Back to Journals » Infection and Drug Resistance » Volume 17
Multiple Blood Culture Sampling, Proper Antimicrobial Choice, and Adequate Dose in Definitive Therapy Supported by the Antimicrobial Stewardship Team Could Decrease 30-Day Sepsis Mortality Rates
Authors Saito N , Tsuchiya J, Itoga M , Okamura Y, Tsuyama H, Kimura M, Inoue F, Kimura T, Ozaki H, Tono Y, Minakawa S , Tomita H
Received 21 October 2023
Accepted for publication 11 January 2024
Published 22 January 2024 Volume 2024:17 Pages 207—219
DOI https://doi.org/10.2147/IDR.S445917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Norihiro Saito,1– 3 Junichiro Tsuchiya,1,3 Masamichi Itoga,1,2 Yuji Okamura,1,4 Hiromasa Tsuyama,1,4 Masahiko Kimura,1,3 Fumio Inoue,1,3 Toshiyuki Kimura,1 Hiromi Ozaki,1 Yuka Tono,1,4 Satoko Minakawa,1,3 Hirofumi Tomita2,3,5
1Division of Infection Control and Prevention, Hirosaki University Hospital, Hirosaki, Aomori, Japan; 2Department of Clinical Laboratory Medicine, Hirosaki University Graduate School of Medicine, Hirosaki, Aomori, Japan; 3Division of Clinical Laboratory, Hirosaki University Hospital, Hirosaki, Aomori, Japan; 4Division of Pharmacy, Hirosaki University Hospital, Hirosaki, Aomori, Japan; 5Department of Cardiology and Nephrology, Hirosaki University Graduate School of Medicine, Hirosaki, Aomori, Japan
Correspondence: Norihiro Saito, Division of Infection Control and Prevention, Hirosaki University Hospital, 53 Hon-cho, Hirosaki, Aomori, 036-8563, Japan, Tel +81-172-33-5111, Email [email protected]
Objective: This study aimed to identify factors that should be focused on by the antimicrobial stewardship team for treating patients with sepsis, by investigating the mortality of patients with sepsis within 30 days and the mortality-related factors in our hospital over a 10-year period from the perspective of appropriate antimicrobial use.
Methods: Factors associated with 30-day mortality were investigated using hierarchical multiple logistic regression in 1406 patients with pathogen-identified sepsis in Hirosaki University Hospital. These factors were clinical data, microbiological data, antimicrobials used in empiric and definitive therapies, presence/absence of ineffective use, underdosing as evaluated using Monte Carlo simulation, and practice of de-escalation.
Results: The ineffective use of antimicrobials in empiric therapy and the underdosing and ineffective use in definitive therapy were significantly associated with 30-day mortality (odds ratio [OR] = 2.70, 3.72, and 3.65, respectively). Multiple blood culture sampling was inversely associated with these inappropriate antimicrobial uses. Every year, the 30-day mortality rate has been decreasing, in line with the increase in multiple blood culture sampling and de-escalation; the inappropriate use of antimicrobials has also decreased.
Conclusion: Multiple blood culture sampling, proper choice of antimicrobial, and using an adequate dose in definitive therapy could decrease the 30-day mortality rate in patients with sepsis and these factors could be supported by the antimicrobial stewardship team.
Keywords: sepsis, antimicrobial stewardship, Monte Carlo simulation, de-escalation
Introduction
In districts distant from large cities in Japan, clinical specialists for infectious diseases are limited. Hirosaki University Hospital located at the northern end of the main island of Japan had no such specialist for several years until 2012. Therefore, education about appropriate antimicrobial use was insufficient at that time. Inappropriate or suboptimal utilization of antimicrobials can lead to increased length of stay, multidrug-resistant infections, and mortality.1 The Core Elements of Hospital Antibiotic Stewardship Programs 2019 reported that approximately 30% of all antimicrobials prescribed in acute care hospitals in the United States were either unnecessary or suboptimal.2 Our Infection Control Team (ICT), which includes four antimicrobial stewardship team (AST) members, started engaging in pragmatic education regarding proper antimicrobial use (including the practice of multiple blood culture sampling) for all clinicians and ward pharmacists in 2013. Antimicrobial stewardship programs (ASPs) aim to achieve optimal clinical outcomes, ensure cost-effectiveness, and minimize unintended consequences, such as toxic effects and resistant pathogen development.1,3
Sepsis management has remained challenging, with the administration of antimicrobials requiring not only proper choice but also quantitatively appropriate doses and dosages. As such, pharmacokinetic/pharmacodynamic (PK/PD) parameters have been established using changes in blood antimicrobial concentrations and minimum inhibitory concentration (MIC) against the pathogen. However, such parameters are not always provided. Therefore, a statistical technique called Monte Carlo simulation has been used to determine better definitive treatments.4
This study aimed to retrospectively investigate the practice of multiple blood culture sampling, bacteria detected in blood cultures, appropriateness of antimicrobial choice, doses and dosages in the empiric and definitive use of antimicrobials, and practice of de-escalation and to analyze their associations with 30-day mortality among patients with sepsis. Moreover, we sought to examine annual changes in these factors as performance indices for the ASP.
