. Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19(22):6074–6083. doi:10.1158/1078-0432.CCR-12-2603
49. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. doi:10.1016/j.ccr.2013.02.013
50. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi:10.1016/j.immuni.2014.06.010
51. Han S, Wang W, Wang S, et al. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics. 2021;11(6):2892–2916. doi:10.7150/thno.50928
52. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. doi:10.1038/s41577-019-0127-6
53. Li X, Pan J, Li Y, et al. Development of a Localized Drug Delivery System with a Step-by-Step Cell Internalization Capacity for Cancer Immunotherapy. ACS Nano. 2022;16(4):5778–5794. doi:10.1021/acsnano.1c10892
54. Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202(2):203–207. doi:10.1084/jem.20050810
55. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi:10.1146/annurev-immunol-020711-074950
56. Ma Y, Pitt JM, Li Q, Yang H. The renaissance of anti-neoplastic immunity from tumor cell demise. Immunol Rev. 2017;280(1):194–206. doi:10.1111/imr.12586
57. Ma Y, Mattarollo SR, Adjemian S, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2014;74(2):436–445. doi:10.1158/0008-5472.CAN-13-1265
58. Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–290. doi:10.1172/JCI21583
59. Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–715. doi:10.1158/0008-5472.CAN-13-1342
60. He Z, Zhou H, Zhang Y, et al. Oxygen-boosted biomimetic nanoplatform for synergetic phototherapy/ferroptosis activation and reversal of immune-suppressed tumor microenvironment. Biomaterials. 2022;290:121832. doi:10.1016/j.biomaterials.2022.121832
61. Jiao X, Sun L, Zhang W, et al. Engineering oxygen-deficient ZrO(2-x) nanoplatform as therapy-activated “immunogenic cell death (ICD)” inducer to synergize photothermal-augmented sonodynamic tumor elimination in NIR-II biological window. Biomaterials. 2021;272:120787. doi:10.1016/j.biomaterials.2021.120787
62. Zuo W, Fan Z, Chen L, et al. Copper-based theranostic nanocatalysts for synergetic photothermal-chemodynamic therapy. Acta Biomater. 2022;147:258–269. doi:10.1016/j.actbio.2022.05.030
63. Meneveau MO, Sahli ZT, Lynch KT, Mauldin IS, Slingluff CL Jr. Immunotyping and Quantification of Melanoma Tumor-Infiltrating Lymphocytes. Methods Mol Biol. 2021;2265:515–528. doi:10.1007/978-1-0716-1205-7_36
64. Kishton RJ, Sukumar M, Restifo NP. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017;26(1):94–109. doi:10.1016/j.cmet.2017.06.016
65. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi:10.1038/nm1622
66. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi:10.1146/annurev-immunol-032712-100008
67. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi:10.1126/science.342.6165.1432
68. Wiede F, Lu KH, Du X, et al. PTP1B Is an Intracellular Checkpoint that Limits T-cell and CAR T-cell Antitumor Immunity. Cancer Discov. 2022;12(3):752–773. doi:10.1158/2159-8290.CD-21-0694
69. Juneja VR, McGuire KA, Manguso RT, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214(4):895–904. doi:10.1084/jem.20160801
70. Cassano R, Cuconato M, Calviello G, Serini S, Trombino S. Recent Advances in Nanotechnology for the Treatment of Melanoma. Molecules. 2021;26(4):785. doi:10.3390/molecules26040785
