Back to Journals » Infection and Drug Resistance » Volume 16
Mycobacterium tuberculosis Intra-Host Evolution Among Drug-Resistant Tuberculosis Patients Failing Treatment
Authors Perumal R , Khan A, Naidoo K , Ngema SL , Nandlal L, Padayatchi N , Dookie N
Received 17 February 2023
Accepted for publication 29 April 2023
Published 9 May 2023 Volume 2023:16 Pages 2849—2859
DOI https://doi.org/10.2147/IDR.S408976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Rubeshan Perumal,1,2 Azraa Khan,1 Kogieleum Naidoo,1,2 Senamile L Ngema,1 Louansha Nandlal,1,2 Nesri Padayatchi,1,2 Navisha Dookie1,2
1Centre for the AIDS Programme of Research in South Africa (CAPRISA), University of KwaZulu-Natal, Durban, KwaZulu Natal, South Africa; 2South African Medical Research Council (SAMRC) – CAPRISA HIV-TB Pathogenesis and Treatment Research Unit, Durban, KwaZulu Natal, South Africa
Correspondence: Rubeshan Perumal, Doris Duke Medical Research Institute (2nd Floor), Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Private Bag X7, Congella, Durban, 4013, South Africa, Tel +27 31 260 4555, Fax +27 31 260 4566, Email [email protected]
Background: Understanding Mycobacterium tuberculosis (Mtb) intra-host evolution of drug resistance is important for successful drug-resistant tuberculosis (DR-TB) treatment and control strategies. This study aimed to characterise the acquisition of genetic mutations and low-frequency variants associated with treatment-emergent Mtb drug resistance in longitudinally profiled clinical isolates from patients who experienced DR-TB treatment failure.
Patients and Methods: We performed deep Whole Genome Sequencing on 23 clinical isolates obtained longitudinally across nine timepoints from five patients who experienced DR-TB treatment failure enrolled in the CAPRISA 020 InDEX study. The minimum inhibitory concentrations (MICs) were established on the BACTEC™ MGIT 960™ instrument on 15/23 longitudinal clinical isolates for eight anti-TB drugs (rifampicin, isoniazid, ethambutol, levofloxacin, moxifloxacin, linezolid, clofazimine, bedaquiline).
Results: In total, 22 resistance associated mutations/variants were detected. We observed four treatment-emergent mutations in two out of the five patients. Emerging resistance to the fluoroquinolones was associated with 16- and 64-fold elevated levofloxacin (2– 8 mg/L) and moxifloxacin (1– 2 mg/L) MICs, respectively, resulting from the D94G/N and A90V variants in the gyrA gene. We identified two novel mutations associated with elevated bedaquiline MICs (> 66-fold): an emerging frameshift variant (D165) on the Rv0678 gene and R409Q variant on the Rv1979c gene present from baseline.
Conclusion: Genotypic and phenotypic resistance to the fluoroquinolones and bedaquiline was acquired in two out of five patients who experienced DR-TB treatment failure. Deep sequencing of multiple longitudinal clinical isolates for resistance-associated mutations coupled with phenotypic MIC testing confirmed intra-host Mtb evolution.
Keywords: tuberculosis, phenotypic resistance, drug resistance acquisition, whole genome sequencing, emerging mutations, bedaquiline
Introduction
Drug resistance in TB (DR-TB) is of particular importance as it represents the single largest cause of mortality due to antimicrobial resistance (AMR).1 It is a challenging disease, requiring complex multidrug treatment over a relatively long duration to achieve relapse-free cure. Treatment failure for DR-TB patients is now defined as the permanent change or termination of a patient’s treatment regimen due to evidence of drug resistance, lack of culture conversion, or culture reversion.2 In 2018, treatment failure was experienced by 35% of people with multidrug-resistant TB (MDR-TB) in South Africa, which is substantially higher than the global treatment failure rate of approximately 8.5%.3 Extensively drug-resistant TB (XDR-TB) is defined as additional resistance to at least one of the fluoroquinolones (FQs) and either bedaquiline (BDQ) or linezolid (LZD).2 Current phenotypic drug susceptibility testing (DST) for FQs, BDQ and LZD is limited and resistance is determined at only the critical concentration (CC) of drugs, as opposed to the determination of a range of minimum inhibitory concentrations (MICs) which are more useful for therapeutic decision-making. The absence of routine molecular DST tests for BDQ and LZD presents a further challenge for the early detection and treatment of XDR-TB.
