Back to Journals » Cancer Management and Research » Volume 13
Nano-Silver Medical Antibacterial Dressing Combined with High-Flow Oxygen Therapy Facilitates Ulcer Wound Healing of Superficial Malignant Tumors
Authors Yu D, Yang DX, Li Y, Guan B, Ming Q, Li Y, Zhu YP, Chen LQ, Luo WX
Received 26 September 2021
Accepted for publication 9 November 2021
Published 6 December 2021 Volume 2021:13 Pages 9007—9013
DOI https://doi.org/10.2147/CMAR.S341448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Dan Yu,1,* Di-Xiao Yang,2,* Yao Li,3 Bi Guan,2 Qian Ming,2 Yan Li,2 Yi-Ping Zhu,4 Li-Qing Chen,1 Wei-Xiang Luo5
1Department of Otolaryngology and Hepatobiliary, Chengdu Fifth People’s Hospital, Chengdu, 611130, Sichuan, People’s Republic of China; 2Nursing Department, Chengdu Fifth People’s Hospital, Chengdu, 611130, Sichuan, People’s Republic of China; 3Intensive Care Unit, Chengdu Fifth People’s Hospital, Chengdu, 611130, Sichuan, People’s Republic of China; 4Department of Oncology, Chengdu Wenjiang District People’s Hospital, Chengdu, 611130, Sichuan, People’s Republic of China; 5Department of Nursing, Shenzhen People’s Hospital (2nd Clinical Medical College of Jinan University, First Affiliated Hospital of Southern University of Science and Technology), Shenzhen, 518020, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei-Xiang Luo
Department of Nursing, Shenzhen People’s Hospital (2nd Clinical Medical College of Jinan University, First Affiliated Hospital of Southern University of Science and Technology), Shenzhen, 518020, Guangdong, People’s Republic of China
Tel +86 755-25533018-2887
Email [email protected]
Background: Due to the poor healing of superficial malignant tumor ulcer wounds, patients suffer great pain and significantly reduced quality of life. Related research shows that oxygen therapy can reduce wound bleeding and promote wound healing.
Objective: This study aims to explore the therapeutic effect of nano-silver antibacterial dressing combined with high-flow oxygen therapy on surface malignant tumor ulcers.
Methods: In this retrospective analysis, 64 patients with superficial malignant tumors and ulcer infection were included and divided into the research group and the control group, with 32 cases in each group. After conventional debridement, the control group was treated with vaseline dressing, while the research group was treated with nano-silver medical antibacterial dressing combined with high-flow oxygen therapy. Both groups were treated for 7 days. The frequency of dressing change and the number of times of blood oozing between the two groups after treatment were recorded. The pain, clinical efficacy, and levels of procalcitonin (PCT) and C-reactive protein (CRP) were compared between the two groups before and after treatment.
Results: The dressing changes and blood oozing were less frequent in the research group compared with the control group. The pain score and the levels of PCT and CRP in the research group were lower than those in the control group. The overall response rate was significantly higher in the research group as compared to the control group. All the above differences were statistically significant (P< 0.05).
Conclusion: Nano-silver medical antibacterial dressing combined with high-flow oxygen therapy can reduce the frequency of dressing changes in patients, relieve pain, reduce inflammation, and accelerate the healing of superficial malignant tumor ulcer wounds.
Keywords: nano-silver medical antibacterial dressing, high flow oxygen therapy, body surface malignant tumor, ulcer, wound
Introduction
Malignant tumors (cancer cells) are metastatic and invasive, and cancer cells can spread throughout the body through hematogenous, lymphatic and implantation metastases. Clinically, the most common tumor metastasis sites are lung, liver, brain, bone, etc. Some patients will have metastasis on the body surface, while in some cases, the tumor directly spreads or invades the body surface.1 The appearance of superficial malignant tumor usually indicates that the tumor is in the middle and late stage. However, many patients seek medical treatment only after the superficial tumor has grown, ruptured, and become infected. The ulceration of superficial malignant tumors can lead to wound bleeding, exudation, pain, infection, and scar hyperplasia, which makes the wound unhealed for a long time, greatly reducing the quality of life of patients.2 At present, surgical resection, chemotherapy and radiotherapy are the mainstays of treatment for superficial malignant tumors.3 Clinically, such patients are required to maintain local dryness of the wound, but frequent dressing changes and large surgical wound make it difficult to keep the wound dry; Moreover, chemotherapy is easy to cause a large amount of exudate in the wound to be in a moist state, coupled with the skin damage aggravation resulted from radiotherapy, leading to the susceptible to wound bleeding and infection and consequently delayed wound healing.4 Therefore, it is of great significance to find a treatment method that can reduce the frequency of dressing changes, avoid wound bleeding, promote wound healing, and relieve the pain of patients.
