Back to Journals » Infection and Drug Resistance » Volume 17
Nab-Paclitaxel for Relapsed AIDS-Related Kaposi Sarcoma -A Case Report
Received 10 January 2024
Accepted for publication 23 March 2024
Published 11 April 2024 Volume 2024:17 Pages 1431—1437
DOI https://doi.org/10.2147/IDR.S456286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Lele Yu, Binhai Zhang, Hu Wan
Department II of Infectious Diseases, Hangzhou Xixi Hospital, Hangzhou Sixth People’s Hospital, Hangzhou, Zhejiang, 310023, People’s Republic of China
Correspondence: Hu Wan, Email [email protected]
Introduction: Kaposi sarcoma (KS) incidence has decreased since the initiation of combination antiretroviral therapy (cART), but it remains the most common cancer in people with HIV/AIDS (PWHA). PWHA with advanced immunosuppression who initiate antiretroviral therapy are susceptible to the occurrence of an immune reconstitution inflammatory syndrome (IRIS).
Case Presentation: This report covers the case of a 25-year-old male with AIDS-related KS who relapsed after Liposomal Doxorubicin, but recovered well after administration of nab-paclitaxel (Nab-PTX).
Conclusion: This is a rare case in choosing Nab-PTX to treat relapsed AIDS-KS and get good feedback. We report the case to provide a possible solution to treat AIDS-KS.
Keywords: Kaposi sarcoma, nab-paclitaxel, HIV, case report
Introduction
Kaposi sarcoma (KS) is a multicentric vascular neoplasm of endothelial origin with low-grade malignancy. KS most commonly affects the skin, mucous membrane, lymphatic system, and gastrointestinal tract.1 With the AIDS epidemic in the 1980s and 1990s, its global incidence increased dramatically. Although its incidence has decreased since the initiation of combination antiretroviral therapy (cART), it remains the most common cancer in people with HIV/AIDS (PWHA). The best current treatment strategy is cART alone or in combination with systemic chemotherapy.2 ART is responsible for KS improvement and resolution, but new onset (unmasking KS-IRIS) or sudden progression of preexisting KS (paradoxical KS-IRIS) can occur, even in patients with a low degree of immunocompromise.3 The incidence of KS-related IRIS has been reported to be 6% to 29% depending on the study population and the country.4
Case Presentation
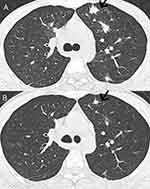
A 25-year-old male presented with dark stools for 9 days. Ulcerated nodules were seen on the roof of the mouth, and black nodules were seen in the eyes and corners of the mouth (Figure 1), which gradually increased over the past 6 months. Neck lymph nodes, axillary lymph nodes, and inguinal lymph nodes were enlargement. Laboratory examination in anti - HIV antibody positive, CD4 T lymphocyte count 2 / mL, HIV RNA - 6.81 * 105 IU/mL. Pulmonary Computed Tomography (CT) showed many small patches, nodules, and patchy high-density shadows, with blurred boundaries and uneven density (Figure 2). Gastrointestinal endoscopy showed no obvious abnormalities. One week after admission to the hospital, the patient’s orbital skin biopsy showed CD34(+) CDX2(-) D2-40(+) HHV8(+) Ki-67(50%+), positive HHV-8 in skin lesions is the gold standard for identification, which was made a definite diagnosis of Kaposi sarcoma (Figure 3). The patient was treated with Liposomal Doxorubicin (20 mg/m2 IV every three weeks) for Kaposi sarcoma by guidelines. Antiviral therapy with tenofovir, lamivudine, and dolutegravir was initiated at the same time.
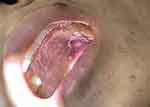 |
Figure 1 Ulcerative nodules on the patient’s roof of the mouth, and black nodules in corners of the mouth. |
 |
Figure 3 The patient’s orbital skin biopsy:CD34(+) CDX2(-) D2-40(+) HHV8(+) Ki-67(50%+). |
After receiving 8 cycles of chemotherapy treatments, the patient’s lung condition and mouth ulcerative nodules improved slowly. CD4 T lymphocyte count increased to 82/mL, HIVRNA <100 IU/mL. However, A new growing neoplasm was seen in the little toe of the left foot(Figure 4). Magnetic resonance imaging (MRI) of the left foot revealed the joint and component bone morphology and signal were as usual, and no changes were observed in the joint space. The morphology and signal intensity of peripheral ligaments, muscle groups, tendons, and tendon sheaths were not changed. There was no obvious abnormality in the arch morphology. The lateral soft tissue of the left foot was swollen. The left foot skin biopsy immunohistochemical showed CD34(+), EBV(-), ERG(+), and HHV8(+), which was taken to suggest Kaposi sarcoma. Considering that the patient was an allergy sufferer, we changed the chemotherapy regimen to albumin-bound paclitaxel (260mg/m2, every three weeks). Up to now, after 3 cycles of chemotherapy, the patient’s new neoplasm of the left foot regressed almost completely(Figure 5). The CD4 T lymphocyte cell counts slowly increased to 188/mL, and viral loads remained below the detectable level. The entire diagnosis and treatment of this case is presented on the timeline (Figure 6).