Several reports have demonstrated the complex and stepwise evolution of Mycobacterium tuberculosis (Mtb), despite stringent adherence to a directly observed treatment (DOT) protocol, from rapidly acquired mutations among DR-TB patients receiving treatment.4–9 Resistance may emerge spontaneously during treatment; however, intra-host evolution of drug can only be successfully studied in longitudinal isolates from patients with persistently positive cultures over several months. Intra-host evolution in the presence of antimicrobials has been shown to be far more complex as exposure to antibiotics may modulate observed bacterial mutation rates in M tb. Under selective drug pressure, intra-host evolution may be especially consequential as emerging resistance is acquired to drugs within a limited multi-drug regimen. Acquired resistance to a core drug in the regimen could result in a regimen being compromised or rendered completely ineffective. Black et al demonstrated that a rapidly changing genetic environment contributed to evolution and diversity of Mtb in longitudinal isolates from two RIF mono-resistant TB cases which evolved to MDR-TB over time.10 Evolution of MDR-TB to XDR-TB which was observed in seven longitudinal isolates from two patients in a previous study resulted from the acquisition of 17 resistance-conferring mutations of varying frequencies over 32 months of treatment.11 The evolution of drug susceptible TB to XDR-TB was documented in a single reported case.6 Stepwise acquisition of resistance was reported for INH, RIF, FQs, streptomycin (STR), ethionamide (ETH), amikacin (AMI) and ethambutol (EMB) from nine sequential isolates collected over 3.5 years.6
At the population level, while most cases of DR-TB result from transmission of resistant strains, the genesis of all new resistance occurs through intra-host evolution whether by spontaneous mutation or under selective drug pressure.12 Clinical simulations with a hollow-fibre TB model suggest that intra-host evolution of resistance could occur despite perfect adherence due to pharmacokinetic variability alone.13 Understanding how M tb populations evolve within the host, and mechanisms which underpin this evolution, may allow for more robust treatment regimens and TB control strategies. Mtb evolution patterns remain poorly understood, partly due to the limited availability of advanced deep genetic sequencing techniques required to illustrate the emergence of low-frequency variants, as well as the paucity of longitudinal MIC data in DR-TB cohorts.
Bedaquiline is a core drug in the current treatment of DR-TB, however we have little understanding of the biological mechanisms driving resistance to this important drug. It is hypothesized that many dynamic low-frequency variants on the Rv0678 gene at baseline disappear when mutations on the atpE gene are subsequently acquired.14,15 However, other studies reported the emergence of Rv0678 variants amongst BDQ treated patients, resulting in >8-fold increases in BDQ MIC and poor treatment outcomes.16,17 An E49 frameshift variant in the Rv0678 gene with an allelic frequency of 72% at baseline increased to 96% after two months of treatment and was associated with an eight-fold increase in BDQ MIC.17 Greater clarity is needed on the mutations and variants associated with BDQ resistance and their impact on phenotypic drug susceptibility.
Newer sequencing methods such as whole genome, targeted, and deep sequencing are increasingly used in the clinical setting to assess the emergence of resistance during treatment and to differentiate Mtb lineages, mixed strain infections and genetic heterogeneity.18–20 Higher depths of coverage allow for in-depth analyses of intra-host Mtb evolution.21–23 Thus, the aim of this study was to characterize the accumulation of mutations associated with Mtb drug resistance using deep whole genome sequencing (WGS) on clinical isolates obtained longitudinally from patients who experienced DR-TB treatment failure and to determine the association between these genetic mutations and the MICs against eight TB drugs.
Materials and Methods
Clinical Isolates
This sub-study was performed on stored clinical isolates obtained from patients in the CAPRISA 020 InDEX randomized control trial (BREC/00001887/2020, NCT03237182). Written informed consent for the storage and use of samples was obtained from patients at the time of enrolment into the parent InDEX study. As part of the InDEX study, clinical specimens underwent decontamination processing, GeneXpert MTB/RIF (Cepheid), MTBDRplus and MTBDRsl (Hain LifeSciences), liquid culture (BACTEC™ MGIT 960™), phenotypic DST for a selected drug panel and standard WGS. Ethical approval for this sub-study was obtained from the University of KwaZulu Natal’s Biomedical Research Ethics Committee (BREC/00001181/2020).
