Back to Journals » Infection and Drug Resistance » Volume 10
Mycoplasma genitalium infections: current treatment options and resistance issues
Authors Sethi S, Zaman K, Jain N
Received 18 May 2017
Accepted for publication 27 July 2017
Published 1 September 2017 Volume 2017:10 Pages 283—292
DOI https://doi.org/10.2147/IDR.S105469
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Sunil Sethi, Kamran Zaman, Neha Jain
Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Abstract: Mycoplasma genitalium is one of the important causes of non-gonococcal urethritis. Rising incidence and emerging antimicrobial resistance are a major concern these days. The poor clinical outcomes with doxycycline therapy led to the use of azithromycin as the primary drug of choice. Single-dose azithromycin regimen over a period of time was changed to extended regimen following studies showing better clinical cures and less risk of resistance development. However, emerging macrolide resistance, either due to transmission of resistance or drug pressure has further worsened the management of this infection. The issues of drug resistance and treatment failures also exist in cases of M. genitalium infection. At present, the emergence of multidrug-resistant (MDR) M. genitalium strains is an alarming sign for its treatment and the associated public health impact due to its complications. However, newer drugs like pristinamycin, solithromycin, sitafloxacin, and others have shown a hope for the clinical cure, but need further clinical trials to optimize the therapeutic dosing schedules and formulate appropriate treatment regimens. Rampant and inappropriate use of these newer drugs will further sabotage future attempts to manage MDR strains. There is currently a need to formulate diagnostic algorithms and etiology-based treatment regimens rather than the syndromic approach, preferably using combination therapy instead of a monotherapy. Awareness about the current guidelines and recommended treatment regimens among clinicians and local practitioners is of utmost importance. Antimicrobial resistance testing and global surveillance are required to assess the efficacy of current treatment regimens and for guiding future research for the early detection and management of MDR M. genitalium infections.
Keywords: Mycoplasma genitalium, non-gonococcal urethritis, antimicrobial resistance, azithromycin, moxifloxacin, doxycycline
Introduction
Though more than 3 decades have passed since the isolation of Mycoplasma genitalium, its management still remains an enigma for clinicians worldwide.1 The organism’s fastidious nature and slow growth have been a major hurdle in the diagnosis and in vitro antibiotic susceptibility studies.2 Fortunately, nucleic acid amplification techniques (NAAT) emerged as a breakthrough for the diagnosis and prevalence studies.3,4 Following the development of diagnostic polymerase chain reaction (PCR) techniques, M. genitalium has got its recognition as an established cause of sexually transmitted infections (STIs).5,6 In recent years, there has been mounting evidence demonstrating the association of STI syndromes with M. genitalium infection.7,8 According to the UK National guidelines for management of non-gonococcal urethritis (NGU), the prevalence of Chlamydia trachomatis and M. genitalium infection among patients with NGU ranges from 11% to 50% and 6% to 50%, respectively.9 Further, reiterating the association of M. genitalium in STIs, is its inclusion under the heading “emerging issues” in the 2015 Centers for Disease Control and Prevention guidelines for treatment of STIs.10 Several reasons responsible for this alarming problem include lack of international consensus on the treatment strategy, co-infection with human immunodeficiency virus (HIV), non-judicious use of macrolides for community-acquired pneumonia and poor adherence to treatment regimens among patients and their partners.11,12
The lack of peptidoglycan in M. genitalium precludes the use of antibiotics acting on the cell wall.1 Other classes of antibiotics that have proven useful are tetracyclines, macrolides and quinolones. The dosage and regimens used for these drugs have differed in various parts of the world giving rise to increasing resistance to these antibiotics, especially to macrolides and quinolones.13,14 Though resistance in other STI pathogens like gonococcus has increased insidiously, resistance in M. genitalium has emerged at a relatively greater speed belying its small size.15 This could be because of the likely paucity of DNA repair systems that might foster emergence of resistance mutations. Increasing resistance in M. genitalium is a worrisome matter. Further aggravating the problem is the paucity of rapid, reliable and cost-effective assays for detecting resistance against more than one class of antibiotics, simultaneously.10,12,15 The 2016 European guidelines for the management of NGU state that testing males with urethritis for M. genitalium along with simultaneous detection of macrolide resistance can improve the cure rate. At present, there is an ongoing search for newer antibiotics to treat NGU as a syndrome and cover all the implicated organisms through a single antimicrobial agent. Till now, pristinamycin is the only drug that has been shown to be effective against M. genitalium that is resistant to both macrolides and quinolones.16 With the rising prevalence of M. genitalium among urethritis patients in certain European regions and higher rates of the asymptomatic carrier state in certain HIV-positive patients (especially in men who have sex with men, MSM), there is an imminent need for newer antibiotics and diagnostic assays for rapid diagnosis and management of M. genitalium infections.17,18
Review of the microbiology of M. genitalium infections and resistance issues
