Back to Journals » International Journal of General Medicine » Volume 16
N-Cadherin Expression with Metastasis of Neck Lymph Nodes in Patients with Nasopharyngeal Carcinoma
Authors Yusuf M , Pradana YPA, Rahmawati R, Farhat F , Kusumastuti EH, Ekoputro JW
Received 21 November 2022
Accepted for publication 14 March 2023
Published 21 March 2023 Volume 2023:16 Pages 1029—1037
DOI https://doi.org/10.2147/IJGM.S393863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Muhtarum Yusuf,1,2 Yogi Putra Adhi Pradana,1,2 Rosydiah Rahmawati,1,2 Farhat Farhat,3 Etty Hary Kusumastuti,2,4 Jeffi Wahyu Ekoputro5
1Department of Otorhinolaryngology, Head, and Neck Surgery, Airlangga University, Surabaya, East Java, Indonesia; 2Department of Otorhinolaryngology, Head, and Neck Surgery, Dr. Soetomo General Teaching Hospital, Surabaya, East Java, Indonesia; 3Department of Otorhinolaryngology, Head, and Neck Surgery, Sumatera Utara University, Medan, North Sumatera, Indonesia; 4Department of Pathology Anatomy, Airlangga University, Surabaya, East Java, Indonesia; 5Muhammadiyah Surabaya University, Surabaya, East Java, 60113, Indonesia
Correspondence: Muhtarum Yusuf, Department of Otorhinolaryngology, Head, and Neck Surgery, Airlangga University, Jl. Mayjend Prof. Dr. Moestopo No. 6-8, Surabaya, Jawa Timur, 60285, 60286, Indonesia, Tel +62 031 5501078, Email [email protected]
Background: Lymph node (LN) metastases were one characteristic of negative progress of NPC patient despite its advanced therapeutic approaches. One mechanism for the occurrence of epithelial to mesenchymal transition (EMT)-mediated metastases is by increasing N-cadherin expression. The purpose of this research is to determine investigating N-cadherin expression against metastatic LN in NPC cases.
Methods: Samples were taken by unproportionate stratified random sampling. N-cadherin expression was examined using immunohistochemistry methods. N-cadherin expression was assessed visually by binocular light microscopy. We analyzed these data using Mann–Whitney U-test to examine N-cadherin expression and lymph node metastases.
Results: A strong expression was found in N3 group by 63.6%; 27.3% in the N2 group and 9.1% in the N1 group. In patients with NPC N0 or without lymph node metastases, N-cadherin expression is 0%. The expression of N-cadherin is indeed an indicator of the occurrence of lymph node metastases in NPC with a statistically significant analysis of p = 0.026 (p < 0.05).
Conclusion: There were correlations between N-cadherin expression and lymph node metastasis on NPC patients.
Keywords: N-cadherin, nasopharyngeal carcinoma, cancer, immunohistochemistry
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy that causes global health problems with 130,000 new cases and more than 70,000 deaths in 2018.1,2 Unlike other neck and head malignancies, NPC is characterized by its ease of progress and metastasis. NPC has the highest tendency to metastasize to regional lymph nodes (LN). According to the anatomy of the submucosal avalvular lymph capillary network, the incidence of LN metastases is quite large, around 70%.3
NPC is a disease with a wide ethnic and geographic distribution, with the highest prevalence in South China and Southeast Asia. In the last decade, hypotheses regarding the genetic and molecular mechanisms of carcinogenesis in NPC such as DNA, RNA and protein regulation have been developed. This raises a new view that NPC is not only a genetic disease but also a disease with a complex pathology involving the primary, circulating, metastases, and multidirectional ecosystem.4
The challenge of NPC therapy in the form of recurrence and metastases is an indicator of poor prognosis, because it is the primary cause of mortality for NPC sufferers.4 Yet, the successful prognosis of NPC therapy is based on anatomic aspects, which include tumor node metastases (TNM).5 NPC patients usually have different biologic characteristics and therapeutic results, although at the same TNM stage.6 Currently, developing the addition of non-anatomic biomarkers is related to the characteristics of molecular biomarkers of cancer cells. This molecular biomarker will add more accurate predictive information on metastatic risk factors.5