For this study, we initially identified forty longitudinal clinical isolates across nine timepoints (collected between 2017 and 2019) from six DR-TB patients within the parent InDEX study who had remained culture positive despite at least six months of treatment. For each patient, we selected all the available longitudinal isolates from baseline up until the last positive culture isolate. For each isolate, we performed culture reviving in liquid mycobacterial growth indicator tube (MGIT) or solid Middlebrook 7H11 agar plates (MediaMage Company Pty (LTD), Johannesburg, South Africa). After excluding three contaminated isolates from one patient, 23/37 isolates from five of the patients were successfully regrown (Figure 1). We performed deep WGS on all 23 isolates and MICs on BACTEC™ MGIT 960™ on 15/23 longitudinal isolates at six timepoints. Patient information (demographic, medical history, HIV status, chest X-ray results, etc.) was extracted from electronic medical records (DFdiscover Data Management) from the CAP020 InDEX study database.
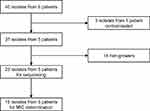 |
Figure 1 Study flow diagram. Abbreviation: MIC, minimum inhibitory concentration. |
Culture and Purity Checks
Inoculation of isolates in MGIT and three quality control/purity checks are described in the supplementary material (Supplementary Methods). A susceptible strain of Mtb (H37Rv, ATCC 27294) was used as the positive control.
Minimum Inhibitory Concentrations
Fifteen longitudinal isolates, based on availability and regrowth, were sub-cultured into fresh MGIT tubes using 0.5 mL of the MGIT suspension (1:5 saline dilution), 0.1 mL of drug and 0.8 mL of oleic acid-albumin-dextrose-catalase (OADC). Most drug stock concentrations were purchased from MediaMage to obtain the following seven concentrations (mg/L) in MGIT (CCs recommended by WHO are bolded): INH (0.125, 0.25, 0.5, 1, 2, 4, 8), RIF (0.25, 0.5, 1, 2, 4, 8, 16), EMB (0.625, 1.25, 2.5, 5, 10, 20, 40), levofloxacin (LEVO) (0.125, 0.25, 0.5, 1, 2, 4, 8), moxifloxacin (MOXI) (0.062, 0.125, 0.25, 0.5, 1, 2, 4), LZD (0.125, 0.25, 0.5, 1, 2, 4, 8) and clofazimine (CFZ) (0.125, 0.25, 0.5, 1, 2, 4, 8).24,25 Bedaquiline was purchased from Adooq® (TMC-207, A12327) and nine concentrations were made up by MediaMage (0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16). A positive/growth control containing no drug, MGIT suspension (1:100 saline dilution) and 0.8 mL OADC, was used alongside each isolate. Details on loading of MGIT tubes and MIC interpretations are found in the supplementary material (Supplementary Methods).
Whole Genome Sequencing and Data Analysis
DNA was extracted from colonies of 23 isolates grown on 7H11 plates using the cetyltrimethylammonium bromide (CTAB) method as described in the supplementary material (Supplementary Methods). Libraries were prepared using MiSeq library preparation kit. Deep sequencing of ~200× depth of coverage was aimed to detect variants at <10% frequency on the NextSeq sequencer (Illumina, San Diego, CA, USA). NextSeq 300 cycles kit was used with read lengths of 2×150 base pairs. Library preparation and sequencing were performed by Inqaba biotech. Raw sequencing data was analyzed on the CLC Genomics workbench, version 22 (Qiagen) using a TB specific pipeline including the Basic Variant detection tool created and supplied by Dr Camus Nimmo. Low quality reads were trimmed with a trim quality limit of 5% excluding the PE and PPE regions and then mapped to the H37Rv reference genome (NC_000962.3). Analysis was performed in parallel on TB Profiler for lineage typing. Mutations with a frequency of ≥75% were referred to as fixed mutations, while a mutant at any frequency was referred to as a variant.
Statistical Analysis
We performed all statistical analysis on IBM SPSS statistics version 27 (SPSS Inc., Chicago, IL, USA). Clinical and demographic results were reported using mean and ranges.