In recent years, nano-silver antibacterial dressing, which can accelerate the proliferation of epidermal cells and rebuild blood vessels, has been gradually applied in clinical treatment. By accelerating the migration and proliferation of endothelial cells in patients’ blood vessels and the synthesis of fibronectin, it accelerates the generation of fibroblasts, which is conducive to cell mitosis and the growth of granulation tissue on the wound, so as to remodel the wound and improve the quality of healing.5–7 In addition, oxygen therapy has gradually been applied to the treatment of epidermal ulcer infection in recent years.8–10 Studies have found that oxygen therapy can reduce wound bleeding and promote wound healing.11 However, there are few studies on the application of nano-silver antibacterial dressing combined with high-flow oxygen therapy in the treatment of ulcer wounds of superficial malignant tumors.
Therefore, we treated patients with superficial malignant tumor wound infection in our hospital with nano-silver antibacterial dressing combined with high-flow oxygen therapy to explore the therapeutic effect of the combination therapy.
Materials and Methods
Clinical Data
This is a retrospective study. Sixty-four patients with superficial malignant tumors and ulcerated infections admitted to the Chengdu Fifth People’s Hospital from June 2019 to June 2021 were selected as the research participants. Inclusion criteria: (1) Patients with confirmed superficial malignant tumors by pathological examination. (2) Patients with tumor surface ulceration and infection. (3) Those without allergy to the drugs used in this study. (4) Those who voluntarily participate and sign the informed consent. Exclusion criteria: (1) Patients during pregnancy or lactation. (2) Patients with severe heart, liver, kidney and other primary diseases and severe immune system diseases. (3) Patients with mental illness, or those who do not cooperate with treatment or have poor treatment compliance. This study was approved by the medical ethics committee of Chengdu Fifth People’s Hospital and was in compliance with the principles laid down in the Declaration of Helsinki.
In the control group (n=32), the wound was covered with vaseline dressing. The research group (n=32) was treated with nano-silver medical antibacterial dressing combined with high-flow oxygen therapy. The male to female ratio in the research group was 10:22, and the average age was 46–66 (52.46 ± 6.73) years. The male to female ratio in the control group was 12:20, and the average age was 45–67 (52.81 ± 6.97) years. The tumor site and ulceration area on the body surface are shown in Table 1. The comparison of general data such as age, sex, BMI, and tumor site showed no significant difference between the two groups (P>0.05), indicating that the two groups were comparable.
 |
Table 1 General Information of Patients |
Methods of Wound Treatment
Both groups of patients were disinfected and debrided according to conventional methods. Specifically, the wound was first cleaned with hydrogen peroxide to remove necrotic tissue, carrion, and purulent secretions, and the operation was carried out gently to avoid wound bleeding as much as possible. After washing the wound and surrounding skin, the wound surface was dried gently with sterile dry gauze, and then disinfected with 2% iodine tincture. In the control group, the wound was covered with vaseline dressings after disinfection and debridement. The drainage situation on the wound surface was observed, and the dressing was changed immediately when the exudation was too much. In the research group, the wound was exposed after disinfection and debridement, and cotton balls were placed on the normal skin around the wound and in the depression of the wound to absorb the exudate. Cotton balls were replaced as soon as they were soaked.
A transparent plastic cup that can cover the wound was prepared, and 3–5 small round holes with a diameter of 5 mm were made in the middle area of the bottom of the cup. The wound was covered with the plastic cup to form a space for local oxygen therapy, and the transparent plastic cup was fixed with adhesive tape. Then, the oxygen device was connected with an oxygen content of 100%, and the oxygen flow was adjusted in the range of 6–8 L/min. The wound was treated with blowing oxygen for 30 minutes. Intermittent oxygen blowing was maintained for more than 12 hours every day, and debridement and dressing were performed before oxygen blowing every day. During non-oxygen blowing period, the wound was covered with nano-silver medical antibacterial dressing (Zeyuan Medical Equipment Co., Ltd., Xinxiang, China, 9×15). All patients were treated for 7 d.