The first isolation of M. genitalium dates back to 1980 by Tully et al from 2 male patients suffering from NGU.1 Taxonomically, it belongs to the family Mycoplasmataceae and order Mycoplasmatales, with the class Mollicutes (mollis: soft, cutis: skin) containing the 2 genera Mycoplasma and Ureaplasma.19 They are probably the Gram-positive bacteria, likely from the clostridia group.20,21 Regarded as the smallest free-living organisms, they were first observed under the transmission electron microscope.1,6,22 With a genome size of only 580 kb, M. genitalium became the second organism to be completely sequenced following Haemophilus influenzae.23 M. genitalium utilizes glucose as a substrate for survival by phosphorylating it with the help of enzyme glyceraldehyde-3-phosphate dehydrogenase to generate adenosine triphosphate.21 Multiple virulence factors are present in the organism that help in the pathogenesis of genital infections. Adhesion is mediated by the proteinaceous terminal tip organelle consisting of MgPa protein and P32 (MG318) protein that are bound to the cell membrane.24,25 The enzymatic activity of glyceraldehyde-3-phosphate dehydrogenase helps in adhesion to the vaginal and cervical mucosa, while another enzyme methionine sulfoxide reductase also increases the virulence.26,27 The immune system evasion by the antigenic variation in the membrane proteins limits the host humoral system from generating an immune response against the organism.28,29 The 2 components of MgPa protein, P110 and P140 undergo genetic variation thus generating novel proteins that are not recognized by the immune system.20,30
Overview of the epidemiology, transmission and natural history of the infection
Epidemiology
Since its discovery in 1980, little progress was initially made regarding the M. genitalium clinical associations and diagnosis.1,2 Its fastidious nature makes it extremely difficult to isolate from clinical specimens. In cultures, it takes several weeks or even months to grow, which makes it further difficult to demonstrate its association with the clinical symptoms.31 However, the implementation of the Vero cell co-culture technique helped in the isolation, clinical association and understanding of the mechanisms of resistance.32 In early 1990, a PCR-based diagnostic assay was created to detect M. genitalium in clinical samples, these assays with better sensitivity empowered many studies demonstrating the association of M. genitalium with STI syndromes in both men and women.3,4,6 M. genitalium infection rate varies with different population groups investigated for the study. In a population with low-risk sexual and high-risk sexual behavior practices, the infection rates are ~2% and 7%, respectively.33 Among the NGU group, the infection rate varies with geographical region and time period ranges from 6% to 50%.9
In men, M. genitalium infection is strongly associated with NGU and nonchlamydial NGU with estimated pooled odds ratio of 5.5 and 7.6, respectively. 6 The prevalence of M. genitalium infection in men with NGU varies from ~10% to 25%.6 M. genitalium infection positivity has been reported to be ~41% and 50% in men with persistent or recurrent and chronic NGU (duration of symptoms >30 days), respectively.34,35 In women, the association of M. genitalium infection with clinical signs and symptoms seems to be less strong than in men.5 In females, its association with pelvic inflammatory disease, cervicitis, preterm labor, spontaneous abortion and tubal infertility have been demonstrated by several studies.5,36–40
Transmission
M. genitalium is primarily transmitted by the sexual route as first studied by Keane et al who reported a concordance rate of 58% for M. genitalium infections among 39 couples, which was higher than the rate for Chlamydia trachomatis.41 Similarly, Manhart et al also studied the transmission among young adults and showed that the risk was higher with vaginal intercourse.42 Besides this route, M. genitalium has also been demonstrated in the anorectal samples through culture and NAAT, with a significant relationship between positive urethral samples and dysuria in MSM.43,44 Edlund et al also established transmission through the penile–anal sexual route.45 Vertical transmission is still an unestablished route of transmission; however, M. genitalium has been isolated from the respiratory tract of newborns.46 It was hypothesized that it may influence the transmission of HIV infections following its isolation from the blood of an HIV-positive patient.47 Moreover, an in vitro study showed that adherence of M. genitalium to HIV-infected cells triggers the release of virus from these cells.48 Consequently, incomplete eradication will increase the likelihood of HIV transmission. Though orogenital contact can lead to transmission of the organism, it is less likely due to the low carriage rate in the oropharynx.49 The clinical features of M. genitalium infection are shown in Table 1.
  | Table 1 Signs, symptoms and complications of Mycoplasma genitalium infection Note: Data from references 50 to 56. |
Natural history
Due to the slow growth rate and difficulty in isolating this organism, very few studies have documented the natural course of M. genitalium infection in literature. A study from Nairobi, Kenya involving a total of 258 female sex workers revealed that 17%, 9% and 21% of M. genitalium infections persisted after 3, 5 and 7 months, respectively.57 In contrast to this, a similar study among female sex workers in Uganda revealed that 55% of the subjects cleared the infection within 3 months and that the infection clearance rates at the end of the sixth and twelfth month were 83% and 93%, respectively. Moreover, HIV-positive women cleared the infection more slowly in comparison with HIV-negative women, and the infection recurred in 39% patients after clearance.58 A community-based study conducted in London revealed that multiple sexual partners and the presence of bacterial vaginosis are independent predisposing factors for M. genitalium infection.59 The authors also reported that 26% of women who were positive for M. genitalium infection at the initiation of the study showed persistent infection after 12–21 months.59 The incidence of persistent or recurrent NGU due to M. genitalium has been found to be 41% in men after doxycycline treatment failure and ~50% of men were M. genitalium positive in chronic symptomatic NGU.34,35
A controversy exists regarding the association of M. genitalium infection with circumcision. In a study conducted in Kenya, 13.4% of uncircumcised men had M. genitalium infection when compared with 8.2% of circumcised men.60 On the contrary, a study from England showed no relationship between the 2 conditions.61 The association of M. genitalium with male infertility is also not exactly known. A meta-analysis of 307 infertile males, pointed out a possible role of M. genitalium in male infertility.62 Hence, further studies are required to unequivocally prove its role in male infertility.