71. Song M, Liu C, Chen S, Zhang W. Nanocarrier-Based Drug Delivery for Melanoma Therapeutics. Int J Mol Sci. 2021;22(4):1873. doi:10.3390/ijms22041873
72. Rautaniemi K, Zini J, Lofman E, et al. Addressing challenges in the removal of unbound dye from passively labelled extracellular vesicles. Nanoscale Adv. 2021;4(1):226–240. doi:10.1039/d1na00755f
73. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28(6):690–714. doi:10.1016/j.ccell.2015.10.012
74. Florencio KGD, Edson EA, Fernandes K, et al. Chromomycin A(5) induces bona fide immunogenic cell death in melanoma. Front Immunol. 2022;13:941757. doi:10.3389/fimmu.2022.941757
75. Lu Y, Ma X, Chang X, et al. Recent development of gold(I) and gold(III) complexes as therapeutic agents for cancer diseases. Chem Soc Rev. 2022;51(13):5518–5556. doi:10.1039/d1cs00933h
76. Guo J, Yu Z, Das M, Huang L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano. 2020;14(4):5075–5089. doi:10.1021/acsnano.0c01676
77. Li L, Li Y, Yang CH, et al. Inhibition of Immunosuppressive Tumors by Polymer-Assisted Inductions of Immunogenic Cell Death and Multivalent PD-L1 Crosslinking. Adv Funct Mater. 2020;30(12):9081. doi:10.1002/adfm.201908961
78. Kuai R, Yuan W, Son S, et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4(4):eaao1736. doi:10.1126/sciadv.aao1736
79. Meng F, Wang J, He Y, et al. A single local delivery of paclitaxel and nucleic acids via an immunoactive polymer eliminates tumors and induces antitumor immunity. Proc Natl Acad Sci U S A. 2022;119(22):e2122595119. doi:10.1073/pnas.2122595119
80. Hu M et al . (2021). Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer ImmunochemotherapyImmunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. Acs Nano, 15(2), 3123–3138. 10.1021/acsnano.0c0968110.1021/acsnano.0c09681.s001
81. Zhu M, Yang M, Zhang J, et al. Immunogenic Cell Death Induction by Ionizing Radiation. Front Immunol. 2021;12:705361. doi:10.3389/fimmu.2021.705361
82. Knijnenburg TA, Wang L, Zimmermann MT, et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23(1):239–254 e6. doi:10.1016/j.celrep.2018.03.076
83. McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–217. doi:10.1038/s41568-020-0246-1
84. Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. doi:10.1038/nature23470
85. Welsh J, Bevelacqua JJ, Dobrzyński L. Abscopal Effect Following Radiation Therapy in Cancer Patients: a New Look from the Immunological Point of View. J Biomed Phys Eng. 2020;10(4):537–542. doi:10.31661/jbpe.v0i0.1066
86. Diamond JM, Vanpouille-Box C, Spada S, et al. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol Res. 2018;6(8):910–920. doi:10.1158/2326-6066.CIR-17-0581
87. Zhou Z, Ni K, Deng H, Chen X. Dancing with reactive oxygen species generation and elimination in nanotheranostics for disease treatment. Adv Drug Deliv Rev. 2020;158:73–90. doi:10.1016/j.addr.2020.06.006
88. Zhang M, Song R, Liu Y, et al. Calcium-Overload-Mediated Tumor Therapy by Calcium Peroxide Nanoparticles. Chem. 2019;5(8):2171–2182. doi:10.1016/j.chempr.2019.06.003
89. Sia J, Szmyd R, Hau E, Gee HE. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: a Primer. Front Cell Dev Biol. 2020;8:41. doi:10.3389/fcell.2020.00041
90. Huang Y, Dong Y, Zhao J, Zhang L, Kong L, Lu JJ. Comparison of the effects of photon, proton and carbon-ion radiation on the ecto-calreticulin exposure in various tumor cell lines. Ann Transl Med. 2019;7(20):542. doi:10.21037/atm.2019.09.128