The cadherin family includes a number of glycoproteins that play a role in cell adhesion via calcium channels. Apart from mediating cell adhesion, cadherins are also important for the cell cycle. It can maintain cell homeostasis through the formation of cadherin-catenin complexes.6 Based on their distribution in tissues, the classic family of cadherins is divided into [1] E-cadherin was located in epithelial tissue; [2] N-cadherin in nerve tissue, muscle and fibroblasts; [3] P-cadherin was found in the placenta; [4] R-cadherin was found in the retina; and [5] M-cadherin in muscles.7
N-cadherin consists of five intracellular domains. To fulfill its function in order to maintain cell homeostasis, the actin cytoskeleton requires the formation of a cadherin-catenin complex to become mature cells. In observational studies, N-cadherin is rarely expressed in normal epithelial cells but is upregulated in bizarre cells such as cancer cells. The increase in expression of this molecule is shown in the case of breast, prostate, urothelium and pancreatic cancer and is associated with poor progression.8
N-cadherin induces cell motility and migration through its interaction with epidermal growth factor receptor −1 (EGFR). In addition, N-cadherin is also able to stimulate MAPK/ERK (Mitogen‑activated protein kinase/extracellular signal‑regulated kinases) signaling pathways that play a role in carcinogenesis. In this era of advanced technology, some signal activation pathways that play a role in the malignancy process have been found. The activation of this carcinogenesis signal can be useful in the development of targeted therapy with good response in cancer patients. Targeted therapy is able to inhibit signaling pathways of carcinogenesis. An in vitro observation showed that N-cadherin inhibition was able to destabilize the cell structure. In the case of malignancy, N-cadherin antagonists show potential targeted therapy in inhibiting cell adhesion and modulation signals, thereby inhibiting tumor metastasis.6,9,10
As metastases occur, changes in E-cadherin levels reverse those of N-cadherin. E-cadherin expression was progressively decreased but increased for N-cadherin in NPC patients with multiple LN metastases.11,12 Therefore, the potential for targeted therapy of N-cadherin as a cadherin family with structural cell homeostatic function deserves to be investigated, considering that the pattern of LN metastases in NPC cases is a significant predictor of prognosis and disease free survival. Given that the rate of LN metastases in NPC cases is quite high, a biomarker that can become a therapeutic target is promising in the era of cancer treatment. Currently, the potential and its relationship have not been studied extensively. This is an updated review of biomarkers investigating N-cadherin expression against metastatic LN in NPC cases.
Methods
The research was located in the outpatient department Otorhinolaryngology, Head, and Neck Surgery, Dr. Soetomo Hospital Surabaya. This research used analytical design through medical records. Samples were taken by unproportionate stratified random sampling with neck LN metastases in NPC patients. Pathological samples were obtained from the primary tumor so that it can cover patients from N0 to N3. These samples were taken from the paraffin block of patients who had been diagnosed with NPC through a biopsy and head and neck CT scan from 2018 to 2021.
N-cadherin expression was defined as a brown color in the cytoplasm and cell membrane as a result of IHC staining using the rabbit polyclonal antibody Anti-N Cadherin ab1820313 (dilution, 1:1500; Abcam®, Cambridge–UK) from a paraffin block of NPC tissue. Two anatomical pathology specialists (EHK and RDL) carried out evaluation of N-cadherin expression out visually under a binocular light microscope with 200x magnification on immunohistochemical examination at the Anatomic Pathology Installation of RSUD Dr. Soetomo Surabaya. Assessment of N-cadherin expression by looking at the intensity of N-cadherin staining was scored 0 (no staining), 1 (light yellow staining), 2 (yellow staining) and 3 (brown staining). We assigned positive cell percentage scores as 0 (<5%), 1 (5‑25%), 2 (26‑50%) and 3 (≥51%). The staining intensity score multiplied by the percentage-positive cells score to give a final score (0, 1, 2, 3, 4, 6 or 9), and the final score of a sample is an average of 10 microscopic fields. Scores ≤4 are recognized as weak expressions of N-cadherin and scores ≥6 are recognized as strong expressions of N-cadherin.13 The data scale of this variable is nominal.