Observation Indexes
(1) The frequency of dressing changes and number of times of blood oozing during treatment were recorded in both groups. (2) The Visual Analogue Scale (VAS)12 was used to evaluate the patient’s pain, with 0 indicating no pain is 0 and 10 points indicating the most unbearable pain. A score of 0–3 indicates mild pain that does not affect sleep, 4–6 means moderate pain that slightly affects sleep, and a score of 7–10 indicates severe pain that severely affects sleep. (3) Evaluation of treatment efficacy. Markedly effective: The wound area is reduced by ≥50%, the number of times of blood oozing and exudate are obviously reduced, and the stench is significantly reduced. Effective: 10%≤wound area reduction <50%, the number of bleeding and exudate are reduced, and the stench is reduced. Ineffective: The wound area has not been reduced, the number of times of blood oozing, the exudate, and the stench have not been reduced. Total effective rate (%) = (markedly effective + effective) cases/total cases × 100%. (4) After treatment, 5 mL of fasting venous blood was drawn from all patients and centrifuged at 2500 r/min for 10 min to collect the upper serum. The level of procalcitonin (PCT) was determined by semi-quantitative solid phase immunoassay (micro-spot immunoassay instrument (E-308)). The level of C-reactive protein (CRP) was determined using the immunosingle diffusion method (Beckman Coulter (IMMAGE800) automatic specific protein analyzer).
Statistical Processing
SPSS 23.0 statistical software (EasyBio Technology Co., Ltd., Beijing, China) was used for statistical analysis, and GraphPad Prism 8.0 (Universal Biotech Co.,Ltd., Shanghai, China) was used for image rendering. Count data were expressed by percentages (%) and compared by χ2 test. The measurement data were represented by (mean ± SD), and t test was used for comparison. P<0.05 was considered as statistically significant difference.
Results and Discussion
Comparison of Dressing Change Frequency and Number of Times of Blood Oozing Between the Two Groups
The dressing change frequency (times) was (19.96±3.50) in the research group and (30.75±3.71) in the control group; The number of times of blood oozing was (11.09±2.62) in the research group and (17.90±3.58) in the control group. The data showed that the dressing change frequency and the number of times of blood oozing in the research group were significantly lower than those in the control group, and the differences were statistically significant (P<0.05, Figure 1).
 |
Figure 1 Comparison of dressing change frequency (A) and number of times of blood oozing (B) between the research group (n=32) and the control group (n=32). Note: ****P<0.0001. |
Comparison of VAS Score Between the Two Groups
After treatment, the VAS score reduced in both groups, and the VAS score of the research group (2.25±0.67) was lower than that of (3.84±0.76) in the control group (t=8.843, P<0.0001, Figure 2).
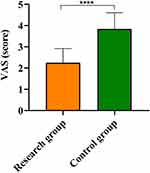 |
Figure 2 Comparison of Visual Analogue Scale (VAS) between the research group (n=32) and the control group (n=32). Note: ****P<0.0001. |
Comparison of Clinical Effect Between the Two Groups
In the research group, the treatment was markedly effective in 13 cases (40.63%), effective in 17 cases (53.12%), and ineffective in 2 cases (6.25%). In the control group, markedly effective was observed in 5 cases (15.63%), effective in 16 cases (50.0%), and ineffective in 11 cases (34.37%). The total effective rate of treatment in the research group was 93.75%, which was obviously higher than that of 65.63% in the control group (Z=−3.024, P=0.002, Figure 3).
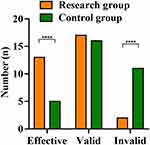 |
Figure 3 Comparison of clinical effects between the research group (n=32) and the control group (n=32). Note: ****P<0.0001. |
Comparison of Inflammatory Factors PCT and CRP Between the Two Groups
After treatment, the levels of PCT and CRP in the research group were (0.95±0.33) ng/mL and (43.60±10.86) mg/L respectively, while those in the control group were (2.90±0.96) ng/mL and (71.91±11.32) mg/L, respectively. The levels of PCT and CRP in the research group were significantly lower than those in the control group, and the differences were statistically significant (P <0.05, Figure 4).
 |
Figure 4 The levels of procalcitonin (PCT) (A) and C-reactive protein (CRP) (B) in the study group (n=32) were significantly lower than those in the control group (n=32). Note: ****P<0.0001. |
Discussion
Superficial malignant tumors refer to malignancies that grow or metastasize to the skin or superficial soft tissues under the skin. Due to the characteristics of malignant tumors, the wound surface after the rupture of superficial malignant tumors is prone to produce a large amount of exudate and blood, which makes it in a moist state, providing a good growth environment for microorganisms and thus leading to infections. Moreover, the infection will invade the surrounding normal tissues, making the wound larger and harder to heal.13 In order to reduce the pain of patients and improve their quality of life, this article explored the therapeutic effect of nano-silver antibacterial dressing combined with high-flow oxygen therapy on superficial malignant tumor ulcers.
