Back to Journals » Drug Design, Development and Therapy » Volume 17
Nalbuphine May Be Superior to Sufentanil in Relieving Postcesarean Uterine Contraction Pain in Multiparas: A Retrospective Cohort Study
Authors Zheng K , Chen B , Sun J
Received 12 January 2023
Accepted for publication 1 May 2023
Published 8 May 2023 Volume 2023:17 Pages 1405—1415
DOI https://doi.org/10.2147/DDDT.S394664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Kang Zheng,1,2 Bingwei Chen,3 Jie Sun4
1Department of Anesthesiology, Nanjing Pukou District Hospital of Chinese Medicine, Nanjing, People’s Republic of China; 2Central Laboratory, Pukou District of Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 3Department of Epidemiology and Biostatistics, School of Public Health, Southeast University, Nanjing, People’s Republic of China; 4Department of Anesthesiology, Southeast University Zhongda Hospital, Nanjing, People’s Republic of China
Correspondence: Jie Sun, Department of Anesthesiology, Southeast University Zhongda Hospital, Nanjing, 210009, People’s Republic of China, Tel +86 25 83262523, Fax +86 25 83262526, Email [email protected]
Purpose: Postcesarean pain remains a major complaint from puerperium women who have undergone cesarean section, especially uterine contraction induced visceral pain. The optimal opioid for pain relief after cesarean section (CS) is still unclear. The goal of this study was to compare the analgesic effect of Nalbuphine to Sufentanil in patients who underwent CS.
Patients and Methods: In this single-center retrospective cohort study, we included patients who received Nalbuphine or Sufentanil Patient-Controlled Intravenous Analgesia (PCIA) after CS between 1 January 2018 and 30 November 2020. Data on a Visual Analog Scale (VAS) at uterine contraction, at rest, and at movement, analgesic consumption, and side effects were collected. We performed logistic regression to identify predictors of severe uterine contraction pain.
Results: A total of 674 patients were identified in the unmatched cohort, and 612 patients in the matched one. Compared to the Sufentanil group, lower VAS-contraction was recorded in the Nalbuphine group in both the unmatched and matched cohorts, the mean difference (MD) on POD1 was 0.35 (95% CI: 0.17 to 0.54, p< 0.001) and 0.28 (95% CI: 0.08 to 0.47, p< 0.001), respectively, and the MD of POD2 was 0.12 (95% CI: 0.03 to 0.40, P=0.019) and 0.12 (95% CI: 0.03 to 0.41, P=0.026), respectively. On POD1 but not POD2, VAS-movement was lower in the Nalbuphine group as compared to the Sufentanil group. No difference was found between VAS-rest on POD1 and POD2 in both unmatched and matched cohorts. Less analgesic consumption, and side effects were recorded in the Nalbuphine group. Logistic regression indicated that multipara and analgesic consumption were risk factors for severe uterine contraction pain. In subgroup analysis, VAS-contraction was meaningfully reduced in the Nalbuphine group compared with the Sufentanil group in multipara patients, but not primiparas.
Conclusion: Compared to Sufentanil, Nalbuphine may provide better analgesia on uterine contraction pain. The superior analgesia may only exhibit in multiparas.