Methods
Participants and Sepsis Diagnosis
From 2011 until 2020, 1581 patients with bacteremia (age, 16–90 years) were hospitalized at Hirosaki University Hospital and diagnosed with sepsis as defined below. Patients with an uncertain prognosis 30 days after being diagnosed with sepsis, as well as those who experienced sudden death due to cardiovascular disorders, massive gastrointestinal hemorrhage, or stroke, were excluded.
The diagnosis of sepsis was essentially based on bacteremia confirmed by at least a single blood culture sample with any manifestation of infectious disease. The presence of Staphylococcus species (except for S. aureus), Bacillus species, Corynebacterium species, Propionibacterium species, or Clostridium perfringens in only one culture bottle indicated contamination, with such cases being excluded. When the aforementioned bacteria were positive in multiple bottles and the attending physician administered antimicrobials in consideration of clinical findings (eg, increased inflammatory markers with symptoms such as high fever and shivering and/or sequential organ failure assessment [SOFA] score ≥ 25), the patient was considered having sepsis. Even a single detection from multiple bottles for bacteria other than those mentioned earlier was considered significant, with such cases considered as sepsis. When a combination of several antimicrobials was used in the same patient for multiple targeted pathogens that could not be covered with a single antimicrobial, antimicrobial targeting of the most likely causative bacteria was conducted according to previous results obtained from blood and any other specimen cultures. Ultimately, 1406 cases were analyzed in this study.
Data Collection
All clinical data collected from the clinical records of patients from September 26th, 2021 to December 30th, 2021 were accessed for this research.
Multiple blood culture sampling was defined as conducting two sets of blood cultures in a single collection. We defined malignant diseases as including cancers and blood disorders such as leukemia.
Blood samples were cultured using the BacT/ALERT® 3D Blood Culture System (bioMérieux, Tokyo, Japan). Pathogens were identified using the VITEK®2 system (bioMérieux) from 2011 to 2014 and a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry–based Biotyper (Bruker Daltonics GmbH, Bremen, Germany) together with the Microscan WalkAway®96 plus system (Beckman Coulter, CA, USA) from 2015.
Definition of Ineffective Use or Underdosing of Antimicrobials
WalkAway was used for measuring MICs according to the Clinical and Laboratory Standards Institute (CLSI) guideline of each year. Antimicrobial susceptibility evaluation was based on the breakpoint set for each bacterial species according to CLSI, using the following classifications: “Susceptible”, “Intermediate”, and “Resistant”. The “Ineffective use of antimicrobial” was defined as “Intermediate” or “Resistant” susceptibility to the pathogens. We defined methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative staphylococci (MRCNS), extended-spectrum beta-lactamase (ESBL)-producing enterobacterium, AmpC-producing enterobacterium, carbapenem-resistant enterobacterium, and multidrug-resistant Pseudomonas aeruginosa as multidrug-resistant bacteria. Vancomycin-resistant enterococci and multidrug-resistant Acinetobacter baumannii were not detected in this study.
PK/PD parameters were calculated according to age, sex, body weight, serum creatinine (Cr), MIC, and doses and dosages by using the “BMs-Pod_ver8.05” application for Monte Carlo simulation.6–8 This application can calculate the PK/PD parameters and target attainment percentage (TA%) of an objective PK/PD parameter value. The PK/PD parameters included the percentage time above the MIC (%TAM) for β-lactams; area under the curve (AUC)/MIC for fluoroquinolones, vancomycin, teicoplanin, and linezolid; and peak serum concentration (Cpeak)/MIC for aminoglycosides. As the objective PK/PD parameter value, %TAM was set at 50% for penicillin, 60% for cephalosporin and cefmetazole, and 40% for carbapenem;9 the AUC/MIC was set at 125, 400, 15, and 100 for fluoroquinolones,10,11 vancomycin,12,13 teicoplanin,14,15 and linezolid,16–18 respectively; and Cpeak/MIC was set at 9 for aminoglycosides.19–21 A TA% of less than 80% to the objective PK/PD parameter value indicated “underdosing of antimicrobial” according to previous data.9
Definition of de-Escalation
We defined de-escalation as a reduction in the number of multiple antimicrobials or a change to another agent with a narrower spectrum within 5 days from the first empiric antimicrobial administration. Beta-lactams, fluoroquinolones, and aminoglycosides were divided into three classes: very broad, moderately broad, and narrow (Table 1). Details regarding this classification are described in the Discussion section. Conversely, the addition of another antimicrobial to the empiric therapy or a change in the first antimicrobial to a broader-spectrum agent within 5 days from the first administration indicated escalation.