Diagnosis
The indications for laboratory testing for M. genitalium as per the 2016 European guidelines have been shown in Table 2. The slow growth rate and fastidious nature of M. genitalium make its isolation very difficult. Jensen et al also developed a method for the isolation of this organism using Friis medium.32 However, culture techniques remain cumbersome and hence, NAAT-based methods targeting the MgPa gene are the main tools for diagnosis.3,4 However, the commercial diagnostic assays are limited and also not widely available in many countries where the syndromic management of NGU is followed. The specimens to be taken depend on the signs and symptoms, including urethral swab, urine, endocervical swab, endometrial biopsy and anal sample. Swabs made of calcium alginate, dacron or polyester with aluminum or plastic shafts are preferable for collecting clinical samples. SP4-based broth culture media is considered to be a good transport as well as a culture medium for M. genitalium and was developed by Tully et al.1 Another medium that has been used widely for culturing M. genitalium is pleuropneumonia-like organisms broth with added supplements.63 Commercial kits for diagnosing M. genitalium infection are available but none of them have received US Food and Drug Administration (FDA) approval for diagnostic use. Le Roy et al evaluated 2 such commercial kits: TIB MOLBIOL LightMix kit (Roche Diagnostics, Risch-Rotkreuz, Switzerland) targeting the mg219 gene and the Diagenode real-time PCR kit (Diagenode, Liège, Belgium) targeting the gap gene.64 These kits have a sensitivity of 92.6% and 87%, respectively, with a specificity of 100%. InvaderPlus technology-based assay targeting the 16S rRNA gene of M. genitalium carried out on urine samples demonstrated a lower detection limit of 10 DNA copies per reaction.65
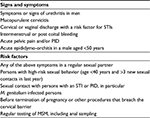  | Table 2 Indications for laboratory testing for Mycoplasma genitalium as per the 2016 European guidelines Note: Data adapted from Jensen et al.70 Abbreviations: MSM, men who have sex with men; PID, pelvic inflammatory disease; STI, sexually transmitted disease. |
Resistance issues
Often termed as the “naked bacteria”,66 M. genitalium is not susceptible to antibiotics that inhibit cell wall formation. Besides the limited range of antibiotics present for managing this organism, the lack of easy and routine methods to determine antimicrobial susceptibility further adds to the problem. However, the inhibition of M. genitalium growth in Vero cell cultures provides an opportunity to determine antimicrobial susceptibility by comparing the proportion of DNA level of M. genitalium controls growing under identical culture conditions.67,68 Tetracyclines, especially doxycycline are still used widely for treating NGU but gradually their cure rates have decreased and resistant isolates were identified.69 Macrolides, especially azithromycin were the second group to be used extensively for managing M. genitalium infections, but again the regimens used were a matter of controversy with present guidelines discouraging the use of a single 1 g dose regimen.70 Quinolones were initially active against macrolide-resistant organisms but reports of resistance to moxifloxacin are available in recent times.71 Newer drugs like josamycin and pristinamycin are being used nowadays for the treatment of multidrug-resistant (MDR) organisms, but only in certain limited geographic regions.70 The following descriptions will elaborate the resistance pattern against each antibiotic class separately.
Tetracyclines
Doxycycline was the most extensively used drug among this group but showed poor response rates in the range of 30%–40%.72,73 Ironically, in vitro data from one study showed that minimum inhibitory concentration (MIC)50 and MIC90 of doxycycline for M. genitalium were 0.25 and 1 mg/L, respectively, contradictory to the clinical efficiency.74 To date, neither any specific mutations nor the exact explanation for this discrepancy has been reported.15
Macrolides
The prominently used macrolide for M. genitalium infection is azithromycin. Traditionally, 2 regimens of azithromycin have been used. Single-dose regimen of 1 g and extended-dose regimen of 1.5 g were given over a period of 5 days. The latter regimen had shown efficacy in Mycoplasma pneumoniae infection, and hence it was predicted that it would be more effective against the slowly growing M. genitalium too; so, it was adopted in many European countries.73,75 The cure rate with the single-dose regimen was initially 85% but this gradually declined as per the studies from various geographic regions.72,73 A study by Manhart et al showed only a 40% cure rate in subjects compared with 30% cure rate in those receiving doxycycline.76 Similarly, another study from Melbourne (Australia) by Twin et al showed a decrease in cure rate from 84% (2005–2007) to 69% (2007–2009).77 In Greenland, where chlamydial infections are common and a single dose of azithromycin is used for its treatment, almost all M. genitalium infections are caused by macrolide-resistant strains.78 Recent guidelines discourage use of the single 1 g dose regimen in light of the emerging macrolide resistance associated with this regimen.70,79 A study by Anagrius et al showed that none of the 77 patients treated with the extended regimen developed macrolide resistance.80 In another study, comparing both regimens in macrolide-susceptible strains showed new onset resistance in 6.5% of subjects receiving the extended regimen and 10% in those receiving the single-dose regimen.81 The probable cause for the failure of the single-dose regimen may be related to the load of organisms in the affected individuals as shown in a study by Bissessor et al where an increase in the organism load by every log10 was associated with chances of failure of this regimen.16 Moreover, injudicious use of this regimen without confirming the eradication of M. genitalium might have given rise to spontaneous mutations in the surviving organisms. Ever since the first report of macrolide resistance in 2006, there has been a rise in the resistance rate.82 As per a recent study among 946 subjects from different geographic regions of USA, the rate of