91. Snyder AG, Hubbard NW, Messmer MN, et al. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci Immunol. 2019;4(36):2004. doi:10.1126/sciimmunol.aaw2004
92. Wang HH, Wu ZQ, Qian D, et al. Ablative Hypofractionated Radiation Therapy Enhances Non-Small Cell Lung Cancer Cell Killing via Preferential Stimulation of Necroptosis In Vitro and In Vivo. Int J Radiat Oncol Biol Phys. 2018;101(1):49–62. doi:10.1016/j.ijrobp.2018.01.036
93. Huang Z, Wang Y, Yao D, Wu J, Hu Y, Yuan A. Nanoscale coordination polymers induce immunogenic cell death by amplifying radiation therapy mediated oxidative stress. Nat Commun. 2021;12(1):145. doi:10.1038/s41467-020-20243-8
94. Wang Y, Ding Y, Yao D, et al. Copper-Based Nanoscale Coordination Polymers Augmented Tumor Radioimmunotherapy for Immunogenic Cell Death Induction and T-Cell Infiltration. Small. 2021;17(8):e2006231. doi:10.1002/smll.202006231
95. Zheng P, Ding B, Jiang Z, et al. Ultrasound-Augmented Mitochondrial Calcium Ion Overload by Calcium Nanomodulator to Induce Immunogenic Cell Death. Nano Lett. 2021;21(5):2088–2093. doi:10.1021/acs.nanolett.0c04778
96. Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9(1). doi:10.1136/jitc-2020-001926
97. He C, Duan X, Guo N, et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499. doi:10.1038/ncomms12499
98. Chen C, Ni X, Jia S, et al. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv Mater. 2019;31(52):e1904914. doi:10.1002/adma.201904914
99. Yang G, Lu SB, Li C, et al. Type I macrophage activator photosensitizer against hypoxic tumors. Chem Sci. 2021;12(44):14773–14780. doi:10.1039/d1sc04124j
100. Mao D, Hu F, Yi Z, et al. AIEgen-coupled upconversion nanoparticles eradicate solid tumors through dual-mode ROS activation. Sci Adv. 2020;6(26):eabb2712. doi:10.1126/sciadv.abb2712
101. Chen Z, Liu L, Liang R, et al. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano. 2018;12(8):8633–8645. doi:10.1021/acsnano.8b04371
102. Ding Y, Sun Z, Gao Y, et al. Plasmon-Driven Catalytic Chemotherapy Augments Cancer Immunotherapy through Induction of Immunogenic Cell Death and Blockage of IDO Pathway. Adv Mater. 2021;33(34):e2102188. doi:10.1002/adma.202102188
103. Liu D, Chen B, Mo Y, et al. Redox-Activated Porphyrin-Based Liposome Remote-Loaded with Indoleamine 2,3-Dioxygenase (IDO) Inhibitor for Synergistic Photoimmunotherapy through Induction of Immunogenic Cell Death and Blockage of IDO Pathway. Nano Lett. 2019;19(10):6964–6976. doi:10.1021/acs.nanolett.9b02306
104. Yang W, Zhang F, Deng H, et al. Smart Nanovesicle-Mediated Immunogenic Cell Death through Tumor Microenvironment Modulation for Effective Photodynamic Immunotherapy. ACS Nano. 2020;14(1):620–631. doi:10.1021/acsnano.9b07212
105. Wang Y, Meng HM, Li Z. Near-infrared inorganic nanomaterial-based nanosystems for photothermal therapy. Nanoscale. 2021;13(19):8751–8772. doi:10.1039/d1nr00323b
106. Wu D, Zhou J, Chen X, et al. Mesoporous polydopamine with built-in plasmonic core: traceable and NIR triggered delivery of functional proteins. Biomaterials. 2020;238:119847. doi:10.1016/j.biomaterials.2020.119847
107. Zhu Y, Hoh HY, Qian S, et al. Ultrastable Zinc Anode Enabled by CO(2)-Induced Interface Layer. ACS Nano. 2022;16(9):14600–14610. doi:10.1021/acsnano.2c05124
108. Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal Therapy Generates a Thermal Window of Immunogenic Cell Death in Neuroblastoma. Small. 2018;14(20):e1800678. doi:10.1002/smll.201800678
109. Ma Y, Zhang Y, Li X, et al. Near-Infrared II Phototherapy Induces Deep Tissue Immunogenic Cell Death and Potentiates Cancer Immunotherapy. ACS Nano. 2019;13(10):11967–11980. doi:10.1021/acsnano.9b06040
110. Xie L, Li J, Wang G, et al. Phototheranostic Metal-Phenolic Networks with Antiexosomal PD-L1 Enhanced Ferroptosis for Synergistic Immunotherapy. J Am Chem Soc. 2022;144(2):787–797. doi:10.1021/jacs.1c09753
111. Jiang Y, Huang J, Xu C, Pu K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat Commun. 2021;12(1):742. doi:10.1038/s41467-021-21047-0
112. Shaterabadi Z, Nabiyouni G, Soleymani M. Physics responsible for heating efficiency and self-controlled temperature rise of magnetic nanoparticles in magnetic hyperthermia therapy. Prog Biophys Mol Biol. 2018;133:9–19. doi:10.1016/j.pbiomolbio.2017.10.001
113. El-Boubbou K. Magnetic iron oxide nanoparticles as drug carriers: clinical relevance. Nanomedicine. 2018;13(8):953–971. doi:10.2217/nnm-2017-0336
114. Stea B, Kittelson J, Cassady JR, et al. Treatment of malignant gliomas with interstitial irradiation and hyperthermia. Int J Radiat Oncol Biol Phys. 1992;24(4):657–667. doi:10.1016/0360-3016(92)90711-p
115. Deger S, Taymoorian K, Boehmer D, et al. Thermoradiotherapy using interstitial self-regulating thermoseeds: an intermediate analysis of a phase II trial. Eur Urol. 2004;45(5):574–579. doi:10.1016/j.eururo.2003.11.012
116. Liu X, Zheng J, Sun W, et al. Ferrimagnetic Vortex Nanoring-Mediated Mild Magnetic Hyperthermia Imparts Potent Immunological Effect for Treating Cancer Metastasis. ACS Nano. 2019;13(8):8811–8825. doi:10.1021/acsnano.9b01979
117. Adkins I, Sadilkova L, Hradilova N, Tomala J, Kovar M, Spisek R. Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. Oncoimmunology. 2017;6(5):e1311433. doi:10.1080/2162402X.2017.1311433
118. Hergt R, Andra W, d’Ambly CG, et al. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans Magn. 1998;34(5):3745–3754. doi:10.1109/20.718537
119. Jordan A, Scholz R, Wust P, Fähling H, Roland F. Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater. 1999;201(1):413–419. doi:10.1016/S0304-8853(99)00088-8
120. Jang JT, Lee J, Seon J, et al. Giant Magnetic Heat Induction of Magnesium-Doped gamma-Fe(2) O(3) Superparamagnetic Nanoparticles for Completely Killing Tumors. Adv Mater. 2018;30(6):56.
121. Liu X, Yan B, Li Y, et al. Graphene Oxide-Grafted Magnetic Nanorings Mediated Magnetothermodynamic Therapy Favoring Reactive Oxygen Species-Related Immune Response for Enhanced Antitumor Efficacy. ACS Nano. 2020;14(2):1936–1950. doi:10.1021/acsnano.9b08320
122. Heshmati Aghda N, Torres Hurtado S, Abdulsahib SM, Lara EJ, Tunnell JW, Betancourt T. Dual Photothermal/Chemotherapy of Melanoma Cells with Albumin Nanoparticles Carrying Indocyanine Green and Doxorubicin Leads to Immunogenic Cell Death. Macromol Biosci. 2022;22(2):e2100353. doi:10.1002/mabi.202100353
123. Konda P, Roque Iii JA, Lifshits LM, et al. Photodynamic therapy of melanoma with new, structurally similar, NIR-absorbing ruthenium (II) complexes promotes tumor growth control via distinct hallmarks of immunogenic cell death. Am J Cancer Res. 2022;12(1):210–228.