Keywords: Nalbuphine, patient-controlled intravenous analgesia, postcesarean pain, uterine contraction pain, multiparous patients
Introduction
In developed countries and east Asia, an estimated 20–40% of childbirths are performed by cesarean section (CS), and the CS rate continues to increase worldwide.1,2 CS is associated with moderate to severe postoperative pain, and poorly controlled pain contributes to increased adverse effects, including postpartum depression, poor newborn care, and prolonged hospital stay.3,4 Postcesarean patients suffered from nociceptive pains in forms of somatic pain and visceral pain, which is dissimilar to most postoperative pains.5 The complicated pain pattern makes postcesarean pain distinctive and intractable.6 Intermittent uterine contraction pain, as the uterus returns to its pre-pregnancy condition, usually evokes severe pain and patient complaints.7–10 This visceral pain is often intensified by widely used uterotonic drugs and uterine massage, for promoting uterine involution, prophylaxis, and treatment of postpartum hemorrhage.5
Despite the pain management after CS having drawn more and more attention in the last decades, there are remaining plenty of debatable issues waiting for further discussion. In the context of multimodal analgesia, neuraxial analgesia is often the preferred technique for patients undergoing CS, since its effectiveness and widespread use.11 However, some nonnegligible side effects (eg, urinary retention, hypotension, etc), concern over economic efficiency and other practical issues limited its worldwide popularization and application, especially in developing countries.12 Intravenous opioid administration, peripheral nerve block, acupuncture, and other techniques have been tested for better pain control in postcesarean patients.13,14 As an alternative to neuraxial analgesia, Patient-Controlled Intravenous Analgesia (PCIA) has proven its effectiveness in postoperative pain control after CS, although it is rarely done in many parts of the world as a routine part of a multi-modal analgesic regimen.15
PCIA offers fast-onset systematic pain relief and allows to titrate analgesics according to analgesia demand.16 Optimizing the formula of PCIA may significantly benefit pain relief for patients after CS.17 Many systemic drugs were tested in patients who underwent CS, including synthetic and natural opioids, NSAIDs, and other medicines. Opioid is the core component of postoperative pain management in most operation types, including CS surgery.18 In the postcesarean setting, no individual drug was clearly proved to be superior in terms of uterine contraction pain relief and general analgesia.11 Recently, one RCT study performed by Sun et al indicated that intravenous Nalbuphine, κ-receptor agonist and μ-receptor antagonistic opioid provided better uterine contraction analgesia as compared to pure μ-receptor agonist Sufentanil.19 However, the sampling bias and the lack of generalizability limited the extrapolation of the result in evaluating population-based health interventions.20 Therefore, we performed this study to further compare analgesic effect of Nalbuphine and Sufentanil in a historical perspective. In this trial, we aimed to: 1) compare the analgesic effect of equi-analgesic Nalbuphine and Sufentanil PCIA on postcesarean uterine contraction pain; and 2) decipher the characteristics of patients who benefit from the superior analgesic.
Methods
Ethical Considerations
In the present trial, all procedures were conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and local relevant regulations.21 The protocol of the present study was approved by the ethics committee of Zhongda Hospital, Southeast University (No. 2020ZDSYLL255-P01). Regarding patient data confidentiality, all collected data were well organized and preserved within the hospital’s medical record system and all identifiable information was not collected for the present study. The protocol of collecting and analyzing data was approved by the ethic committee of Southeast University Zhongda Hospital.
Study Design and Participants
This trial was conducted in the Department of Anesthesiology of Zhongda Hospital, Southeast University in Nanjing, China. We collected clinical data of postcesarean patients applied either Nalbuphine or Sufentanil PCIA in our hospital from 1 January 2018 to 30 November 2020. Patients were eligible for this trial based on the following criteria: 1) age >18 years; 2) American Society of Anesthesiology (ASA) physical status I, II, or III; 3) performed elective or emergency CS surgery; and 4) applied Nalbuphine or Sufentanil postoperation PCIA. Exclusion criteria included: 1) a history of chronic pain disorder, or chronic opioid usage; 2) insufficient clinical data; and 3) patients who transferred to ICU for postoperative care. The following PCIA formulas were applied: 16 mg ondansetron and equi-analgesic potencies of Sufentanil 2 µg/kg or Nalbuphine 2 mg/kg were dissolved into 100 mL normal saline,22 both groups of patients were managed with a continuous dose of 2 mL/h and bolus dose of 2 mL, with a lock-out of 15 min. According to the group assignment, 0.1 µg/kg Sufentanil or 1 mg/kg Nalbuphine were given as rescue analgesia. Oral paracetamol 500 mg every 8 hours were given to patients in both groups.
Data Collection
All data were obtained from the hospital’s medical record system and postoperative follow-up system according to relevant regulations. All collected data were validated and analyzed by well-trained reviewers with a standardized data collection form. The data set included demographic characteristics, Visual Analog Scale (VAS) at uterine contraction (VAS-contraction), VAS at rest (VAS-rest), VAS at movement (VAS-movement), PCIA injections and attempts, rescue analgesia, analgesic consumption, flatus, and side effects. Clinical data were collected on the first and second day after CS (POD1 and POD2). The VAS score was a single one assessed by a nurse at the indicated timepoint.