Our data is obtained from the medical records of patients who have been diagnosed with NPC from 2018 to 2021. Prior to starting the sampling, the patients had given informed consent to the procedure and had consented to it. Ethical approval (0071/KEPK/IX/2019) for this study was obtained by the ethics committee of RSUD Dr Soetomo Surabaya. This study also follows the guidelines outlined in the Declaration of Helsinki.
The inclusion criteria are as follows: [1] Having paraffin block of nasopharyngeal biopsy material, [2] Histopathology of non-keratinizing undifferentiated carcinoma,14 [3] Have medical record data CT-scan head and neck focus of the nasopharyngeal with and without contrast. Exclusion criteria: [1] Paraffin blocks that were damaged or did not meet the requirements during the immunohistochemical staining process or during the reading of the results. [2] Have undergone radiotherapy or chemotherapy (see data: https://doi.org/10.6084/m9.figshare.21159232.v2). The paraffin block of the study patients was obtained after meeting the inclusion criteria and then examined in the anatomical pathology laboratory, Faculty of Medicine, Airlangga University, Surabaya.
The minimum sample size formula used in this study:15
= 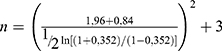 = 60.98 rounded to 61 patients.
= 60.98 rounded to 61 patients.
Note:
n = minimum sample size required in this study
Z1-α/2 = standard deviation of α; α = 0.05 and confidence interval (CI) 95% known as Z1-α/2 value 1.96
Z1-β = power test; for β = 80%, known as Z1-β value 0.84
r = 0.35216
This study used a sample size of 61. Due to the disproportionate number of NPC samples with neck lymph node metastases N0, the sample selection in this study was by unproportionate stratified random sampling. The stratum used is metastatic cervical lymph nodes (N0, N1, N2, and N3) in NPC patients. The results obtained were large samples of N0 as many as 7 patients, N1 as many as 8 patients, N2 as many as 25 patients and N3 as many as 21 patients. The distribution of research samples was based on gender, the number of male samples was 44 patients (72.1%) and female samples were 17 patients (27.9%). The ratio between men and women is 2.58:1. All samples were from WHO III category, namely non-keratinizing undifferentiated carcinoma. Statistical analysis using Mann–Whitney U-test to see the relationship between N-cadherin expression and metastasis.
Results
NPC staging is assessed based on three key components: tumor size (T), neck lymph node nodule or metastases (N) and the presence of distant metastases (M). The most common tumor size in this study was T4, which was 33 patients (54.1%). The most nodules were N2, which was 25 patients (41.0%). The most distant metastases were M0, ie, 50 patients (82.0%). The NPC stage was based on the eighth edition of AJCC classification. In this study, it was found that stage IV was the most common, namely 41 patients (67.2%), followed by stage III as many as 13 patients (21.3%), stage II as many as 7 patients (11,5%), and there were no patients with NPC with stage I (Table 1). All results of this study are based on WHO histopathological classification type III, namely non-keratinizing undifferentiated carcinoma.
 |
Table 1 Characteristic of the Subjects |
The results of examination of N-cadherin expression on various confounding variables such as primary tumors, distant metastases, and stages of NPC patients can be seen in Table 2.
 |
Table 2 The Results of Examination of N-Cadherin Expression on Various Confounding Variables |
The results of a statistical analysis of the relationship between N-cadherin expression with primary tumors (T1, T2, T3, and T4) and stage (Stage I, II, III, IV) in NPC patients using Mann–Whitney U-test showed p = 0.603 and p = 0.098. The results of a statistical analysis of the relationship between N-cadherin expression with distant metastases classification (M0 and M1) with Chi-Square test obtained p = 1000. Means, expression of N-cadherin with primary tumor, distant metastases, and stage in NPC patients did not have a significant relationship (p<0.05).