124. Liu X, Feng Y, Xu J, et al. Combination of MAPK inhibition with photothermal therapy synergistically augments the anti-tumor efficacy of immune checkpoint blockade. J Control Release. 2021;332:194–209. doi:10.1016/j.jconrel.2021.02.020
125. Jia Y, Shi K, Dai L, et al. Gold Nanorods and Polymer Micelles Mediated Dual TLR Stimulators Delivery System CPG@Au NRs/M-R848 Regulate Macrophages Reprogramming and DC Maturation for Enhanced Photothermal Immunotherapy of Melanoma. Small Methods. 2023;7(5):e2201087. doi:10.1002/smtd.202201087
126. Ma S, Liang X, Yang N, et al. Boosting cancer immunotherapy by biomineralized nanovaccine with ferroptosis-inducing and photothermal properties. Biomater Sci. 2023;11(2):518–532. doi:10.1039/d2bm01126c
127. Feng ZH, Li ZT, Zhang S, et al. A combination strategy based on an Au nanorod/doxorubicin gel via mild photothermal therapy combined with antigen-capturing liposomes and anti-PD-L1 agent promote a positive shift in the cancer-immunity cycle. Acta Biomater. 2021;136:495–507. doi:10.1016/j.actbio.2021.09.052
128. Liu Q, Chen F, Hou L, et al. Nanocarrier-Mediated Chemo-Immunotherapy Arrested Cancer Progression and Induced Tumor Dormancy in Desmoplastic Melanoma. ACS Nano. 2018;12(8):7812–7825. doi:10.1021/acsnano.8b01890
129. Su Z, Xiao Z, Huang J, et al. Dual-Sensitive PEG-Sheddable Nanodrug Hierarchically Incorporating PD-L1 Antibody and Zinc Phthalocyanine for Improved Immuno-Photodynamic Therapy. ACS Appl Mater Interfaces. 2021;13(11):12845–12856. doi:10.1021/acsami.0c20422
130. Zhao X, Zhang J, Chen B, Ding X, Zhao N, Xu FJ. Rough Nanovaccines Boost Antitumor Immunity Through the Enhancement of Vaccination Cascade and Immunogenic Cell Death Induction. Small Methods. 2023;7(5):e2201595. doi:10.1002/smtd.202201595
131. Li M, Guo R, Wei J, et al. Polydopamine-based nanoplatform for photothermal ablation with long-term immune activation against melanoma and its recurrence. Acta Biomater. 2021;136:546–557. doi:10.1016/j.actbio.2021.09.014
132. Yan T, Yang K, Chen C, et al. Synergistic photothermal cancer immunotherapy by Cas9 ribonucleoprotein-based copper sulfide nanotherapeutic platform targeting PTPN2. Biomaterials. 2021;279:121233. doi:10.1016/j.biomaterials.2021.121233
133. Zhang Y, Guo C, Liu L, et al. ZnO-based multifunctional nanocomposites to inhibit progression and metastasis of melanoma by eliciting antitumor immunity via immunogenic cell death. Theranostics. 2020;10(24):11197–11214. doi:10.7150/thno.44920
134. Tang H, Xu X, Chen Y, et al. Reprogramming the Tumor Microenvironment through Second-Near-Infrared-Window Photothermal Genome Editing of PD-L1 Mediated by Supramolecular Gold Nanorods for Enhanced Cancer Immunotherapy. Adv Mater. 2021;33(12):e2006003. doi:10.1002/adma.202006003
135. Zhu J, Chang R, Wei B, et al. Photothermal Nano-Vaccine Promoting Antigen Presentation and Dendritic Cells Infiltration for Enhanced Immunotherapy of Melanoma via Transdermal Microneedles Delivery. Research (Wash D C). 2022;2022:9816272. doi:10.34133/2022/9816272
136. Hu D, Xu H, Zhang W, et al. Vanadyl nanocomplexes enhance photothermia-induced cancer immunotherapy to inhibit tumor metastasis and recurrence. Biomaterials. 2021;277:121130. doi:10.1016/j.biomaterials.2021.121130
137. Li Z, Xiang J, Zhang Q, et al. An engineered hydrogel with low-dose antitumor drugs enhances tumor immunotherapy through tumor interstitial wrap. Front Bioeng Biotechnol. 2022;10:1072393. doi:10.3389/fbioe.2022.1072393
138. Medrano RFV, Salles TA, Dariolli R, et al. Potentiation of combined p19Arf and interferon-beta cancer gene therapy through its association with doxorubicin chemotherapy. Sci Rep. 2022;12(1):13636. doi:10.1038/s41598-022-17775-y
139. Yang C, Ming Y, Zhou K, et al. Macrophage Membrane-Camouflaged shRNA and Doxorubicin: a pH-Dependent Release System for Melanoma Chemo-Immunotherapy. Research (Wash D C). 2022;2022:9768687. doi:10.34133/2022/9768687