Outcomes
The primary outcome of this trial was the VAS-contraction. Secondary outcomes included VAS-rest, VAS-movement, PCIA injections and attempts, rescue analgesia, analgesic consumption, and flatus. Side effects outcome included pruritus, postoperative nausea and vomiting (PONV), and uroschesis.
Statistical Analysis
Since missing data were inevitable in a historical study, multiple imputations were performed for missing values. Patients were excluded if postoperative missing data were greater than 25%. The Kolmogorov–Smirnov test was employed for normality of data distribution analysis. Quantitative data were expressed in the form of mean (standard deviation, SD) or median (interquartile range, IQR). Categorical variables were presented as frequencies and proportions. Demographic data, VAS, and analgesic consumptions were analyzed by Student’s t-test or Mann–Whitney test depending on whether the data were distributed normally or not. Pearson’s chi-square test or Fisher’s exact test was performed for the comparison of categorical variables, eg, the incidence of flatus and PONV. In the matched cohort, we applied propensity score matching (PSM) with a ratio of 1:1 in order to balance the covariates and reducing bias in groups as previously described.23
For further deciphering the relationship between postoperative uterine contraction pain and covariates, we defied that VAS-contraction <4 was mild pain, and VAS-contraction ≥4 was moderate to severe pain. The presentation of moderate to severe pain was considered a dependent outcome variable. Logistic regression analysis was performed to explore the risk factors for moderate to severe uterine contraction pain. The following covariates were included in our analysis: age, body weight, height, parous or not, ASA status, type of anesthesia, and rescue analgesia. The adjusted odds ratio (aOR) and 95% confidence interval (CI) were calculated, and we applied a forest plot for displaying results.
All statistical analyses were performed with SPSS 25 and R 4.1. A two-sided p<0.05 was considered statistically significant for all comparisons.
Results
Baseline Characteristics
We retrospectively collected the clinical data of 1082 patients; 180 cases were excluded as they did not employ Nalbuphine or Sufentanil PCIA, 228 cases were excluded as they met the exclusion criteria. In the end, we identified 674 patients, including 306 patients in the Sufentanil group and 368 in the Nalbuphine group (Figure 1).
In the initial unmatched cohort, significant differences were found in height [163 (158–167) vs 164 (159–168); p=0.013], blood loss in surgery [310 (270–340) vs 320 (275–360); p=0.038], and volume of lochia 12 h after CS [100 (75–145) vs 95 (70–135); p=0.022) (Table 1). The propensity-score is calculated with a multivariate logistic regression model, 1:1 PSM according to the baseline characteristics for minimizing selection bias. After matching, each group has 306 patients. In the matched cohort, no significant differences were found in demographic and baseline data between the Sufentanil group and the Nalbuphine group.
 |
Table 1 Demographics and Characteristics of Participants |
Primary Outcome
In both the unmatched cohort and the matched cohort, VAS-contraction of the Nalbuphine group is meaningfully lower than those of the Sufentanil group on both POD1 and POD2 [Unmatched cohort: POD1: 3.3 (1.2) vs 3.6 (1.3); p<0.001, MD: 0.3, 95% CI: 0.17–0.54. POD2: 3.2 (1.0) vs 3.4 (1.4); p=0.019, MD: 0.2, 95% CI: 0.03–0.40. Matched cohort: POD1: 3.3 (1.2) vs 3.6 (1.3); p<0.001, MD: 0.3, 95% CI: 0.08–0.47. POD2: 3.2 (1.0) vs 3.4 (1.4); p=0.026, MD: 0.2, 95% CI: 0.03–0.40] (Table 2).
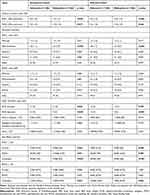 |
Table 2 Primary, Secondary, and Side Effects Outcomes |
Secondary Outcomes
On POD1, VAS-movement of the Nalbuphine group was significantly lower than those of the Sufentanil group in both unmatched and matched cohorts [Unmatched cohort: 2.5 (0.7) vs 2.8 (1.1); p<0.001; Matched cohort: 2.5 (0.7) vs 2.8 (1.1); p<0.001], but not on POD2. Comparable VAS-rest were found in unmatched and matched cohorts (Table 2).