Results
Patient Demographics and Treatment History
Six patients experienced treatment failure in the INDEX study and were eligible for the evaluation of intra-host evolution. Culture revival was successful in 23 isolates across 2–9 timepoints from 5 patients (Figure 1). All patients had microbiological confirmation of DR-TB diagnosis by GeneXpert, MTBDRplus and MTBDRsl, phenotypic DST on a selected drug panel, and routine WGS. MIC was successfully determined for 15/23 isolates. All patients experienced delayed culture/microbiological conversion; however, all but one patient achieved cure at study end. All five patients were HIV positive and had extensive bilateral disease on chest radiograph at baseline. Two of the five patients had previous history of TB disease and TB treatment. The mean age was 33.6 (range 23–41) years, mean weight was 55.8 (range 45.4–74.0) kg and mean BMI was 19.9 (range 16.1–27.5) kg/m2 (Table 1). The clinical course and treatment history for each patient are outlined in the supplementary material (Supplementary Clinical Course and Treatment History).
 |
Table 1 Baseline Demographic and Clinical Data of the Five Patients Failing Treatment |
Rising Minimum Inhibitory Concentrations and Emerging Variants During Treatment
Two out of the five patients (3 and 5) developed treatment emergent FQ and BDQ resistance. The range of MICs over time demonstrating the development of elevated FQ and BDQ MICs during treatment are depicted in Table 2; the associated treatment-emergent variants are described in Table 3. Patient 3 exhibited a ≥16-fold increase in LEVO and MOXI MICs at month 8 due to the emergence of an A90V minor variant on the gyrA gene with 22% frequency (Supplementary Figure 1). Patient 5 exhibited a ≥66-fold elevation in LEVO MIC of 8 mg/L at month 7 due to the acquisition of two gyrA variants; the D94N and D94G variants appeared at 32.35% and 62.16% frequencies, respectively, at month 4, before the D94G mutation outcompeted the population by month 7 (100% frequency) (Supplementary Figure 2). This month 7 isolate also exhibited ≥66-fold elevation in BDQ MIC of 4 mg/L, with no effect on CFZ MIC, presumably due to the D165 frameshift variant on the Rv0678 gene which emerged with a frequency of 10.14% at month 4 and increased to 84.62% by month 7. Patients 1–4 demonstrated fluctuating phenotypic susceptibility to BDQ throughout the duration of treatment with MICs ≤0.25 mg/L. Table 2 shows the MIC profiles of the full set of 15 longitudinal isolates from the five DR-TB patients for eight anti-TB drugs. Table 3 shows the resistance associated mutations/variants detected across the 23 sequenced longitudinal isolates from the five patients.
Baseline Mutations/Variants and Minimum Inhibitory Concentrations
In total, there were 22 mutations/variants detected in all 23 longitudinal isolates (Table 3). Eighteen baseline mutations associated with RIF, INH, ETH, EMB, CFZ and pyrazinamide (PZA) resistance were identified. All baseline phenotypes bearing mutations/variants were resistant to the respective tested drugs, except for H445N mutation in rpoB for RIF (patient 2) and R409Q mutation in Rv1979c for CFZ (patient 5) which were susceptible (Table 2 and Supplementary Discordant Results). Additionally, phenotypic resistance to E173fs mutation in pncA for PZA resistance was not confirmed since MICs for PZA were not conducted. Each isolate’s mutations and frequencies are discussed in the supplementary material. No mutations were found in genes involved in resistance to AMI, LZD, capreomycin (CAP), p-aminosalicylic acid and cycloserine. Results of lineage typing, median depths of coverage, and the phylogenetic tree are discussed in the supplementary material (Supplementary Genotypic Profiles, Supplementary Table 1, and Supplementary Figure 3).
Discussion
We examined the dynamics of intra-host Mtb populations in relation to successive changes in drug resistance profiles from longitudinal isolates obtained from the same patient. In this study, we report on 22 resistance associated mutations/variants from five patients who experienced failing DR-TB treatment, in whom 18 baseline mutations conferred resistance to RIF, INH, ETH, EMB, PZA and CFZ. By characterizing their longitudinal isolates, emerging genotypic and phenotypic resistance was demonstrated in two of the five patients during treatment. There was no evidence of reinfection with another strain at any timepoint, therefore drug resistance was solely due to the in vivo acquisition of resistance within the host. Thus, it is highly likely that ineffective sterilization and inadequate drug pressure contributed to the evolution of resistance.