Table 3 shows an increase in the number of cells that expressed weak and strong N-cadherin expression in neck lymph node metastases N0, N1, N2, and N3. The strong expression of N-cadherin was 63.6% in the N3 group, 27.3% in the N2 group, 9.1% in the N1 group, and 0% in the N0 group of all N-cadherin strong expressions. Weak expression of N-cadherin was found to be 28.0% in the N3 group, 44.0% in the N2 group, 14% in the N1 group, and 14% in the N0 group from all weak N-cadherin expressions. Cadherin expression in NPC tissue can be seen by staining the tissue with immunohistochemical techniques (Figure 1).
 |
Table 3 The Results of Examination of N-Cadherin Expression in Various Neck Lymph Node Metastases in NPC Patients |
 |
Figure 1 Results of staining of N-cadherin on NPC tissue by immunohistochemical technique at 200x magnification. There are 3 pictures attached as figure 1 which represents staining results with immunohistochemical technique. (A). Visible light yellow intensity outward appearance on the cytoplasm and cell membranes. Shows weak expression of N-cadherin (B). It looks yellow intensity on the cytoplasm and cell membranes. Shows a weak expression of N-cadherin (C). The intensity of the brown color appears on the cytoplasm and cell membranes. Shows strong N-cadherin expression. |
The results of a statistical analysis of the relationship between N-cadherin expression and cervical lymph node metastases (N0, N1, N2, and N3) in NPC patients using The Mann–Whitney U-test showed p = 0.026. Means and expression of N-cadherin with cervical lymph node metastases were found to have a significant relationship (p < 0.05). There is a relationship between N-cadherin expression and cervical lymph node metastasis (N0, N1, N2 and N3) in NPC patients.
Discussion
The aim of this study was to describe the relationship between N-cadherin expression and LN metastasis in NPC cases. This relationship is a potential for estimating prognosis and targeted therapy for cases of metastatic NPC.
In the case of NPC, metastasis is still the primary cause of death, even though the mode of therapy has made significant progress.8 Patients with NPC have a poor prognosis when NPC cells are able to migrate, despite receiving a combination of therapy with radiation and chemotherapy.17,18 The successful prognosis of NPC therapy so far is based on anatomic aspects, which include TNM stage, histopathology, and immune response of the patient. Identification of more accurate prognostic indicators can assist clinicians in identifying NPC patients who are at high risk for distant metastases, local regional recurrences, and death. Many experts combine non-anatomical prognostic factors that can reflect tumor behavior to improve the parameters of TNM. These factors can be useful as biomarkers for personal patient risk classification, especially in assessing metastatic risk and determining the intensity of therapy. One of the biomarkers associated with metastases and a worse prognosis in patients with NPC is N-cadherin expression.13,14
This study proved that there was a relationship between N-cadherin expression and cervical LN metastasis (N0, N1, N2, and N3) in NPC patients with p = 0.026 (Table 3). The results of the data analysis mean that the higher the expression of N-cadherin also increases cervical LN metastases. Thus, this study succeeded in proving the hypothesis of this study.