macrolide-resistant M. genitalium infection was 50.8% in females and 42% in male subjects.83 The mechanism of macrolide resistance involves an alteration in the ribosomal proteins that prevent the binding of the drug to the ribosomes. Mutations have been identified in V region of the 23S rRNA and the L4 and L22 ribosomal components.84,85 The predominant mutations identified in the 23S rRNA gene are A2058G, A2059G and A2058T; with the latter being the most common.86 The injudicious use of azithromycin for respiratory tract infections and single copy number of the gene are likely responsible for rising macrolide resistance. Thus, newer macrolides like josamycin have been tried for the management of azithromycin-resistant M. genitalium infection. However, mutations associated with resistance in josamycin have also been reported recently.87 It has been noticed that A2058G and A2059G mutations lead to resistance to the 15-membered macrolides such as azithromycin. Also, it has been found that the same A2059G mutation leads to high-level resistance to the 16-membered macrolides too, such as josamycin. In addition to this, mutation at position A2062 of the 23S rRNA gene can lead to high-level resistance to josamycin (16-membered macrolide) but surprisingly, not to azithromycin (15-membered macrolide), suggesting a difference in the binding site. However, to date, it is unknown whether A2058G/A2059G and A2062G mutation can co-occur in the 23S rRNA gene.87
Fluoroquinolones
Moxifloxacin, a fourth-generation fluoroquinolone, has been the most frequently used second-line drug. Its use for management of these infections was reported for the first time in 2006 and many initial studies had shown a cure rate approaching 100%.88,89 But, recent reports document treatment failures with moxifloxacin, especially in the Asia-Pacific region, with many subjects having an infection with strains resistant to both macrolides and fluoroquinolones.16,71 Another study from Japan showed an increasing rate of fluoroquinolone resistance among M. genitalium isolates, with a rise from 20% in 2011 to 47% in 2013.13
The first report of a mutation associated with moxifloxacin resistance in M. genitalium was from Sydney, Australia.71 Mutations in the DNA gyrase genes (gyrA and gyrB) and topoisomerase IV genes (parC and parE) are associated with resistance. A study from Japan, identified mutations in the quinolone resistance determining regions of the parC gene as the cause of resistance in moxifloxacin and other fourth-generation quinolones.90 The mutations in the positions Ser83 and Asp87 (MG numbering) are found in the resistant isolates. The moxifloxacin resistance rate varies in different parts of the world; a rising trend (47%) has been noted in Japan while a lower incidence of 5% in London (UK) and 15% in an Australian STI clinic has been reported.91,92 Despite the reported resistance mutation, no correlation has been established between the rising MIC values and treatment failure rates.
Detection of antimicrobial resistance (AMR)
All strains of M. genitalium isolated from clinical samples before 2003 were susceptible to macrolides but since then the number of resistant strains has increased. With this in mind, all samples with a positive result in NAAT for M. genitalium should ideally be tested for macrolide resistance mutations. With the increasing macrolide resistance, there is a need for an assay to diagnose Mycoplasma and detect macrolide resistance simultaneously. Recently, a multiplex assay named MG 23S assay was developed that employs novel PlexZyme™ and PlexPrime™ technology to diagnose M. genitalium infection and detect 5 mutations involved in macrolide resistance.93 A total of 400 samples were evaluated with this assay and the results were compared with the reference quantitative PCR method with high-resolution melt analysis. The sensitivity for M. genitalium diagnosis and mutation detection was shown to be 99.1% and 97.4%, respectively, and the specificity for the same was 98.5% and 100%, respectively.93 Use of such assays, should be helpful in choosing the appropriate antibiotics for managing the infection. Further data and research will decide the possible future use of this kit for diagnosis. Mutations mediating resistance to moxifloxacin can also be detected by molecular methods that are based on parC gene sequencing.74,91,94 However, there does not exist a fine correlation between various mutations in parC and in vitro moxifloxacin resistance.91 To date, no commercial assay has been approved by FDA due to the lack of validation of these developed platforms. Recently, an automated Aptima platform targeting the 16S rRNA is under comprehensive validation and may generate superior results.15
Management of M. genitalium infection
Patients with M. genitalium infection are advised to maintain abstinence from unprotected intercourse until both sexual partners have completed the treatment and are symptom-free. Both sexual partners should be screened for other STIs and informed about the risk of transmission and imminent complications. In cases where a partner does not get tested, the same treatment is to be offered as given to the index patient. A test of cure should also be performed routinely for all patients in view of the increasing prevalence of macrolide resistance, which may exist prior to initiation of therapy or can evolve during therapy with a macrolide.70 Furthermore, M. genitalium infection during pregnancy can jeopardize the health of the fetus as well as the mother, especially in terms of susceptibility to preterm labor and spontaneous abortions.5 The problem is further aggravated by the absence of safe options for treatment of infection caused by macrolide-resistant strains during pregnancy, hence treatment of such infections is often withheld till completion of pregnancy. Pristinamycin, due to its safety profile, has proven to be a ray of hope for treatment of such resistant infections during pregnancy. The neonates of infected patients should be observed for development of conjunctivitis and respiratory tract infections.70
Current treatment options