Emerging Resistance
Emerging resistance was detected towards FQs and BDQ in two of the five MDR-TB patients who experienced treatment failure. The FQs are the most vulnerable to acquired resistance and the accompanying risk of treatment failure. FQs are commonly used to treat a range of common bacterial infections, therefore previous exposure and pre-treatment resistance are likely and not always established at the time of diagnosis and treatment selection. The emerging gyrA variants detected in our study, A90V and D94G/N, are among the most frequently occurring mutations associated with FQ resistance globally and in South Africa.26 As observed in our study, the A90V and D94G/N mutations are associated with low and high levels of phenotypic resistance, respectively.27–29 Several studies have also shown these gyrA variants to emerge during treatment and to be associated with treatment failure.7,9,30,31 In this study, we report on clonal interference between the D94G and D94N variants in patient 5 at month 4 with frequencies of 62.16% and 32.35%, respectively, until month 7 when only the D94G variant was present at 100% frequency and dominated the population (Figure 2). While treatment failure was already reported clinically and microbiologically at month 6 for patient 3, we did not detect resistance acquisition genotypically and phenotypically until month 8. A possible explanation for this was our inability to detect low-frequency variants due to our low sequencing depth.
Resistance to Bedaquiline
Two longitudinal isolates from one patient harboured Rv0678 and Rv1979c variants. The Rv1979c gene is implicated in CFZ resistance only, while the Rv0678 gene is involved in cross-resistance between BDQ and CFZ.16,32,33 The Rv0678 gene is implicated in drug efflux out of the Mtb cell, raising the MICs of many drugs.34 In patient 5, the emerging frameshift variant on the Rv0678 gene (D165) has not been previously reported to our knowledge, therefore its association with resistance is unknown. Since only BDQ MIC was elevated and not CFZ, this variant may be a phylogenetic marker and requires further investigation (Figure 2). The R409Q SNP in the Rv1979c gene was recently found in 30 different patient isolate strains from the CRyPTIC consortium unpublished dataset, and was shown to be associated with phenotypic resistance to BDQ (MIC = 0.25–0.5 mg/L) and borderline susceptibility to CFZ (MIC = 0.06–0.5 mg/L) using the UKMYC5/6 method (CC of 0.25 mg/L for BDQ and CFZ).35 Mutations in the Rv1979c gene have thus far only been implicated in CFZ resistance and not BDQ resistance, possibly indicating another cross-resistance mechanism between BDQ and CFZ along this gene.36 Frameshift mutations inhibit the repressor activity of Rv0678 leading to the overexpression of efflux pump activity, cause dramatic structural and functional changes of the protein, and ultimately elevated MICs and phenotypic resistance to BDQ.15,37 Elevated BDQ MIC at month 7, when both these mutations became fixed, may be evidence that these novel mutations have a direct phenotypic impact on BDQ. However, other studies reported that DR-TB clinical isolates with elevated BDQ MICs harboured no mutations in the atpE, Rv0678 or pepQ genes.38,39 This implies other genetic or host-related factors may be involved in BDQ resistance. The lack of an increased CFZ MIC despite both Rv0678 and Rv1979c mutations, is surprising. Our finding was in contrast to other studies, which found complete cross-resistance on the Rv0678 gene between BDQ and CFZ.14,33,37,40–43 However, recent unpublished data from the CRyPTIC consortium revealed 25 BDQ resistant isolates with Rv0678 mutations were also not cross-resistant to CFZ, challenging previous conclusions.35 We do not fully understand the mechanistic interaction of BDQ with Mtb. A recent systematic review revealed the difficulty in finding an association or correlation of genotypic and phenotypic resistance for BDQ.43 This challenges any developments in molecular drug susceptibility tests and complicates the detection of resistance to this core drug used for treating DR-TB. Bedaquiline’s mechanism of action in various Mtb metabolic states necessitates further in vivo and host interaction studies.