The existence of a relationship between N-cadherin expression and neck LN metastases in NPC patients indicates that metastasis can be predicted from the strong and weak N-cadherin expression. Strong expression of N-cadherin in NPC tumor cells results in higher cervical lymph node metastasis in NPC patients. The results of this study are similar to the research of Sunet al (2017) who found there was a positive relationship between high expression of N-cadherin and β-catenin with metastasis and poor prognosis of NPC cases.13,19 Research by Luo et al stated that strong cytoplasmic N-cadherin expression is positively associated with high N classification, distant metastases and advanced clinical stages.14
Mechanisms of involvement of the cadherin family in metastasis are through the cadherin switching mechanism. Cadherin switching is the process of decreasing E-cadherin expression and increasing N-cadherin.14 The process that occurs in the epithelial-mesenchymal transition (EMT) causes a loss of cell contact and cell polarity, which disrupts the epithelial architecture and gives mesenchymal cells in the competence of migration and invasion.20 Epithelial–mesenchymal transition is regulated by some transcription factors zinc-finger protein (SNAIL1/2 and ZEB 1/2), helix-loop-helix protein (TWIST1/2) and SLUG protein.21 EMT transcription factors, such as SNAIL1/2, TWIST, and SLUG will increase N-cadherin, and decrease E-cadherin expression. Decreased expression of E-cadherin is associated with disruption of epithelial and the formation of a mesenchymal phenotype like malignant cell character. E-cadherin-mediated adherens bonds are released due to the downregulation of E-cadherin, while N-cadherin forms relatively weak adherens bonds. Neural cadherins cause loss of stable adhesive junctions in epithelial cells and loss of normal polarity between cells.6
An in vitro study by Luo et al showed that there was an upregulation of N-cadherin in NPC tissue but there was no increase in its expression in non-tumor nasopharyngeal epithelium. Maeda et al presented that upregulation of N-cadherin within the cell is important for breast epithelial cell migration during TGF-β1-induced EMT.14 N-cadherin translocation in the nucleus contributes to the invasive characteristics of carcinoma cells by upregulating MMP-9 (Matrix metalloproteinase-9).22 When the extracellular matrix wall is degraded by MMP-9, tumor cells tend to move and invade easily.7 Metastasis can be via the blood vessels (hematogenous pathway) after neovascularization or via the lymphatic system (lymphogenous pathway) after lymphangiogenesis. Angiogenesis and lymphangiogenesis can be routes of metastasis to metastasize to distant organs. Lymphatic capillaries (10–60 m) are larger than blood capillaries (5–20 m) and leak relatively easily than blood vessels, making lymphatic capillaries the easiest route for the spread of cancer cells. Hematogenous spread of the tumor originates from the blood vessels, while the lymphatic spread originates from the lymph vessels begins through regional lymph nodes.23
Malignant epithelial cells have greater motility during the EMT process, so they can escape from the primary site and metastasize to regional lymph nodes in the tumor environment. Luo et al found increased cytoplasmic or nuclear N-cadherin expression at the invasion margins in some samples, especially at tumor budding sites. They also reported that increased expression of nuclear N-cadherin can stratify the survival time of NPC patients at advanced tumor stages (III–IV). Patients with NPC diagnosed at stages III–IV were at risk of local recurrence and metastasis after radiotherapy. Thus, N-cadherin can be used as a potential biomarker for patients with advanced disease.14
Expression of N-cadherin found in tumor cells can enhance cell survival, migration and invasion of cancer cells through signal cascades. The cross-linking of N-cadherin with other membrane proteins (integrin, FGFR and γ-secretase) activates cascades that regulate transcription, proliferation and cell invasion. Due to its role in cell survival, motility, invasion and angiogenesis, N-cadherin has been suggested as a potential target for cancer therapy. Inhibition of N-cadherin function is thought to weaken tumor cell invasion and angiogenesis.24
The same thing reported by Sun et al showed that high expression of N-cadherin or β-catenin is closely associated with metastasis and poorer prognosis in NPC patients. These results suggest that N-cadherin and β-catenin can be used as valuable biomarkers during monitoring of patients with NPC. Sun et al also mentioned that inactivation of N-cadherin will inhibit cell migration and metastasis, thus making N-cadherin a potential molecular target in gene therapy for malignancy.13 Thus, the expression of N-cadherin is an important step in the metastases of epithelial cell malignancies and disease progression.8 Unfortunately, this study did not evaluate the relationship between N-cadherin expression and survival of NPC patients.
The results of a statistical analysis of the relationship between N-cadherin expression with tumor classification (T1, T2, T3 and T4) and stage (Stage I, II, III, IV) did not show a significant relationship with p = 0.603 and p = 0.098 (Table 3). The results of a statistical analysis of the relationship between N-cadherin expression and metastatic classification (M0 and M1) with Chi-Square test also did not show a significant relationship with p = 1.000. This confirms that the expression of N-cadherin only has a significant relationship with cervical lymph node metastases (N0, N1, N2 and N3) in patients with NPC.