 |
Table 1 A Spectrum Classification for β-Lactams, Fluoroquinolones, and Aminoglycosides |
Data Analysis
Data were analyzed using BellCurve for Excel version 3.22 (Social Survey Research Information Co., Saitama, Japan). The clinical characteristics were compared between two groups, using Student’s t-test or Welch’s t-test (when data variances were unequal) for parametric data and the chi-square test for alternative data (Table 2).
 |
Table 2 A Comparison of Characteristics Between the Recovery Group and the Death Group (Within 30 Day After the Onset of Bacteremia) |
Associations between certain factors and mortality within 30 days after the bacteremia onset were determined using hierarchical multiple logistic regression analysis (Table 3). Here, 30-day mortality (recovery = 0; death = 1) was a dependent variable. Initially, various factors (ie, clinical data, laboratory data including microbiological data, antimicrobials used as empiric and definitive therapies) were entered as independent variables, except for the underdosing and ineffective use of antimicrobials, escalation, and de-escalation. Thereafter, these independent variables were narrowed down to significant factors through multiple logistic regression analysis using forward–backward stepwise selection (stepwise method) (Model 1). Factors identified as significant in Model 1 and other additional factors were entered as independent variables, which were then analyzed by multiple logistic regression using the enter method (Model 2). Next, the difference in goodness of fit between adjacent models were evaluated using the change in “-2 log likelihood” values along with the change in degrees-of-freedom values. The variance inflation factor (VIF) was used to test for potential multicollinearity. None of the regression analyses produced VIF values exceeding 10, indicating no multicollinearity in our models. The significance level was set at 0.05.
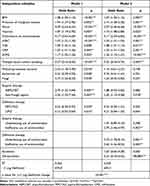 |
Table 3 Hierarchical Multiple Logistic Regression Analysis Predicting the Mortality (Recovery = 0, Death = 1) Within 30 Days After the Onset of Bacteremia |
Results
A total of 1406 patients were diagnosed with sepsis, of which 179 died (12.7%). Table 2 lists the characteristics of the recovery and death groups (within 30 days after the onset of bacteremia). The death group had significantly more frequent clinical symptoms of shock, hypoxia, and consciousness disturbances (72.1%, 70.9%, and 55.9%, respectively) than the recovery group (30.2%, 34.2%, and 15.3%, respectively). Values for white blood cell (WBC), creatinine (Cr), total bilirubin (T-Bil), and C-reactive protein (CRP) (14,480 ± 19,344/µL, 2.19 ± 5.75 mg/dL, 2.95 ± 5.83 mg/dL, and 14.7 ± 10.3 mg/dL) were also significantly higher in the death group than in the recovery group (10,333 ± 7605/µL, 1.35 ± 1.53 mg/dL, 1.17 ± 2.11 mg/dL, and 9.9 ± 10.1 mg/dL, respectively), but the platelet counts were lower (134.1 ± 112.3 vs 194.4 ± 134.0×103 /μL). Furthermore, the death group had significantly higher SOFA scores than the recovery group (5.5 ± 2.7 vs 2.6 ± 2.2). Conversely, the practice of multiple blood culture sampling was significantly more common in the recovery group than in the death group (88.8% vs 79.3%). In the cultured pathogens, only fungi were significantly more frequent in the death group than in the recovery group (12.3% vs 5.0%). Regarding empiric therapy, antifungal agents were used more frequently in the death group than in the recovery group (16.2% vs 8.0%). In definitive therapy, meropenem (MEPM) and antifungal agents were used more frequently in the death group (31.3% and 17.3%) than in the recovery group (21.4% and 9.0%, respectively). The underdosing and ineffective use of antimicrobials in empiric therapy were more frequent in the death group (22.9% and 30.2%) than in the recovery group (8.1% and 10.8%, respectively), similar to those in definitive therapy (death group: 24.6% and 15.6%; recovery group: 7.1% and 3.2%, respectively). Escalation was also more frequent in the death group (7.3%) than in the recovery group (2.7%), whereas de-escalation was less frequent in the death group (4.5% vs 19.4%).