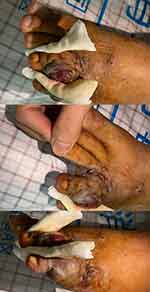 |
Figure 4 A new growing neoplasm was seen in the little toe of the left foot. |
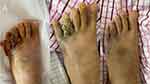 |
Figure 5 Changes in left foot Kaposi sarcoma after nab-paclitaxel chemotherapy, 1 cycle (A), 2 cycles (B), 3 cycles (C). |
 |
Figure 6 The timeline shows the entire diagnosis and treatment process of this case. |
Discussion
Kaposi sarcoma (KS) is a multicentric vascular neoplasm of endothelial origin with low-grade malignancy. It is pathologically characterized by abnormal proliferation of blood vessels and progression to skin and visceral damage. KS is one of the most common malignancies in people living with HIV and is considered to be an AIDS-related malignancy. In untreated AIDS patients, KS progresses rapidly, and the average survival time after diagnosis is less than 1 year. With highly active antiretroviral therapy (HAART), 84% of patients survive for more than 2 years.5 With the widespread use of combined antiretroviral therapy, the incidence of AIDS-KS has decreased significantly. However, although HIV viral load is suppressed and CD4+ T cell counts are considerable, there are still some patients with KS who have poor treatment effects.
In the post-cART era, acquired immunodeficiency syndrome related to Kaposi sarcoma (AIDS - KS) epidemiology is changing, and in the context of immune reconstitution inflammatory syndrome incidence increased. The best current treatment strategy is cART alone or in combination with systemic chemotherapy, and there is emerging evidence supporting a staged stratified treatment regimen to guide its use. In addition, several new targeted therapies for Kaposi sarcoma are under investigation.
The current first-line therapy is pegylated liposomal doxorubicin (PLD) or other liposomal anthracyclines.6 Paclitaxel monotherapy has also shown significant activity against Kaposi sarcoma. Randomized trials comparing paclitaxel with PLD have shown slightly higher response rates and longer progression-free survival (PFS) with paclitaxel but markedly increased toxicity. Given its efficacy and side effect profile, pegylated doxorubicin is the currently preferred first-line therapy in advanced disease. Paclitaxel remains an alternative first-line option.6–9
Taxanes are a diverse class of chemotherapeutic agents comprising paclitaxel, docetaxel, cabazitaxel, and albumin-bound paclitaxel (nab-paclitaxel), which work by stabilizing the microtubules. The resulting cell cycle inhibition during the G2/M phase activates the cellular apoptosis pathways.10 Paclitaxel was the first drug in this class to receive FDA approval in 1992 for the treatment of ovarian cancer after failure of first-line or late-stage therapy. It was subsequently approved for metastatic breast cancer, AIDS-related Kaposi sarcoma, non-small cell lung cancer, and bladder cancer. Nab-paclitaxel is used in the treatment of advanced pancreatic cancer, breast cancer, and non-small cell lung cancer.11
Since PTX was insoluble in water, the injection solution was dissolved in a mixture of polyoxyethylene castor oil and absolute ethanol at a ratio of 50–50 (v /v). The clinical use of paclitaxel has been greatly limited due to the adverse effects of polyoxyethylene castor oil, such as severe allergic reactions, delayed neurological disorders, nephrotoxicity, and cardiotoxicity, as well as the dose limitation in patients who are intolerant to alcohol. Albumin-bound paclitaxel is a nanoparticle formulation of paclitaxel that binds to albumin during its passage through the bloodstream and therefore does not cause allergy during infusion.12
Gardner’s study shows that paclitaxel disposition is subject to considerable variability depending on the formulation used. Nab-paclitaxel produced significantly higher anti-tumor activity than solvent-based paclitaxel because systemic exposure to unbound paclitaxel is likely a driving force behind tumoral uptake.13 British scholars have found that the concentration of Nab-PTX in serum is 10 times that of ordinary paclitaxel, and the intratumoral concentration of Nab-PTX is 33% higher than the same dose of ordinary PTX.14 The administration of nab-PC as first-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) was efficacious and resulted in a significantly improved ORR versus sb-PC, achieving the primary endpoint.15 There has been a meta-report showing that Nab-PTX can improve ORR compared with paclitaxel and should be given priority when aiming to reduce tumor load in breast cancer.16 Nab‐PTX, approved by the FDA in 2012 as the first line of neoadjuvant therapy for pancreatic cancer, is widely used in clinical work and significantly improves outcomes.17
In this case, the patient relapsed after 8 cycles of Liposomal Doxorubicin chemotherapy, but tumor regression was observed by nab-paclitaxel. Given that PTX is classified as the second-line agent, as well as the curative effect of Nab-PTX is better than PTX in other tumors, we try Nab-PTX to treat recurrent KS with excellent results. The therapeutic dose is referenced for the dose for breast cancer and non-small cell lung cancer. This is a rare case in choosing Nab-PTX to treat relapsed AIDS-KS and get good feedback. We report the case to provide a possible solution to treat relapsed AIDS-KS.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation. For data inquiries, please contact [email protected].