This study only selected patients with NPC with WHO type III histopathology. Therefore, NPC with WHO type III histopathology was found in 95% of all cases in endemic areas.1 Based on by Kentjono (2003) research at Otorhinolaryngology, Head, and Neck Surgery RSUD Dr. Soetomo Surabaya in 2000, we obtained WHO histopathology of NPC types I, II and III, respectively, at 5.59%, 8.04% and 85.66%.25 The histopathological type of tumor has great potential to influence the prognosis. In research by Cheng et al, a study of 149 patients with NPC revealed that WHO type II histology was a poor prognostic factor for locoregional control. Research by Hwang et al shows that locoregional control and survival rates in NPC patients with WHO type III are better than WHO type II or WHO type I, with 5-year survival rates of 68%: 29%: 0%.26 The composition and structure of transcription factors that affect EMT vary by physiology and pathology, and depending on the cell or histopathological type.27 We can only consider this study for patients with NPC with WHO type III only. For NPC patients with WHO type I and WHO type II histopathology, further research needs to be done.
There are several limitations of this study. This study only examined the histopathology of non-keratinizing undifferentiated carcinoma and WHO type III NPC. This study proves that there are several other factors that have not been taken into account in this study, which are indicators of the immune response of patients with NPC.
Conclusion
This study proves that there is a relationship between N-cadherin expression and neck LN metastases (N0, N1, N2, and N3) in NPC patients. Stronger expression of N-cadherin was followed by an increase in neck lymph node metastases in patients with NPC. Thus, this study succeeded in proving the research hypothesis. There is a relationship between N-cadherin expression and neck LN metastases (N0, N1, N2, and N3) in NPC patients, indicating that we can predict tumor metastases from strong and weak N-cadherin expression. Strong N-cadherin expression in NPC tumor cells results in higher neck LN metastases in NPC patients. This study can be used as an indicator of the prognostic factor for cervical lymph node metastases in NPC and proves that N-cadherin is a potential targeted therapy. In accordance with the majority type of NPC, this study only selected NPC patients with WHO type III histopathology. Further research is needed to take into account the indicator factors of the immune response of NPC patients as well as research samples from other WHO types.
Data Sharing Statement
The data set is available online on https://doi.org/10.6084/m9.figshare.21159232.v2.
Disclosure
All authors declare neither any conflict of interest nor any grants/sponsorships were received for this work.
References
1. Chiang CL, Guo Q, Ng WT, et al. Prognostic factors for overall survival in nasopharyngeal cancer and implication for TNM staging by UICC: a systematic review of the literature. Front Oncol. 2021;11. doi:10.3389/fonc.2021.703995
2. Fles R, Indrasari SR, Herdini C, et al. Effectiveness of a multicentre nasopharyngeal carcinoma awareness programme in Indonesia. BMJ Open. 2016;6(3):e008571. doi:10.1136/bmjopen-2015-008571
3. Xu Y, Huang T, Fan L, Jin W, Chen X, Chen J. Patterns and prognostic value of lymph node metastasis on distant metastasis and survival in nasopharyngeal carcinoma: a surveillance, epidemiology, and end results study, 2006–2015. J Oncol. 2019;2019:1.
4. Luo W. Nasopharyngeal Carcinoma Ecology Theory: cancer as Multi- dimensional Spatiotemporal “Unity of Ecology and Evolution” Pathological Ecosystem. MDPI; 2022.
5. Liu M-T, Chen M-K, Huang -C-C, Huang C-Y. Prognostic value of molecular markers and implication for molecular targeted therapies in nasopharyngeal carcinoma: an update in an era of new targeted molecules development. World J Oncol. 2015;6(1):243–261. doi:10.14740/wjon610w
6. Yu W, Yang L, Li T, Zhang Y. Cadherin signaling in cancer: its functions and role as a therapeutic target. Front Oncol. 2019;9. doi:10.3389/fonc.2019.00989
7. Cao Z, Wang Z, Leng P. Biomedicine & Pharmacotherapy Aberrant N-cadherin expression in cancer. Biomed Pharmacother. 2019;118(16):109320. doi:10.1016/j.biopha.2019.109320
8. Mrozik KM, Blaschuk OW, Cheong CM, Zannettino ACW, Vandyke K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer. 2018;18(1):1–16. doi:10.1186/s12885-018-4845-0
9. Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(6):727–735. doi:10.1242/jcs.000455
10. Breier G, Grosser M, Rezaei M. Endothelial cadherins in cancer. Cell Tissue Res. 2014;355(3):523–527. doi:10.1007/s00441-014-1851-7
11. Lou PJ, Chen WP, Lin CT, DePhilip RM, Wu JC. E-, P-, and N-cadherin are Co-expressed in the nasopharyngeal carcinoma cell line TW-039. J Cell Biochem. 2000;76(1):161–172. doi:10.1002/(SICI)1097-4644(20000101)76:1<161::AID-JCB16>3.0.CO;2-W
12. Liu Q, Wang Y, Jing S-H, Wang X-L, Cheng Y-J, Wu F-P. [Expression of E-cadherin in nasopharyngeal carcinoma and its relationship with cervical lymph node metastasis]. Zhonghua Zhong Liu Za Zhi. 2010;32(6):425–428. Chinese.