140. Huang SW, Wang ST, Chang SH, et al. Imiquimod Exerts Antitumor Effects by Inducing Immunogenic Cell Death and Is Enhanced by the Glycolytic Inhibitor 2-Deoxyglucose. J Invest Dermatol. 2020;140(9):1771–1783 e6. doi:10.1016/j.jid.2019.12.039
141. Yu N, Ding M, Wang F, et al. Near-infrared photoactivatable semiconducting polymer nanocomplexes with bispecific metabolism interventions for enhanced cancer immunotherapy. Nano Today. 2022;46:101600. doi:10.1016/j.nantod.2022.101600
142. Su Z, Xiao Z, Wang Y, et al. Codelivery of Anti-PD-1 Antibody and Paclitaxel with Matrix Metalloproteinase and pH Dual-Sensitive Micelles for Enhanced Tumor Chemoimmunotherapy. Small. 2020;16(7):e1906832. doi:10.1002/smll.201906832
143. Yerragopu AK, Vellapandian C. Chemoimmunotherapy with doxorubicin and caffeine combination enhanced ICD induction and T-cell infiltration in B16F10 melanoma tumors. J Biochem Mol Toxicol. 2023;37(5):e23327. doi:10.1002/jbt.23327
144. Wang C, Shi X, Song H, et al. Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials. 2021;268:120579. doi:10.1016/j.biomaterials.2020.120579
145. Fu X, Shi Y, Zang H, et al. Combination of oxaliplatin and POM-1 by nanoliposomes to reprogram the tumor immune microenvironment. J Control Release. 2022;347:1–13. doi:10.1016/j.jconrel.2022.04.041
146. Fan Y, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ. Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017;17(12):7387–7393. doi:10.1021/acs.nanolett.7b03218
147. Liu Q, Zhu H, Tiruthani K, et al. Nanoparticle-Mediated Trapping of Wnt Family Member 5A in Tumor Microenvironments Enhances Immunotherapy for B-Raf Proto-Oncogene Mutant Melanoma. ACS Nano. 2018;12(2):1250–1261. doi:10.1021/acsnano.7b07384
148. Shan CK, Du YB, Zhai XT, et al. Pingyangmycin enhances the antitumor efficacy of anti-PD-1 therapy associated with tumor-infiltrating CD8(+) T cell augmentation. Cancer Chemother Pharmacol. 2021;87(3):425–436. doi:10.1007/s00280-020-04209-7
149. Li Q, Chen C, Kong J, Li L, Li J, Huang Y. Stimuli-responsive nano vehicle enhances cancer immunotherapy by coordinating mitochondria-targeted immunogenic cell death and PD-L1 blockade. Acta Pharm Sin B. 2022;12(5):2533–2549. doi:10.1016/j.apsb.2021.11.005
150. Li C, Zhang Y, Yan S, et al. Alternol triggers immunogenic cell death via reactive oxygen species generation. Oncoimmunology. 2021;10(1):1952539. doi:10.1080/2162402X.2021.1952539
151. Xie L, Wang G, Sang W, et al. Phenolic immunogenic cell death nanoinducer for sensitizing tumor to PD-1 checkpoint blockade immunotherapy. Biomaterials. 2021;269:120638. doi:10.1016/j.biomaterials.2020.120638
152. Gong Y, Chen M, Tan Y, et al. Injectable Reactive Oxygen Species-Responsive SN38 Prodrug Scaffold with Checkpoint Inhibitors for Combined Chemoimmunotherapy. ACS Appl Mater Interfaces. 2020;12(45):50248–50259. doi:10.1021/acsami.0c13943
153. Prieto K, Cao Y, Mohamed E, et al. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 2019;5:134. doi:10.1038/s41420-019-0214-2
154. Yang C, He B, Zheng Q, et al. Nano-encapsulated tryptanthrin derivative for combined anticancer therapy via inhibiting indoleamine 2,3-dioxygenase and inducing immunogenic cell death. Nanomedicine. 2019;14(18):2423–2440. doi:10.2217/nnm-2019-0074
155. Kalus P, De Munck J, Vanbellingen S, et al. Oncolytic Herpes Simplex Virus Type 1 Induces Immunogenic Cell Death Resulting in Maturation of BDCA-1(+) Myeloid Dendritic Cells. Int J Mol Sci. 2022;23(9):2165. doi:10.3390/ijms23094865
156. Zhou B, Shi B, Jin D, Liu X. Controlling upconversion nanocrystals for emerging applications. Nat Nanotechnol. 2015;10(11):924–936. doi:10.1038/nnano.2015.251
157. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi:10.1056/NEJMoa1503093
158. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