Table 3 shows the identified predictors of mortality within 30 days after the bacteremia onset through hierarchical multiple logistic regression analysis. The first regression analysis using a stepwise method recognized the factors listed in Model 1 as candidate mortality predictors. Model 1 showed that in empiric therapy, body mass index (BMI), malignant disease presence, shock, hypoxia, consciousness disturbances, WBC, T-Bil, CRP, SOFA score, multidrug-resistant bacteria, ampicillin/sulbactam (ABPC/SBT), and antifungal agents demonstrated significant positive associations with 30-day mortality. The multiple blood culture sampling practice, piperacillin/tazobactam (PIPC/TAZ) and cefmetazole (CMZ) in definitive therapy showed significant negative associations with 30-day mortality. With the addition of six factors (ie, underdosing and ineffective use of antimicrobial in empiric and definitive therapies, escalation, and de-escalation), Model 2 showed that the ineffective use of antimicrobials in empiric therapy and underdosing and ineffective use in definitive therapy exhibited significant positive associations with 30-day mortality, whereas de-escalation showed a negative association. Notably, the p-value for the change in Δ−2 log-likelihood was less than 0.001, suggesting that Model 2’s accuracy was significantly superior to that of Model 1.
Ultimately, Model 2 identified the following as significant factors associated with mortality: BMI (odds ratio [OR], 1.07), malignant disease presence (OR, 2.15), shock (OR, 3.97), hypoxia (OR, 1.74), consciousness disturbances (OR, 4.17), WBC (OR, 1.03), T-Bil (OR, 1.08), CRP (OR, 1.03), SOFA score (OR, 1.26), multiple blood culture sampling practice (OR, 0.42), antifungal agents in empiric therapy (OR, 2.30), ineffective use of antimicrobials in empiric therapy (OR, 2.70), underdosing and ineffective use of antimicrobials in definitive therapy (OR, 3.72 and 3.65), and de-escalation (OR, 0.23).
Therefore, multiple logistic regression analysis predicting the ineffective use of antimicrobials in empiric therapy was conducted using the stepwise method. Multidrug-resistant bacteria (OR, 6.83), Enterococcus species (OR, 2.46), and fungi (OR, 3.32) had significant positive associations with ineffective use in empiric therapy (Table 4). Regarding definitive therapy, Enterococcus species (OR, 2.08), carbapenems except for MEPM (ie imipenem/cilastatin, doripenem, biapenem, panipenem/betamipron) (OR, 2.95), and ABPC/SBT (OR, 2.56) had significant positive associations with underdosing (Table 5), whereas multidrug-resistant bacteria (OR, 17.0) and Enterococcus species (OR, 4.53) had significant positive associations with ineffective use (Table 6).
 |
Table 4 Multiple Logistic Regression Analysis Predicting Ineffective Use of Antimicrobial in Empiric Therapy |
 |
Table 5 Multiple Logistic Regression Analysis Predicting Underdosing Use of Antimicrobial in Definitive Therapy |
 |
Table 6 Multiple Logistic Regression Analysis Predicting Ineffective Use of Antimicrobial in Definitive Therapy |
Figure 1 shows a year-on-year comparison of multiple blood culture sampling, 30-day mortality, percentages of ineffective use in empiric therapy, underdosing and ineffective in definitive therapy, and de-escalation by attending physicians and AST recommendations. Multiple blood culture sampling was very infrequent in 2011–2012 (59.2%) but increased significantly thereafter (84.3%, 90.3%, 96.8%, and 96.0% in 2013–2014, 2015–2016, 2018–19, and 2019–2020, respectively). The 30-day mortality rate was 18.3% in 2011–2012 and decreased subsequently (15.3%, 11.5%, 11.7%, and 9.7% in 2013–2014, 2015–2016, 2017–2018, and 2019–2020, respectively). The ineffective use of antimicrobials in empiric therapy was very frequent in 2011–2012 (21.1%) but decreased significantly thereafter (14.8%, 13.0%, 10.8%, and 9.9% in 2013–2014, 2015–2016, 2017–2018, and 2019–2020). In definitive therapy, underdosing was very frequent in 2011–2012 (18.8%) and 2013–2014 (13.6%) but decreased significantly thereafter (6.3%, 6.7%, and 5.6% in 2015–2016, 2018–2019, and 2019–2020, respectively). Ineffective use was also very frequent in 2011–2012 (8.9%) but decreased significantly thereafter (5.1%, 4.5%, 3.8%, and 3.2% in 2013–2014, 2015–2016, 2018–2019, and 2019–2020, respectively). Moreover, the percentage of de-escalation increased significantly with each passing year (3.8%, 9.3%, 15.4%, 25.6%, and 31.1% in 2011–2012, 2013–2014, 2015–2016, 2017–2018, and 2019–2020, respectively), even after excluding de-escalation according to AST recommendations (2.0%, 7.7%, 7.8%, and 11.5% in 2013–2014, 2015–2016, 2017–2018, and 2019–2020, respectively).