Limitations
Limitations of this study were that many longitudinal isolates of patients did not grow, possibly due to the long period of storage resulting in the organisms dying or remaining in a dormant state. Consequently, the intermediate phenotype and genotype could not be analysed between every timepoint, and the small sample size limited statistical comparisons. To detect patients with treatment failure, we surveyed 160 participants on DR-TB treatment with monthly sputum cultures. In our study, we found only five patients who remained culture positive at month six, and for whom longitudinal isolate evolution could be assessed. Intra-host evolution is uncommon and can only be studied in patients with persistently positive cultures over several months, usually on treatment. Although our sample size was relatively small, we evaluated all patients who remained culture positive at month six within a highly characterised relatively large DR-TB randomised controlled trial cohort. Phenotypic resistance against the drugs PZA, ETH and the injectable agents were not determined due to unavailability of a MGIT DST carrier during the COVID-19 pandemic for PZA, inability to detect the relatively small MIC changes associated with thioamide resistance with thermolabile drugs, and the injectable agents could not be accessed since these are no longer included in current standard treatment regimens. We were also not able to perform MIC replicates due to the large number of drugs we evaluated, and the high costs and infrastructure requirements associated with such testing. However, we used validated laboratory methods with stringent standard operating protocols within a well-established laboratory.
Conclusion
In conclusion, we observed emerging genotypic and phenotypic resistance towards the FQs and BDQ in two out of five patients experiencing DR-TB treatment failure. The association between baseline ETH, PZA and EMB resistance and the acquisition of FQ resistance during treatment needs to be explored using data from larger cohorts. We found that combining deep sequencing with multiple longitudinal isolates from the same patient enabled us to study the dynamic changes of intra-host evolution of Mtb in patients who experienced DR-TB treatment failure.
Improving our understanding of the relationship between phenotypic and genotypic resistance to BDQ is essential for defining clinical breakpoints. Phenotypic resistance testing for new drugs like BDQ, CFZ, LZD and pretomanid (PRT) should be routinely implemented to characterize the level of in vivo resistance in a way that genotypic assays cannot yet determine. Novel short-course regimens such as BPaL (BDQ, PRT and LZD), BPaLM (BDQ, PRT, LZD and MOXI) and BPaMZ (BDQ, PRT, MOXI and PZA) contain a relatively small number of effective drugs, thus resistance to just one or more drugs can have a detrimental impact on clinical outcomes. Early identification of intra-host drug-resistance evolution may be important for guiding treatment and informing public health strategies for TB control.
Ethical Approval
Ethical approval for this sub-study was obtained from the University of KwaZulu Natal’s Biomedical Research Ethics Committee (BREC/00001181/2020). Written informed consent was obtained from human subjects in the CAP 020 InDEX parent study. The study complies with the Declaration of Helsinki.
Acknowledgments
This study was supported by the South African Medical Research Council; the European & Developing Countries Clinical Trials Partnership (EDCTP) Fellowships (TMA2018CDF-2372 and TMA2018SF-2467); the National Research Foundation (NRF) – Thuthuka (TTK1902114157860); and the DSI-NRF Centre of Excellence (CoE) for Biomedical Tuberculosis Research. The funders had no role in study design, data collection, analysis, and interpretation of data, writing and preparation of the manuscript, or decision to submit article for publication.
We acknowledge Dr Camus Nimmo for assistance with the CLC Genomics workbench workflows, Tashmin Rampersad for assistance in lab work, the CAPRISA 020 InDEX study team and Inqaba biotech for sequencing our samples.
Disclosure
The authors report no conflicts of interest in this work.
References
1. O’Neill J. Grande-Bretagne. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. 2014;2014:1.
2. World Health Organization. Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27–29 October 2020. Geneva: World Health Organization; 2020.