No statistical difference was found in sedation, activity, sleep scores, and incidence rate of flatus between patients from the Sufentanil and Nalbuphine groups on both POD1 and POD2 (Table 2).
In the unmatched cohort, less PCIA attempts and PCIA injections were recorded in the Nalbuphine group [PCIA attempts: 12 (5) vs 14 (7); p=0.028. PCIA injections: 9 (5) vs 12 (7); p<0.001]. However, the incidence rate of rescue analgesia and total analgesic consumption were similar. In the matched cohort, PCIA injections in the Nalbuphine group were also significantly reduced compared with this in the Sufentanil group [9 (5) vs 12 (7); p<0.001] (Table 2).
Side Effects Outcomes
In both the unmatched and matched cohorts, significantly less pruritus and PONV were recorded in the Nalbuphine group than the Sufentanil group on POD1, and a marked reduction of uroschesis occurrence on both POD1 and POD2 (POD1: Unmatched cohort: Pruritus: 0% vs 2.3%; p=0.004. PONV: 2.7% vs 8.5%; p=0.001. Uroschesis: 0% vs 6.5%; p<0.001. Matched cohort: Pruritus: 0% vs 2.3%; p=0.008. PONV: 2.6% vs 8.5%; p=0.003. Uroschesis: 0% vs 6.5%; p<0.001. POD2: Uroschesis: Unmatched cohort: 0.5% vs 4.6%; p=0.001. Matched cohort: 0.7% vs 4.6%; p=0.002) (Table 2).
Multiple Logistic Regression and Subgroup Analysis
As we found VAS-contraction was significantly decreased in the Nalbuphine group, we performed multiple logistic regression to decipher the potential factor which may affect uterine contraction induced visceral pain. The results showed that multiparous and increased total analgesic consumption were associated with increased odds of suffering from severe uterine contraction pain (Multiparous: adjusted odds risk (aOR) 1.46, 95% CI: 1.02–2.09, p=0.037; Total analgesic consumption: aOR 1.04, 95% CI: 1.02–1.06; p<0.001) (Figure 2). It hinted that multiparous patients were more likely suffering from severe uterine contraction pain than primiparous ones.
Thus, we performed subgroup analysis according to the parous status (Table 3). In the multipara subgroup, VAS-contraction and VAS-rest were significantly reduced in the Nalbuphine group compared with those in the Sufentanil group on both POD1 and POD2 [POD1: VAS-contraction: 3.2 (1.2) vs 3.9 (1.3); p<0.001. VAS-rest: 1.4 (1.0) vs 1.8 (1.4); p<0.001. POD2: VAS-contraction: 3.2 (0.8) vs 3.6 (1.4); p=0.019. VAS-rest: 1.4 (0.8) vs 1.6 (1.3) vs; p=0.038], and VAS-movement also reduced on POD1 [2.5 (0.7) vs 3.1 (1.3); p<0.001]. Meaningful less PCIA injections were recorded in the Nalbuphine group as compared to the Sufentanil group [9 (8) vs 13 (13); p<0.008]. Meanwhile, primiparous patients had similar VAS-rest, VAS-movement, and VAS-contraction between the Nalbuphine and Sufentanil groups. Both primiparous and multiparous patients had less PCIA injections in the Nalbuphine group than the Sufentanil group, but similar analgesic consumption.
 |
Table 3 Subgroup Analyses of Clinical Characteristics Associated with Pain Management |
Discussion
Our findings suggested that Nalbuphine PCIA may provide better analgesia than equianalgesic Sufentanil on uterine contraction pain after CS surgery in multipara patients, but not primiparas.