In view of the increasing resistance among M. genitalium for macrolides and quinolones, the treatment regimen should be short and convenient to the patient in order to ensure adherence. The most recent European guidelines – 2016 have divided the treatment depending on whether the infection is complicated or not and presence/absence of macrolide resistance among the isolates.70 Similarly, another European guideline for the management of NGU in 2016 has also given a stepwise approach for treatment.9,95 Macrolides are still recommended as the first-line antibiotics for M. genitalium infections. Newer antibiotics like josamycin and pristinamycin have also been included in the guidelines. The recommended therapies as per the European guidelines for management of M. genitalium infections are shown in Table 3.70
  | Table 3 European guidelines for management of Mycoplasma genitalium infection Note: Data from Jensen et al.70 |
Emerging treatment options
Pristinamycin
It is a bactericidal streptogramin used against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium, especially in France. It is effective against macrolide-susceptible M. genitalium and is also used as a third-line agent against MDR strains.16,96 In a Scandinavian trial, patients responded well to this drug and infections were successfully eradicated in 6 patients in Australia.16 The maximal recommended dose is 1 g 4 times a day for 10 days. Due to the high price, lack of clinical registration of drug and patient compliance for the drug issues, this drug has not been established as a second-line drug.15
Josamycin
Besides azithromycin, this is the other macrolide agent that is used as a first-line drug against M. genitalium infection, especially in Russia. A study in 2015 showed that the drug (500 mg 3 times a day for 10 days) eradicated infection in 93.5% male patients with urethritis who had lower M. genitalium load (≤4 g eq/mL [log10]) prior to treatment, while patients in whom load was high (≥6 g eq/mL [log10]), the eradication rate achieved was 50%.87 Resistance has been reported against this agent due to mutation at A2059G and A2062G of the 23S rRNA gene.87
Solithromycin (CEM – 101)
The drug is an extended-spectrum fluoroketolide superior to doxycycline, quinolones and azithromycin, possessing activity against both macrolide-susceptible and -resistant M. genitalium, though cross-resistance exists and mutation at the A2058 position is responsible for higher MIC.97 A clinical cure of 65%–85% has been theoretically estimated in the case of azithromycin resistance infections; however, large-scale clinical trials are needed to further assess the clinical efficacy.
Lefamulin (BC-3781)
This pleuromutilin antibiotic inhibits protein synthesis by interfering with 23s rRNA.98 It has been previously used for a long time in the veterinary industry and is recently being studied for human use. In a study by Paukner et al it was found to be efficacious against MDR bacterial pathogens causing STIs, including M. genitalium.98 Though the drug is advantageous as it is available in both oral and intravenous formulations, more clinical trials are needed in order to evaluate its potential. This drug has successfully cleared the Phase II randomized controlled trial for its use in skin and soft tissue infections.99 However, its clinical efficacy in M. genitalium infections is yet to be evaluated.
Sitafloxacin
This fourth-generation fluoroquinolone may also become a treatment option in the near future. The drug has already been registered for use in Japan for treatment of M. genitalium infection with an overall cure rate of around 95% in recent studies.100,101
Zoliflodacin
Zoliflodacin is a newer spiropyrimidinetrione class of drug and is DNA gyrase/topoisomerase inhibitor. It has been found to be efficacious against Neisseria gonorrhoeae isolates including those resistant to fluoroquinolones. Also, it is equally effective against macrolide- and quinolone-susceptible strains of M. genitalium but both in vivo and in vitro studies regarding its efficacy in MDR strains are lacking.102
Spectinomycin
This aminocyclitol aminoglycoside is used as an alternative treatment for gonococcal infections. This can be a promising option for MDR M. genitalium as Falk and Jensen successfully treated a case of macrolide-resistant M. genitalium urethritis with this drug.103 However, further studies are required to determine the appropriate treatment regimen for this drug.
Future perspectives
The alarming rise in antibiotic resistance among M. genitalium isolates highlights the indiscriminate use of macrolides for respiratory tract infections, lack of consensus on the management of NGU and lack of resources for facile evaluation of AMR in this organism. Henceforth, a national consensus guideline, including the antibiotic policy, diagnostic steps and partner tracing should be framed. A research priority should be the development of an easy, economic and quick diagnostic test that is available at point of care to diagnose M. genitalium infections and resistance simultaneously so that treatment can be optimally guided. Similar to N. gonorrhoeae, dual therapy for M. genitalium infection too, should likely be introduced in the near future.10,104 As the exact role of M. genitalium in conditions such as adverse pregnancy outcome and infertility are not perfectly known, further elaborative studies are required to establish the association.
Conclusion
M. genitalium has emerged as a superbug and the rising resistance in this bacterium with only a few treatment options in hand is an imminent problem. Future research should look toward developing newer antimicrobials and proper management algorithms. Monotherapy should no longer be used. Combination therapy along with AMR testing is the need of the hour. Etiology-based treatment will be a definitive solution to this emerging AMR due to the misuse of antibiotics as a part of syndromic management. National and international surveillance networks need to monitor and place emphasis on the existing prevalence, growing trend of resistance, and testing for AMR in treatment failure cases, which should be increased. Solithromycin and sitafloxacin seem to be promising treatment options and drugs such as lefamulin and zoliflodacin are in the pipeline and should be further evaluated for their efficacy.