3. World Health Organisation. Global Tuberculosis Report 2021. Geneva: World Health Organisation; 2021.
4. Chawla K, Martinez E, Kumar A, Shenoy VP, Sintchenko V. Whole-genome sequencing reveals genetic signature of bedaquiline resistance in a clinical isolate of Mycobacterium tuberculosis. J Glob Antimicrob Resist. 2018;15:103–104. doi:10.1016/j.jgar.2018.09.006
5. de Vos M, Ley SD, Wiggins KB, et al. Bedaquiline microheteroresistance after cessation of tuberculosis treatment. N Engl J Med. 2019;380(22):2178–2180. doi:10.1056/NEJMc1815121
6. Eldholm V, Norheim G, von der Lippe B, et al. Evolution of extensively drug-resistant Mycobacterium tuberculosis from a susceptible ancestor in a single patient. Genome Biol. 2014;15(11):490. doi:10.1186/s13059-014-0490-3
7. Mokrousov I, Akhmedova G, Polev D, Molchanov V, Vyazovaya A. Acquisition of bedaquiline resistance by extensively drug-resistant Mycobacterium tuberculosis strain of Central Asian Outbreak clade. Clin Microbiol Infect. 2019;25(10):1295–1297. doi:10.1016/j.cmi.2019.06.014
8. Polsfuss S, Hofmann-Thiel S, Merker M, et al. Emergence of low-level delamanid and bedaquiline resistance during extremely drug-resistant tuberculosis treatment. Clin Infect Dis. 2019;69(7):1229–1231. doi:10.1093/cid/ciz074
9. Trauner A, Liu Q, Via LE, et al. The within-host population dynamics of Mycobacterium tuberculosis vary with treatment efficacy. Genome Biol. 2017;18(1):71. doi:10.1186/s13059-017-1196-0
10. Black PA, de Vos M, Louw GE, et al. Whole genome sequencing reveals genomic heterogeneity and antibiotic purification in Mycobacterium tuberculosis isolates. BMC Genomics. 2015;16:857. doi:10.1186/s12864-015-2067-2
11. Merker M, Kohl TA, Roetzer A, et al. Whole genome sequencing reveals complex evolution patterns of multidrug-resistant Mycobacterium tuberculosis Beijing strains in patients. PLoS One. 2013;8(12):e82551. doi:10.1371/journal.pone.0082551
12. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. doi:10.1128/MMBR.00016-10
13. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204(12):1951–1959. doi:10.1093/infdis/jir658
14. Ismail N, Peters RPH, Ismail NA, Omar SV. Clofazimine exposure in vitro selects efflux pump mutants and bedaquiline resistance. Antimicrob Agents Chemother. 2019;63(3). doi:10.1128/AAC.02141-18
15. Peretokina IV, Krylova LY, Antonova OV, et al. Reduced susceptibility and resistance to bedaquiline in clinical M. tuberculosis isolates. J Infect. 2020;80(5):527–535. doi:10.1016/j.jinf.2020.01.007
16. Ghodousi A, Rizvi AH, Baloch AQ, et al. Acquisition of cross-resistance to bedaquiline and clofazimine following treatment for tuberculosis in Pakistan. Antimicrob Agents Chemother. 2019;63(9). doi:10.1128/AAC.00915-19
17. Nimmo C, Millard J, Brien K, et al. Bedaquiline resistance in drug-resistant tuberculosis HIV co-infected patients. Eur Respir J. 2020;55(6):1902383. doi:10.1183/13993003.02383-2019
18. Cabibbe AM, Trovato A, De Filippo MR, et al. Countrywide implementation of whole genome sequencing: an opportunity to improve tuberculosis management, surveillance and contact tracing in low incidence countries. Eur Respir J. 2018;51(6):1800387. doi:10.1183/13993003.00387-2018
19. Casali N, Broda A, Harris SR, Parkhill J, Brown T, Drobniewski F. Whole genome sequence analysis of a large isoniazid-resistant tuberculosis outbreak in London: a retrospective observational study. PLoS Med. 2016;13(10):e1002137. doi:10.1371/journal.pmed.1002137
20. Manson AL, Cohen KA, Abeel T, et al. Genomic analysis of globally diverse Mycobacterium tuberculosis strains provides insights into the emergence and spread of multidrug resistance. Nat Genet. 2017;49(3):395–402. doi:10.1038/ng.3767
21. Cancino-Munoz I, Moreno-Molina M, Furio V, et al. Cryptic resistance mutations associated with misdiagnoses of multidrug-resistant tuberculosis. J Infect Dis. 2019;220(2):316–320. doi:10.1093/infdis/jiz104
22. Nimmo C, Brien K, Millard J, et al. Dynamics of within-host Mycobacterium tuberculosis diversity and heteroresistance during treatment. EBioMedicine. 2020;55:102747. doi:10.1016/j.ebiom.2020.102747
23. Vargas R, Freschi L, Marin M, et al. In-host population dynamics of Mycobacterium tuberculosis complex during active disease. Elife. 2021;2021:10.
24. World Health Organization. Technical Report on Critical Concentrations for Drug Susceptibility Testing of Medicines used in the Treatment of Drug-Resistant Tuberculosis. Geneva: World Health Organization; 2018.
25. Ajbani K, Comas I, Coulter C, et al. Technical Report on Critical Concentrations for Drug Susceptibility Testing of Isoniazid and the Rifamycins (rifampicin, Rifabutin and Rifapentine). Geneva: World Health Organization; 2021.