Ethics Approval and Consent to Participate
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Ethics Committee of the Hangzhou Xixi Hospital. The ethics committee approved the waiver in this case report, based on the ethical standards to publish the case details
Consent for Publication
Written informed consent was obtained from the individual, for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
The authors would like to express their gratitude to the staff in the HIV/AIDS ward of the Hangzhou Xixi Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; have drafted, revised, or critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Zhejiang Traditional Chinese Medicine Science and Technology Program of China (Grant number: 2022ZB292).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Grabar S, Costagliola D. Epidemiology of Kaposi’s Sarcoma. Cancers. 2021;13(22):5692. doi:10.3390/cancers13225692
2. Dupin N. Update on oncogenesis and therapy for Kaposi sarcoma. Curr Opin Oncol. 2020;32(2):122–128. doi:10.1097/CCO.0000000000000601
3. Poizot-Martin I, Gigeon S, Palich R, et al. Immune reconstitution inflammatory syndrome associated Kaposi Sarcoma. Cancers. 2022;14(4):986. doi:10.3390/cancers14040986
4. Volkow P, Cesarman-Maus G, Garciadiego-Fossas P, et al. Clinical characteristics, predictors of immune reconstitution inflammatory syndrome and long-term prognosis in patients with Kaposi sarcoma. AIDS Res Ther. 2017;14(1):30. doi:10.1186/s12981-017-0156-9
5. Schneider JW, Dittmer DP. Diagnosis and Treatment of Kaposi Sarcoma. Am J Clin Dermatol. 2017;18(4):529–539. doi:10.1007/s40257-017-0270-4
6. Bower M, Collins S, Cottrill C, et al. British HIV Association guidelines for HIV-associated malignancies 2008. HIV Med. 2008;9(6):336–388. doi:10.1111/j.1468-1293.2008.00608.x
7. Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol. 2014;11(4):223–238. doi:10.1038/nrclinonc.2014.31
8. Krell J, Stebbing J. Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2014;32(5):373–375. doi:10.1200/JCO.2013.53.7126
9. Goff CB, Dasanu CA. Changing therapeutic landscape in advanced Kaposi sarcoma: current state and future directions. J Oncol Pharm Pract. 2023;29(4):917–926. doi:10.1177/10781552221148417
10. Mosca L, Ilari A, Fazi F, et al. Taxanes in cancer treatment: activity, chemoresistance and its overcoming. Drug Resist Updates. 2021;54:100742. doi:10.1016/j.drup.2020.100742
11. Christensen SB. Drugs that changed society: microtubule-targeting agents belonging to taxanoids, macrolides and non-ribosomal peptides. Molecules. 2022;27(17):5648. doi:10.3390/molecules27175648
12. Gallego-Jara J, Lozano-Terol G, Sola-Mart Nez RA, et al. A compressive review about taxol(®): history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
13. Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–4205. doi:10.1158/1078-0432.CCR-07-4592
14. Neesse A, Frese KK, Chan DS, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63(6):974–983. doi:10.1136/gutjnl-2013-305559
15. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a Phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi:10.1200/JCO.2011.39.5848
16. Li BX, Chen XJ, Ding TJ, et al. Potentially overestimated efficacy of nanoparticle albumin-bound paclitaxel compared with solvent-based paclitaxel in breast cancer: a systemic review and meta-analysis. J Cancer. 2021;12(17):5164–5172. doi:10.7150/jca.59794
17. Ramanathan RK, Goldstein D, Korn RL, et al. Positron emission tomography response evaluation from a randomized phase III trial of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. Ann Oncol. 2016;27(4):648–653. doi:10.1093/annonc/mdw020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.