Disclosure
The authors report no conflicts of interest in this work.
References
Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288–1291. | ||
Samra Z, Borin M, Bukowsky Y, Lipshitz Y, Sompolinsky D. Non-occurrence of Mycoplasma genitalium in clinical specimens. Eur J Clin Microbiol Infect Dis.1988;7(1):49–51. | ||
Palmer HM, Gilroy CB, Furr PM, Taylor-Robinson D. Development and evaluation of the polymerase chain reaction to detect Mycoplasma genitalium. FEMS Microbiol Lett. 1991;61(2–3):199–203. | ||
Jensen JS, Uldum SA, Sondergard-Andersen J, Vuust J, Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol. 1991;29(1):46–50. | ||
Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015;61(3):418–426. | ||
Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24(3):498–514. | ||
Sethi S, Singh G, Samanta P, Sharma M. Mycoplasma genitalium: an emerging sexually transmitted pathogen. Indian J Med Res. 2012;136(6):942–955. | ||
Daley GM, Russell DB, Tabrizi SN, McBride J. Mycoplasma genitalium: a review. Int J STD AIDS. 2014;25(7):475–487. | ||
Horner P, Blee K, O’Mahony C, Muir P, Evans C, Radcliffe K; Clinical Effectiveness Group of the British Association for Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27(2):85–96. | ||
Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. Morb Mortal Wkly Rep (MMWR). Recomm Rep. 2015;64(RR3):1–137. | ||
Vandepitte J, Weiss HA, Bukenya J, et al. Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: evidence from a nested case-control study. Sex Transm Infect. 2014;90(7):545–549. | ||
Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: sobering thoughts. Expert Rev Anti Infect Ther. 2014;12(6):715–722. | ||
Kikuchi M, Ito S, Yasuda M, et al. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother. 2014;69(9):2376–2382. | ||
Lau A, Bradshaw CS, Lewis D, et al. The Efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis. 2015;61(9):1389–1399. | ||
Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol. 2017;14(3):139–152. | ||
Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2015;60(8):1228–1236. | ||
Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis. 2014;59(1):24–30. | ||
Bradshaw CS, Fairley CK, Lister NA, Chen SJ, Garland SM, Tabrizi SN. Mycoplasma genitalium in men who have sex with men at male-only saunas. Sex Transm Infect. 2009;85(6):432–435. | ||
Prescott LM, Harley J, Klein DA, editors. Microbiology. 6th ed. New York: McGraw-Hill; 2005. | ||
Jensen JS. Mycoplasma genitalium infections. Diagnosis, clinical aspects, and pathogenesis. Dan Med Bull. 2006;53(1):1–27. | ||
Murry A Stein, Joel B Baseman. The evolving saga of Mycoplasma genitalium. Clin Microbiol Newsl. 2006;28(6):41–48. | ||
Taylor-Robinson D, Gilory CB, Jensen JS. The biology of Mycoplasma genitalium. Venereology. 2000;13:119–127. | ||
Fraser CM, Gocayne JD, White O, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270(5235):397–403. | ||
Reddy SP, Rasmussen WG, Baseman JB. Molecular cloning and characterization of an adherence-related operon of Mycoplasma genitalium. J Bacteriol. 1995;177(20):5943–5951. | ||
Inamine JM, Loechel S, Collier AM, Barile MF, Hu PC. Nucleotide sequence of the MgPa (mgp) operon of Mycoplasma genitalium and comparison to the P1 (mpp) operon of Mycoplasma pneumoniae. Gene. 1989;82(2):259–267. | ||
Alvarez RA, Blaylock MW, Baseman JB. Surface localized glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma genitalium binds mucin. Mol Microbiol. 2003;48(5):1417–1425. | ||
Dhandayuthapani S, Blaylock MW, Bebear CM, Rasmussen WG, Baseman JB. Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J Bacteriol. 2001;183(19):5645–5650. | ||
Moxon ER, Rainey PB, Nowak MA, Lenski RE. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994;4(1):24–33. | ||
Arber W. Genetic variation: molecular mechanisms and impact on microbial evolution. FEMS Microbiol Rev.2000;24(1):1–7. | ||
Ueno PM, Timenetsky J, Centonze VE, et al. Interaction of Mycoplasma genitalium with host cells: evidence for nuclear localization. Microbiology. 2008;154(Pt 10):3033–3041. | ||
Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect. 2006;82(4):269–271. | ||
Jensen JS, Hansen HT, Lind K. Isolation of Mycoplasma genitalium strains from the male urethra. J Clin Microbiol. 1996;34(2):286–291. | ||
McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7(5):e1001324. | ||
Wikstrom A, Jensen JS. Mycoplasma genitalium: a common cause of persistent urethritis among men treated with doxycycline. Sex Transm Infect. 2006;82(4):276–279. | ||
Frolund M, Lidbrink P, Wikstrom A, Cowan S, Ahrens P, Jensen JS. Urethritis-associated Pathogens in Urine from Men with Non-gonococcal Urethritis: A Case-control Study. Acta Derm Venereol. 2016;96(5):689–694. | ||
Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187(4):650–657. | ||
Oakeshott P, Hay P, Taylor-Robinson D, et al. Prevalence of Mycoplasma genitalium in early pregnancy and relationship between its presence and pregnancy outcome. BJOG. 2004;111(12):1464–1467. | ||
Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet. 2002; 359(9308):765–766. | ||