Uterine contraction pain is one of the major complaints in postcesarean patients, especially in the first few days after CS operation.3 As a visceral pain, uterine contraction pain is pathophysiologically different from somatic pain. The process of pain nociception comprises four major segments: transduction, transmission, modulation, and perception.24 The process of modulation and perception are similar between somatic and visceral pain, however, transduction and transmission differ vastly.25 Transduction begins when peripheral terminals of nociceptors are depolarized by stimulants. Nociceptors express an abundance of ion channels, which are essential for depolarization, including voltage-gated calcium channels, transient receptor potential (TRP) cation channels, and purinergic P2X channels.26 In the transduction process of visceral pain, certain ion channel isoforms are activated, eg TRPA1, P2X3, which are different from activated ones in somatic pain.27 Regarding transmission, most of the afferent nerves are large myelinated Aσ fibers in somatic pain. In visceral pain, afferent nerves are composed predominantly of unmyelinated C fibers, numbers of Pacinian corpuscles, and a small amount of myelinated Aσ fibers.28 Viscerosensory axons are polymodal and exhibit mechanosensitivity as well as chemosensitivity and thermosensitivity.29 In the setting of uterine contraction pain, a study by Hsu et al indicated that the pain is primarily triggered by physical contraction of the uterine smooth muscle, transmitted centrally by C fiber exclusively and may be enhanced by the subsequent peripheral and central sensitization.30 Studies indicated that painful sensations from the uterus are conveyed to the spinal cord mainly via the pelvic nerve, and importantly, pelvic splanchnic nerve fibers are markedly more sensitive than hypogastric nerves to uterine mechanostimulation.31
Opioids constitute the core of acute and chronic pain management.18 Choosing the appropriate opioid pharmakon based on characteristics of different types of pain may maximize analgesic effect as a part of a multimodal analgesic regimen. After visceral stimulation, different opioids have distinct impact on pelvic nerve afferent fiber input. A series of animal experiments conducted by Gebhart G.F. and colleagues showed that intravenous κ-receptor agonist but not any other types of opioids attenuated the response of pelvic nerve afferent fibers to colorectal distention stimulations in rabbit.32 The results are verified by Riviere and other researchers in uterine cervical distension rodent models.33 In clinic settings, Schoppmann et al demonstrated that κ-agonist and μ-antagonistic Nalbuphine provides potent analgesia for women overwhelmed with sudden, intense uterine contraction induced labor pain,34 which is consistent with Sun et al’s study and our result.19 The main mechanism is that Nalbuphine acts on κ-opioid receptors and nonopioid molecular targets (eg, sodium channels) which locate on primary sensory afferents nerves, including peptidergic and nonpeptidergic C fibers.35 Activated κ-opioid receptors inhibit voltage-dependent calcium channels, decreasing cAMP levels and blocking the release of glutamate, substance P and other pain neurotransmitters, then preventing activation and sensitization of afferent nerve fibers and blocking the release of pain transmitters.36
The anti-inflammation effects of κ-receptor may contribute to uterine contraction pain relief. Numerous studies have indicated that inflammation plays an important positive role in inducing uterine contraction pain.37 κ-Receptor agonists possess an immunomodulatory function,33 it has been proved in many visceral pain animal models, including uterine infection, cervical pushing, and uterine distension, and the effect was not found in other opioid agonists.38,39 κ-Receptor agonist acts at multiple sites in the inflammatory cascade since the beginning of the inflammation, and exerts the anti-inflammatory actions by restraining expression of pro-inflammation cytokines (eg, TNF-α, NF-κB), promoting production of anti-inflammation cytokines (eg, IL-10), expansion of CD4+ T cells, and other mechanisms.40 In inflammation induced pain models, κ-receptor selective opioid agonist evokes potent increase in pain thresholds after stimulation, reductions in visceromotor responses and afferent nerve activity.33,41
Previous studies indicated that uterine contraction pain increased with maternal parity, and multiparas may be more prone to experiencing severe visceral pain as compared to primiparas.42,43 Our logistic regression analysis also showed that multiparous patients correlated with severe VAS-contraction. The potential mechanism may include uterine smooth muscle damage in previous pregnancy, increased strength and frequency of contractions are necessary for involution, and visceral hyperalgesia.44 Moshiree et al described four potential mechanisms for visceral hypersensitivity:45 1) sensitization of primary afferent innervating the certain viscera; 2) spinal sensitization due to extra impulse input from primary afferent neurons; 3) declining facilitation from brain to spinal cord; and 4) certain selective alteration in cerebral cortical processing of ascending afferent input. Regarding the transduction and transmission process of uterine contraction pain in multiparas, local inflammation, tissue damage can sensitize visceral afferent receptors, especially the mechanically insensitive afferents, and sensitized receptors generate tonic impulse input that may induce spinal sensitization. Hormonal cycling may also play a role in the visceral hypersensitivity in females. Bradesi et al revealed that estrogen plays a vital role in visceral hypersensitivity via tachykinins receptor and other mechanisms.46 Further studies are needed for understanding the mechanism of visceral hypersensitivity. The aggravating uterine contraction pain in multiparas may potentially benefit them more from visceral pain-relieving analgesic as compared to primiparous patients.