Silver ions have a broad-spectrum antibacterial effect. In recent years, nano-silver has been widely used in clinical practice.14,15 Nano-silver refers to the use of nanotechnology to decompose silver into particles with a diameter of about 100 nm, with improved stability and enhanced ability to penetrate the skin compared with silver ions.16 Nano-silver antibacterial dressing is to evenly cover silver nanoparticles on medical purified gauze, and its main mechanism of action is as follows: silver ions can act on bacterial proteins to suppress the activity of bacterial proteins, and inhibit the replication of bacterial DNA, thereby exerting an antibacterial effect.17 The mesh fiber structure of the gauze is beneficial to wound drainage and infiltration; In addition, the release of silver ions in the dressing is long-lasting and slow, which can prolong its antibacterial action time.18 Moreover, as an external dressing, the amount of silver ions entering into the human body is very small, so the harm can be ignored. Oxygen is a necessary condition for maintaining the vitality of human cells and an important element for the regeneration of epidermal cells.19 Hyperbaric oxygen therapy, a special treatment developed in recent years, can improve the blood supply of ischemic and hypoxic tissues, enhance microcirculation, inhibit the growth of anaerobic bacteria, and promote the expression of various growth factors, thus facilitating wound healing.20,21
In this study, the dressing changes and blood oozing were less frequent in patients who used nano-silver antibacterial dressing combined with high-flow oxygen therapy; The pain score of the research group was lower than that of the control group, and the curative effect was better than that of the control group. These results showed that high-flow oxygen therapy can reduce the exudation of ulcerative wounds of superficial malignant tumors and make the wounds in a relatively dry state, which in turn reduces the frequency of dressing changes and number of times of blood oozing, thus alleviating pain and promoting wound healing. Oxygen therapy can promote wound angiogenesis and cell tissue proliferation, providing favorable conditions for wound healing.22 In addition, it can increase the oxygen content of wound microvessels, inhibit the growth and reproduction of bacteria, especially the growth of anaerobic bacteria, and reduce the risk of bacterial infection, contributing to wound healing.23 Oxygen therapy can also promote the synthesis of collagen, and accelerate the formation of blood vessels and the expression of various growth factors, which is conducive to wound healing.24 PCT is an indicator of bacterial and fungal infections, and CRP can reflect infectious inflammation in the body.25 In this study, the levels of PCT and CRP in the research group were significantly lower than those in the control group after treatment. This is related to the strong antibacterial effect of the nano-silver antibacterial dressing, which can effectively prevents the invasion of bacteria and reduces the risk of infection. However, this treatment method requires the configuration of an expensive and bulky hyperbaric oxygen chamber, as well as the whole-process monitoring by professional and technical personnel, resulting in high treatment cost and complex treatment process.
The novelty of this study lies in that we evaluated the therapeutic advantages of nano-silver antibacterial dressing combined with high-flow oxygen therapy in the treatment of superficial malignant tumor wound infection from multiple perspectives of dressing change frequency, number of times of blood oozing, pain, clinical efficacy, as well as PCT and CRP levels, demonstrating that this treatment has exact and high clinical efficacy and is worthy of clinical promotion.