Manhas A, Sethi S, Sharma M, et al. Association of genital mycoplasmas including Mycoplasma genitalium in HIV infected men with nongonococcal urethritis attending STD & HIV clinics. Indian J Med Res. 2009;129(3):305–310. | ||
Rajkumari N, Kaur H, Roy A, Gupta N, Dhaliwal LK, Sethi S. Association of Mycoplasma genitalium with infertility in North Indian women. Indian J Sex Transm Dis. 2015;36(2):144–148. | ||
Keane FE, Thomas BJ, Gilroy CB, Renton A, Taylor-Robinson D. The association of Chlamydia trachomatis and Mycoplasma genitalium with non-gonococcal urethritis: observations on heterosexual men and their female partners. Int J STD AIDS. 2000;11(7):435–439. | ||
Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007;97(6):1118–1125. | ||
Soni S, Alexander S, Verlander N, et al. The prevalence of urethral and rectal Mycoplasma genitalium and its associations in men who have sex with men attending a genitourinary medicine clinic. Sex Transm Infect. 2010;86(1):21–24. | ||
Taylor-Robinson D, Gilroy CB, Keane FE. Detection of several Mycoplasma species at various anatomical sites of homosexual men. Eur J Clin Microbiol Infect Dis. 2003;22(5):291–293. | ||
Edlund M, Blaxhult A, Bratt G. The spread of Mycoplasma genitalium among men who have sex with men. Int J STD AIDS. 2012;23(6):455–456. | ||
Luki N, Lebel P, Boucher M, Doray B, Turgeon J, Brousseau R. Comparison of polymerase chain reaction assay with culture for detection of genital mycoplasmas in perinatal infections. Eur J Clin Microbiol Infect Dis. 1998;17(4):255–263. | ||
Montagnier L, Berneman D, Guetard D, et al. Inhibition de l’infection des souches prototypes du VIH par des anticorps dirigés contre une séquence peptidique du mycoplasme [Infectivity inhibition of HIV prototype strains by antibodies directed against a peptide sequence of mycoplasma]. C R Acad Sci III. 1990;311(12):425–430. French. | ||
Phillips DM, Pearce-Pratt R, Tan X, Zacharopoulos VR. Association of Mycoplasma with HIV-1 and HTLV-I in human T lymphocytes. AIDS Res Hum Retroviruses. 1992;8(11):1863–1868. | ||
Deguchi T, Yasuda M, Yokoi S, et al. Failure to detect Mycoplasma genitalium in the pharynges of female sex workers in Japan. J Infect Chemother. 2009;15(6):410–413. | ||
Anagrius C, Lore B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81(6):458–462. | ||
Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81(1):73–78. | ||
Horner PJ, Taylor-Robinson D. Association of Mycoplasma genitalium with balanoposthitis in men with non-gonococcal urethritis. Sex Transm Infect. 2011;87(1):38–40. | ||
Taylor-Robinson D, Gilroy CB, Horowitz S, Horowitz J. Mycoplasma genitalium in the joints of two patients with arthritis. Eur J Clin Microbiol Infect Dis.1994;13(12):1066–1069. | ||
Lu GC, Schwebke JR, Duffy LB, et al. Midtrimester vaginal Mycoplasma genitalium in women with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2001;185(1):163–165. | ||
Bjornelius E, Jensen JS, Lidbrink P. Conjunctivitis associated with Mycoplasma genitalium infection. Clin Infect Dis. 2004;39(7):e67–e69. | ||
Khattab RA, Abdelfattah MM. Study of the prevalence and association of ocular chlamydial conjunctivitis in women with genital infection by Chlamydia trachomatis, Mycoplasma genitalium and Candida albicans attending outpatient clinic. Int J Ophthalmol. 2016;9(8):1176–1186. | ||
Cohen CR, Nosek M, Meier A, et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34(5):274–279. | ||
Vandepitte J, Weiss HA, Kyakuwa N, et al. Natural history of Mycoplasma genitalium infection in a cohort of female sex workers in Kampala, Uganda. Sex Transm Dis. 2013;40(5):422–427. | ||
Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “New Chlamydia?” A community-based prospective cohort study. Clin Infect Dis. 2010;51(10):1160–1166. | ||
Mehta SD, Gaydos C, Maclean I, et al. The effect of medical male circumcision on urogenital Mycoplasma genitalium among men in Kisumu, Kenya. Sex Transm Dis. 2012;39(4):276–280. | ||
Homfray V, Tanton C, Miller RF, et al. Male circumcision and STI acquisition in Britain: evidence from a National Probability Sample Survey. PLoS One. 2015;10(6):e0130396. | ||
Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology. 2015;3(5):809–816. | ||
Waites KB, T-R. Mycoplasma and Ureaplasma. 9th ed. Washington DC: ASM Press; 2007. | ||
Le Roy C, Pereyre S, Bébéar C. Evaluation of two commercial real-time PCR assays for detection of Mycoplasma genitalium in urogenital specimens. J Clin Microbiol. 2014;52(3):971–973. | ||
Takanashi M, Ito S, Kaneto H, et al. Development and clinical application of an InvaderPlus(R) assay for the detection of genital mycoplasmas. J Infect Chemother. 2015;21(7):516–519. | ||
Roux MC, Hoosen AA. Mycoplasma genitalium: a brief review. South Afr J Epidemiol Infect. 2010;25(4):7–10. | ||
Hamasuna R, Osada Y, Jensen JS. Antibiotic susceptibility testing of Mycoplasma genitalium by TaqMan 5′ nuclease real-time PCR. Antimicrob Agents Chemother. 2005;49(12):4993–4998. | ||