In the multiparous subgroup, but not in primiparas, we found better uterine contraction pain relief in the Nalbuphine group than the Sufentanil one. This is accordance with a study from Liu et al, who found Nalbuphine combined with Sufentanil provided better analgesia than Sufentanil alone in patients who received second CS surgery.47 The explanations might attribute to pharmacological feature of Nalbuphine and mechanism of visceral hyperalgesia. Theory of visceral hyperalgesia indicated that sodium channels and other cation gated channels play a vital role in sensitization of visceral nociceptors.48 Nalbuphine may alleviate visceral hyperalgesia by inactivating primary sensory afferent-located voltage-gated sodium and potassium channels.49 Previous animal studies have proved that visceral hyperalgesia can be alleviated by Nalbuphine.50,51 That may explain, at least partly, why Nalbuphine showed superior analgesic effects in multipara after CS. This study is the first trial indicating that multiparas may benefit more from Nalbuphine more than primiparas in term of postcesarean uterine contraction pain. Yet, as the present trial is a retrospective study, the result should be testified by RCTs in the future.
In this trial, fewer side effects were reported in the Nalbuphine group than in the Sufentanil group. This is expected because Nalbuphine, with antagonistic effects on the μ-receptor, can relieve opioid-induced side effects, eg, PONV, uroschesis.51 Furthermore, a large number of studies reported antipruritic effect of Nalbuphine for acute and chronic pruritus of different etiologies.52
Our study is burdened by various limitations. First, this is a single center study; the external validity of this trial was limited by its inherent selection bias. Second, as a retrospective cohort study, the intrinsic disadvantages are inevitable (eg, poor control over the exposure factor, covariates, and potential confounders). Third, the progression of uterine involution was not recorded; we are not able to answer whether uterine contraction pain relief affect the progression. Fourth, long term follow-up was not performed; whether Nalbuphine has influence on chronic pain after CS was not investigated.
Conclusion
Compared to Sufentanil, Nalbuphine may provide better analgesia on uterine contraction pain in postcesarean patients. Subgroup analysis indicated that the superior analgesia of Nalbuphine was only exhibited in multiparas, but not primiparous patients.
Acknowledgment
The authors thank Dr. Hong Yu for helping in study design, and Dr. Daisy Dai for language editing.
Funding
The authors have no funding in this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Betrán AP, Merialdi M, Lauer JA, et al. Rates of caesarean section: analysis of global, regional and national estimates. Paediatr Perinat Epidemiol. 2007;21(2):98–113. doi:10.1111/j.1365-3016.2007.00786.x
2. Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6(6):3564.
3. Eshkevari L, Trout KK, Damore J. Management of postpartum pain. J Midwifery Womens Health. 2013;58(6):622–631.
4. Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95(2):301–318.
5. Lavand’homme P. Postcesarean analgesia: effective strategies and association with chronic pain. Curr Opin Anaesthesiol. 2006;19(3):244–248.
6. Golzari SE, Nader ND, Mahmoodpoor A. Underlying Mechanisms of Postoperative Pain After Laparoscopic Surgery. JAMA Surg. 2016;151(3):295–296.
7. Deussen AR, Ashwood P, Martis R, Stewart F, Grzeskowiak LE. Relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev. 2020;10:CD004908.