26. Avalos E, Catanzaro D, Catanzaro A, et al. Frequency and geographic distribution of gyrA and gyrB mutations associated with fluoroquinolone resistance in clinical Mycobacterium tuberculosis isolates: a systematic review. PLoS One. 2015;10(3):e0120470. doi:10.1371/journal.pone.0120470
27. Rigouts L, Coeck N, Gumusboga M, et al. Specific gyrA gene mutations predict poor treatment outcome in MDR-TB. J Antimicrob Chemother. 2016;71(2):314–323. doi:10.1093/jac/dkv360
28. Uddin MKM, Ather MF, Nasrin R, et al. Correlation of gyr mutations with the minimum inhibitory concentrations of fluoroquinolones among multidrug-resistant mycobacterium tuberculosis isolates in Bangladesh. Pathogens. 2021;10(11):1422. doi:10.3390/pathogens10111422
29. Zhang X, Chen X, Wang B, et al. Molecular characteristic of both levofloxacin and moxifloxacin resistance in mycobacterium tuberculosis from individuals diagnosed with preextensive drug-resistant tuberculosis. Microbial Drug Res. 2022;28(3):280–287.
30. Chen X, He G, Lin S, et al. Analysis of serial multidrug-resistant tuberculosis strains causing treatment failure and within-host evolution by whole-genome sequencing. mSphere. 2020;5(6). doi:10.1128/mSphere.00884-20
31. Wollenberg KR, Desjardins CA, Zalutskaya A, et al. Whole-genome sequencing of mycobacterium tuberculosis provides insight into the evolution and genetic composition of drug-resistant tuberculosis in Belarus. J Clin Microbiol. 2017;55(2):457–469. doi:10.1128/JCM.02116-16
32. Almeida D, Ioerger T, Tyagi S, et al. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in mycobacterium tuberculosis. Antimicrob Agents Chemother. 2016;60(8):4590–4599. doi:10.1128/AAC.00753-16
33. Hartkoorn RC, Uplekar S, Cole ST. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58(5):2979–2981. doi:10.1128/AAC.00037-14
34. Ghajavand H, Kargarpour Kamakoli M, Khanipour S, et al. High prevalence of bedaquiline resistance in treatment-naive tuberculosis patients and verapamil effectiveness. Antimicrob Agents Chemother. 2019;63(3). doi:10.1128/AAC.02530-18
35. Sonnenkalb L, Carter J, Spitaleri A, et al. Deciphering bedaquiline and clofazimine resistance in tuberculosis: an evolutionary medicine approach. bioRxiv. 2021;2021:436.
36. Zhang S, Chen J, Cui P, Shi W, Zhang W, Zhang Y. Identification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2015;70(9):2507–2510. doi:10.1093/jac/dkv150
37. Andries K, Villellas C, Coeck N, et al. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One. 2014;9(7):e102135. doi:10.1371/journal.pone.0102135
38. Pang Y, Zong Z, Huo F, et al. In vitro drug susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis in Beijing, China. Antimicrob Agents Chemother. 2017;61(10). doi:10.1128/AAC.00900-17
39. Zimenkov DV, Nosova EY, Kulagina EV, et al. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. J Antimicrob Chemother. 2017;72(7):1901–1906. doi:10.1093/jac/dkx094
40. Ismail NA, Omar SV, Joseph L, et al. Defining bedaquiline susceptibility, resistance, cross-resistance and associated genetic determinants: a retrospective cohort study. EBioMedicine. 2018;28:136–142. doi:10.1016/j.ebiom.2018.01.005
41. Kadura S, King N, Nakhoul M, et al. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J Antimicrob Chemother. 2020;75(8):2031–2043. doi:10.1093/jac/dkaa136
42. Ismail N, Ismail NA, Omar SV, Peters RPH. In vitro study of stepwise acquisition of rv0678 and atpE mutations conferring bedaquiline resistance. Antimicrob Agents Chemother. 2019;63(8). doi:10.1128/AAC.00292-19
43. Ismail N, Rivière E, Limberis J, et al. Genetic variants and their association with phenotypic resistance to bedaquiline in Mycobacterium tuberculosis: a systematic review and individual isolate data analysis. Lancet Microbe. 2021;2(11):e604–e16. doi:10.1016/S2666-5247(21)00175-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