Hamasuna R, Jensen JS, Osada Y. Antimicrobial susceptibilities of Mycoplasma genitalium strains examined by broth dilution and quantitative PCR. Antimicrob Agents Chemother. 2009;53(11):4938–4939. | ||
Falk L, Fredlund H, Jensen JS. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex Transm Infect. 2003;79(4):318–319. | ||
Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016;30(10):1650–1656 | ||
Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24(10):822–828. | ||
Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex Transm Infect. 2008;84(1):72–76. | ||
Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48(12):1649–1654. | ||
Jensen JS, Bradshaw C. Management of Mycoplasma genitalium infections – can we hit a moving target? BMC Infect Dis. 2015;15:343. | ||
Unemo M, Endre KM, Moi H. Five-day azithromycin treatment regimen for Mycoplasma genitalium infection also effectively eradicates chlamydia trachomatis. Acta Derm Venereol. 2015;95(6):730–732. | ||
Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis.2013;56(7):934–942. | ||
Twin J, Jensen JS, Bradshaw CS, et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One. 2012;7(4):e35593. | ||
Gesink DC, Mulvad G, Montgomery-Andersen R, et al. Mycoplasma genitalium presence, resistance and epidemiology in Greenland. Int J Circumpolar Health. 2012;71:1–8. | ||
Horner P, Blee K, Adams E. Time to manage Mycoplasma genitalium as an STI: but not with azithromycin 1 g!. Curr Opin Infect Dis. 2014;27(1):68–74. | ||
Anagrius C, Lore B, Jensen JS. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One. 2013;8(4):e61481. | ||
Falk L, Enger M, Jensen JS. Time to eradication of Mycoplasma genitalium after antibiotic treatment in men and women. J Antimicrob Chemother. 2015;70(11):3134–3140. | ||
Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin Failure in Mycoplasma genitalium Urethritis. Emerg Infect Dis. 2006;12(7):1149–1152. | ||
Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium Prevalence, Coinfection, and Macrolide Antibiotic Resistance Frequency in a Multicenter Clinical Study Cohort in the United States. J Clin Microbiol. 2016;54(9):2278–2283. | ||
Shimada Y, Deguchi T, Nakane K, et al. Macrolide resistance-associated 23S rRNA mutation in Mycoplasma genitalium, Japan. Emerg Infect Dis. 2011;17(6):1148–1150. | ||
Diner EJ, Hayes CS. Recombineering reveals a diverse collection of ribosomal proteins L4 and L22 that confer resistance to macrolide antibiotics. J Mol Biol. 2009;386(2):300–315. | ||
Nijhuis RH, Severs TT, Van der Vegt DS, Van Zwet AA, Kusters JG. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70(9):2515–2518. | ||
Guschin A, Ryzhikh P, Rumyantseva T, Gomberg M, Unemo M. Treatment efficacy, treatment failures and selection of macrolide resistance in patients with high load of Mycoplasma genitalium during treatment of male urethritis with josamycin. BMC Infect Dis. 2015;15:40. | ||
Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3(11):e3618. | ||
Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. Int J STD AIDS. 2008;19(10):676–679. | ||
Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in parC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36(3):255–258. | ||
Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58(5):631–637. | ||
Tagg KA, Jeoffreys NJ, Couldwell DL, Donald JA, Gilbert GL. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51(7):2245–2249. | ||
Tabrizi SN, Tan LY, Walker S, et al. Multiplex assay for simultaneous detection of Mycoplasma genitalium and Macrolide Resistance using plexZyme and plexPrime technology. PLoS One. 2016;11(6):e0156740. | ||
Mondeja BA, Rodríguez NM, Barroto B, Blanco O, Jensen JS. Antimicrobial susceptibility patterns of recent cuban Mycoplasma genitalium isolates determined by a modified cell-culture-based method. PLoS One. 2016;11(9):e0162924. | ||
Horner PJ, Blee K, Falk L, van der Meijden W, Moi H. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27(11):928–937. | ||
Renaudin H, Tully JG, Bebear C. In vitro susceptibilities of Mycoplasma genitalium to antibiotics. Antimicrob Agents Chemother. 1992;36(4):870–872. | ||
Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58(6):3151–3156. | ||
Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57(9):4489–4495. | ||
Prince WT, Ivezic-Schoenfeld Z, Lell C, et al. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2013;57(5):2087–2094. | ||
Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18(3):414–418. | ||
Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19(5):941–945. | ||
Waites KB, Crabb DM, Duffy LB, Huband MD. In vitro antibacterial activity of AZD0914 against human Mycoplasmas and Ureaplasmas. Antimicrob Agents Chemother. 2015;59(6):3627–3629. | ||
Falk L, Jensen JS. Successful outcome of macrolide-resistant Mycoplasma genitalium urethritis after spectinomycin treatment: a case report. J Antimicrob Chemother 2017;72(2):624–625. | ||
Committee on Gynecologic Practice. ACOG Committee Opinion No. 645: Dual Therapy for Gonococcal Infections. Obstet Gynecol. 2015;126(5):e95–99. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