8. Hsu H-W, Cheng Y-J, Chen L-K, et al. Differential Analgesic Effect of Tenoxicam on the Wound Pain and Uterine Cramping Pain After Cesarean Section. Clin J Pain. 2003;19(1):55–58.
9. Cai Q, Gong H, Fan M, Chen W, Cai L. The analgesic effect of tramadol combined with butorphanol on uterine cramping pain after repeat caesarean section: a randomized, controlled, double-blind study. J Anesth. 2020;34(6):825–833.
10. Huang YC, Tsai SK, Huang CH, et al. Le ténoxicam intraveineux réduit les crampes utérines à la suite d’une césarienne. Canadian Journal of Anesthesia/Journal Canadien D’anesthésie. 2002;49(4):384–387. doi:10.1007/BF03017327
11. Roofthooft E, Joshi GP, Rawal N, et al. PROSPECT guideline for elective caesarean section: updated systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76(5):665–680. doi:10.1111/anae.15339
12. Tilleul P, Aissou M, Bocquet F, et al. Cost-effectiveness analysis comparing epidural, patient-controlled intravenous morphine, and continuous wound infiltration for postoperative pain management after open abdominal surgery. Br J Anaesth. 2012;108(6):998–1005.
13. Usichenko TI, Henkel BJ, Klausenitz C, et al. Effectiveness of Acupuncture for Pain Control After Cesarean Delivery: a Randomized Clinical Trial. JAMA Netw Open. 2022;5(2):e220517.
14. Sutton CD, Carvalho B. Optimal pain management after cesarean delivery. Anesthesiol Clin. 2017;35(1):107–124.
15. Parker RK, White PF. Epidural Patient-Controlled Analgesia: an Alternative to Intravenous Patient-Controlled Analgesia for Pain Relief After Cesarean Delivery. Anesthesia Analgesia. 1992;75(2):245–251.
16. Nie Z, Cui X, Zhang R, et al. Effectiveness of Patient-Controlled Intravenous Analgesia (PCIA) with Sufentanil Background Infusion for Post-Cesarean Analgesia: a Randomized Controlled Trial. J Pain Res. 2022;15:1355–1364.
17. Chi X, Li M, Mei W, Liao M. Comparison of patient-controlled intravenous analgesia with sufentanil versus tramadol in post-cesarean section pain management and lactation after general anesthesia - a prospective, randomized, double-blind, controlled study. J Pain Res. 2017;10:1521–1527.
18. Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710–1714.
19. Sun S, Guo Y, Wang T, Huang S. Analgesic Effect Comparison Between Nalbuphine and Sufentanil for Patient-Controlled Intravenous Analgesia After Cesarean Section. Front Pharmacol. 2020;11:574493.
20. Sanson-Fisher RW, Bonevski B, Green LW, D’Este C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33(2):155–161.
21. Goodyear MDE, Krleza-Jeric K, Lemmens T. The declaration of Helsinki. Br Med J. 2007;335:624–625.
22. Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(2S):S133.
23. Schulte PJ, Mascha EJ. Propensity score methods: theory and practice for anesthesia research. Anesth Analg. 2018;127(4):1074–1084.
24. Osterweis M, Kleinman A, Mechanic D. Pain and disability: clinical, behavioral, and public policy perspectives. Int J Med. 1987;1:36.
25. Gebhart GF, Bielefeldt K. Physiology of Visceral Pain. Compr Physiol. 2016;6(4):1609–1633.
26. Benarroch EE. Ion channels in nociceptors. Recent Developments. 2015;84(11):1153–1164.
27. Hockley JR, Gonzalez-Cano R, McMurray S, et al. Visceral and somatic pain modalities reveal NaV 1.7-independent visceral nociceptive pathways. J Physiol. 2017;595(8):2661–2679.
28. Boezaart AP, Smith CR, Chembrovich S, et al. Visceral versus somatic pain: an educational review of anatomy and clinical implications. Reg Anesth Pain Med. 2021;46(7):629–636.
29. Sharma GS, Tillisch K. Visceral Pain: mechanisms, Syndromes, and Treatment. In: Pain Care Essentials and Innovations. Elsevier; 2021:45–58.