13. Sun H, Liu M, Wu X, Yang C, Zhang YXZ. Overexpression of N-cadherin and β-catenin correlates with poor prognosis in patients with nasopharyngeal carcinoma. Oncol Lett. 2017;13(3):725–730. doi:10.3892/ol.2017.5645
14. Luo W, Yao K. Molecular characterization and clinical implications of spindle cells in nasopharyngeal carcinoma: a novel molecule-morphology model of tumor progression proposed. PLoS One. 2013;8(12):1–12. doi:10.1371/journal.pone.0083135
15. Browner WS, Newman TB, Hulley S. Estimating sample size and power: applications and examples. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB, editors. Designing Clinical Research. Philadelphia, PA USA: Lippincott Williams and Wilkins; 2013:1–6.
16. Luo WR, Wu AB, Fang WY, Li SY, Yao KT. Nuclear expression of N-cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology. 2012;61(2):237–246. doi:10.1111/j.1365-2559.2012.04212.x
17. Romdhoni AC, Aulia R, Utaminingtyas RP, Suharjono Alderman CP. Correlation of chemotherapy costs with quality of life in nasopharyngeal cancer patients. J Basic Clin Physiol Pharmacol. 2019;30:6.
18. Chou J, Lin Y-C, Kim J, et al. Nasopharyngeal carcinoma--review of the molecular mechanisms of tumorigenesis. Head Neck. 2008;30(7):946–963. doi:10.1002/hed.20833
19. Amelia FI, Yusuf M. Correlation Between ß-catenin expression and staging in nasopharyngeal carcinoma patients. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 1):384–389.
20. Morandi A, Taddei ML, Chiarugi P, Giannoni E. Targeting the metabolic reprogramming that controls epithelial-to-mesenchymal transition in aggressive tumors. Front Oncol. 2017;7:1–19. doi:10.3389/fonc.2017.00040
21. Vergara D, Simeone P, Franck J, et al. Translating epithelial mesenchymal transition markers into the clinic: novel insights from proteomics. EuPA Open Proteomics. 2016;10:31–41. doi:10.1016/j.euprot.2016.01.003
22. Hsu -C-C, Huang S-F, Wang J-S, et al. Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer. 2016;16(1):800. doi:10.1186/s12885-016-2846-4
23. Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol. 2016;39(5):397–410. doi:10.1007/s13402-016-0281-9
24. Mariotti A, Perotti A, Sessa C, Rüegg C. N-cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs. 2007;16(4):451–465. doi:10.1517/13543784.16.4.451
25. Kentjono WA. Perkembangan terkini penatalaksanaan karsinoma nasofaring [Recent developments in the management of NPC Tropical medicine magazine]. Majalah Kedoteran Tropis Indonesia; 2003:1–39.
26. Hwang JM, Fu KK, Phillips TL. Results and prognostic factors in the retreatment of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41(5):1099–1111. doi:10.1016/S0360-3016(98)00164-3
27. Debnath P, Huirem RS, Dutta P, Palchaudhuri S. Epithelial-mesenchymal transition and its transcription factors. Biosci Rep. 2022;42:1. doi:10.1042/BSR20211754
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