Conclusion
Superficial malignant tumor ulcer wounds are caused by inflammatory exudation, resulting in microcirculation disorders and insufficient blood oxygen supply, which reduces the tissue nutrient supply and affects wound healing. Nano-silver medical antibacterial dressing combined with high-flow oxygen therapy can improve the blood oxygen supply of the wound, reduce dressing change frequency and the number of times of blood oozing in patients, and keep the wound dry; Moreover, it can reduce wound exudate, relieve pain, and inhibit the growth of wound bacteria; All of these facilitate the healing of ulcer wounds of superficial malignant tumors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hussein MR. Skin metastasis: a pathologist’s perspective. J Cutan Pathol. 2010;37(9):e1–20. doi:10.1111/j.1600-0560.2009.01469.x
2. Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37(3):633–646. doi:10.1016/j.cll.2017.06.004
3. Scholz SL, Eckstein A, Dekowski D, Esser J, Westekemper H, Steuhl KP. [Current therapies in superficial malignant tumors]. Laryngorhinootologie. 2019;98(10):685–694. Polish. doi:10.1055/a-0960-6490
4. Killick DR, Rowlands AM, Burrow RD, et al. Mast cell tumour and cutaneous histiocytoma excision wound healing in general practice. J Small Anim Pract. 2011;52(9):469–475. doi:10.1111/j.1748-5827.2011.01093.x
5. Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64. doi:10.1016/j.addr.2017.08.001
6. Yang L, Liu F, Chen Y, Liu Z, Zhang G. Research on the treatment of diabetic foot with ulcer based on nano-silver antibacterial dressing. J Nanosci Nanotechnol. 2021;21(2):1220–1229. doi:10.1166/jnn.2021.18680
7. Li S, Liu Y, Huang Z, Kou Y, Hu A. Efficacy and safety of nano-silver dressings combined with recombinant human epidermal growth factor for deep second-degree burns: a meta-analysis. Burns. 2021;47(3):643–653. doi:10.1016/j.burns.2019.12.015
8. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257–268. doi:10.1111/j.1365-2133.2010.09804.x
9. Andrade SM, Santos IC. Hyperbaric oxygen therapy for wound care. Rev Gaucha Enferm. 2016;37(2):e59257. doi:10.1590/1983-1447.2016.02.59257
10. Kimmel HM, Grant A, Ditata J. The presence of oxygen in wound healing. Wounds. 2016;28(8):264–270.
11. de Smet GHJ, Kroese LF, Menon AG, et al. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25(4):591–608. doi:10.1111/wrr.12561
12. Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol. 2014;54(3):241–244. doi:10.1002/jcph.250
13. Xu Z, Hsia HC. The impact of microbial communities on wound healing: a review. Ann Plast Surg. 2018;81(1):113–123. doi:10.1097/SAP.0000000000001450
14. Chugh H, Sood D, Chandra I, Tomar V, Dhawan G, Chandra R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif Cells Nanomed Biotechnol. 2018;46(sup1):1210–1220. doi:10.1080/21691401.2018.1449118
15. Choudhury H, Pandey M, Lim YQ, et al. Silver nanoparticles: advanced and promising technology in diabetic wound therapy. Mater Sci Eng C Mater Biol Appl. 2020;112:110925. doi:10.1016/j.msec.2020.110925
16. Thompson AK, Hackett C, Grady TL, Enyinnia S, Moore QC
17. Park SB, White SB, Steadman CS, et al. Silver-coated magnetic nanocomposites induce growth inhibition and protein changes in foodborne bacteria. Sci Rep. 2019;9(1):17499. doi:10.1038/s41598-019-53080-x
18. Mondal R, Foote M, Canada A, Wiencek M, Cowan ME, Acevedo C. Efficient silver release from ion exchange silver dressings in biologically relevant media. Wounds. 2020;32(1):22–29.
19. Kanayama K, Takada H, Saito N, et al. Hair regeneration potential of human dermal sheath cells cultured under physiological oxygen. Tissue Eng Part A. 2020;26(21–22):1147–1157. doi:10.1089/ten.tea.2019.0329
20. Francis A, Baynosa R. Ischaemia-reperfusion injury and hyperbaric oxygen pathways: a review of cellular mechanisms. Diving Hyperb Med. 2017;47(2):110–117. doi:10.28920/dhm47.2.110-117
21. Memar MY, Yekani M, Alizadeh N, Baghi HB. Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Biomed Pharmacother. 2019;109:440–447. doi:10.1016/j.biopha.2018.10.142
22. Unfirer S, Kibel A, Drenjancevic-Peric I. The effect of hyperbaric oxygen therapy on blood vessel function in diabetes mellitus. Med Hypotheses. 2008;71(5):776–780. doi:10.1016/j.mehy.2008.06.016
23. Park MK, Myers RA, Marzella L. Oxygen tensions and infections: modulation of microbial growth, activity of antimicrobial agents, and immunologic responses. Clin Infect Dis. 1992;14(3):720–740. doi:10.1093/clinids/14.3.720
24. Sammarco MC, Simkin J, Cammack AJ, et al. Hyperbaric oxygen promotes proximal bone regeneration and organized collagen composition during digit regeneration. PLoS One. 2015;10(10):e0140156. doi:10.1371/journal.pone.0140156
25. Hu L, Shi Q, Shi M, Liu R, Wang C. Diagnostic value of PCT and CRP for detecting serious bacterial infections in patients with fever of unknown origin: a systematic review and meta-analysis. Appl Immunohistochem Mol Morphol. 2017;25(8):e61–e69. doi:10.1097/PAI.0000000000000552
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