30. Hsu H-W, Cheng Y-J, Chen L-K, et al. Differential analgesic effect of tenoxicam on the wound pain and uterine cramping pain after cesarean section. Clin J Pain. 2003;19(1):55–58.
31. Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993;69(2):533–544.
32. Borgbjerg FM, Frigast C, Madsen JB, Mikkelsen LF. The effect of intrathecal opioid-receptor agonists on visceral noxious stimulation in rabbits. Gastroenterology. 1996;110(1):139–146.
33. Riviere PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141(8):1331–1334.
34. Schoppmann S, Spiess D, Muller D, Burch A, Zimmermann R, Simoes-Wust AP. Nalbuphine: a candidate for treatment of women overwhelmed with sudden, intense labor pain? J Matern Fetal Neonatal Med. 2021;25:1–3.
35. Joshi SK, Lamb K, Bielefeldt K, Gebhart GF. Arylacetamide κ-opioid receptor agonists produce a tonic-and use-dependent block of tetrodotoxin-sensitive and-resistant sodium currents in colon sensory neurons. J Pharmacology Exp Therapeutics. 2003;307(1):367–372.
36. Snyder LM, Chiang MC, Loeza-Alcocer E, et al. Kappa Opioid Receptor Distribution and Function in Primary Afferents. Neuron. 2018;99(6):1274–1288 e1276.
37. Chen Y, Jiang W, Zhao Y, et al. Prostaglandins for Postpartum Hemorrhage: pharmacology, Application, and Current Opinion. Pharmacology. 2021;106(9–10):477–487.
38. Sandner-Kiesling A, Pan HL, Chen SR, et al. Effect of kappa opioid agonists on visceral nociception induced by uterine cervical distension in rats. Pain. 2002;96(1–2):13–22.
39. Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14(3):249–258.
40. Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111(9):1329–1338.
41. Kapitzke D, Vetter I, Cabot PJ. Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control. Ther Clin Risk Manag. 2005;1(4):279.
42. Holdcroft A, Snidvongs S, Cason A, Doré CJ, Berkley KJ. Pain and uterine contractions during breast feeding in the immediate post-partum period increase with parity. Pain. 2003;104(3):589–596.
43. Duan G, Yang G, Peng J, et al. Comparison of postoperative pain between patients who underwent primary and repeated cesarean section: a prospective cohort study. BMC Anesthesiol. 2019;19(1):189.
44. Taffazoli M, Khadem Ahmadabadi M. Assessment of factors affecting afterpain in multiparous women delivered in Mashhad 17-Shahrivar Hospital, Mashhad, Iran. J Midwifery Reproductive Health. 2014;2(1):60–65.
45. Moshiree B, Zhou Q, Price DD, Verne GN. Central sensitisation in visceral pain disorders. Gut. 2006;55(7):905–908.
46. Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain. 2003;102(3):227–234.
47. Liu J, Lin L, Li X, et al. Nalbuphine 20 mg combined with sufentanil 2 mug/kg exerts a better postoperative analgesia effect in patients undergoing a second cesarean section: a randomised trial. Ann Palliat Med. 2022;11(10):3213–3223.
48. Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care. 2012;6(1):17–26.
49. Liu YY, Hsiao HT, Wang JC, Liu YC, Wu SN. Effectiveness of nalbuphine, a κ-opioid receptor agonist and μ-opioid receptor antagonist, in the inhibition of I(Na), I(K(M)), and I(K(erg)) unlinked to interaction with opioid receptors. Drug Dev Res. 2019;80(6):846–856.
50. Ortiz MI, Ponce-Monter H, Fernandez-Martinez E, et al. Evaluation of the interaction between acemetacin and opioids on the Hargreaves model of thermal hyperalgesia. Pharmacol Biochem Behav. 2007;88(1):47–54.
51. Narver HL. Nalbuphine, a non-controlled opioid analgesic, and its potential use in research mice. Lab Anim (NY). 2015;44(3):106–110.
52. Inan S, Dun NJ, Cowan A. Antipruritic Effect of Nalbuphine, a Kappa Opioid Receptor Agonist, in Mice: a Pan Antipruritic. Molecules. 2021;26(18):5517.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