 |
Figure 1 Year-on-year comparisons of multiple blood culture sampling, 30-day mortality, ineffective use in empiric therapy, underdosing and ineffective use in definitive therapy, and de-escalation. |
Discussion
According to trends in sepsis incidence and outcomes based on a Japanese nationwide database (2010–2017), the inhospital rate and median hospitalization duration were reported to be significant (18.3% and 27 [15–50] days).23 However, whether single or multiple blood sampling data, blood culture data, and information on the antimicrobials used were not reported. Therefore, we investigated the mortality rate of sepsis in 30 days and the factors of mortality in our hospital over 10 years from the perspective of appropriate antimicrobial use.
In comparing between the sepsis recovery group and the death group, all factors included in the SOFA scoring were more prevalent in the death group, obtaining higher SOFA scores. In the multiple logistic regression analysis, shock, hypoxia, consciousness disturbances, T-Bil, WBC, and CRP were associated with 30-day mortality. Therefore, to early detect sepsis and determine the systematic severity, clinicians should pay attention to the abovementioned factors and perform early blood culture and antimicrobial administration.
The practice of multiple blood culture sampling was significantly lower in the death group than in the recovery group. When the patient has an abnormal temperature (>38.0 or <36.0 °C) or is clearly displaying shivering, multiple sets of blood cultures should be collected as rapidly as possible.24 Multiple blood culture sampling was identified as a significant negative factor for 30-day mortality (OR, 0.42). Performing it before antimicrobial treatment is reportedly a major prognostic factor in patients with sepsis.25 Interestingly, this approach was inversely associated with the ineffective use of antimicrobials in empiric therapy, and both underdosing and ineffective use in definitive therapy. Clinicians who do not practice multiple blood culture sampling would tend to be unfamiliar with the proper use of antimicrobials.
In the blood culture results, only fungi were detected more frequently in the death group, and multidrug-resistant bacteria and fungi were identified as significant positive factors for 30-day mortality, and Escherichia coli as a negative factor. Wisplinghoff et al conducted a prospective nationwide surveillance study involving 24,179 cases and reported that the crude rate of sepsis by Candida species was the highest and that by E. coli was the second lowest (the first, coagulase-negative staphylococci).26 In empirical therapy, antifungal agent use was more common in the death group than in the recovery group and was a significant factor in logistic regression analysis predicting mortality. Antifungal agents are often administered before culture results are available in patients with significant immunodeficiency.
Furthermore, the ineffective use of antimicrobials in empiric therapy was identified as a significant factor for 30-day mortality (OR, 2.70). International guidelines recommend the empiric use of broad-spectrum antimicrobial therapy in patients with severe infections to minimize the risk of initial inadequate antimicrobial treatment.27 Before the blood culture results, antimicrobial therapy that does not cover the actual causative organism during empiric therapy is occasionally unavoidable. Multiple logistic regression analysis showed that multidrug-resistant bacteria, Enterococcus species, and fungi had significant positive associations with ineffective use in empiric therapy. Particular attention should be paid to Enterococci such as E. faecium because they may exhibit resistance to carbapenems. To cover all of these microorganisms at the empiric therapy stage, clinicians need to administer not only carbapenems but also anti–MRSA drugs and antifungal agents. However, this intervention poses a risk of overprescribing antimicrobials and an increased risk for multidrug-resistant pathogens, making empiric therapy challenging. If the patient is immunocompromised and all of the mentioned microorganisms are being covered, de-escalation is required after obtaining blood culture results.
Japanese clinicians who are unfamiliar with the current proper use of antimicrobials would tend to prescribe an inappropriately lower antimicrobial dose. Our results demonstrated that underdosing in definitive therapy was a significant factor for 30-day mortality (OR, 3.72). Carbapenems except for MEPM, and ABPC/SBT showed significant positive associations with underdosing in definitive therapy. Although our hospital predominantly utilizes imipenem/cilastatin (IPM/CS) among the carbapenems except for MEPM, the administered dose was often modest, accounting for the adverse effects of renal toxicity and convulsion.28,29 The AST has recommended administering sufficient amounts of MEPM rather than a modest dose of IPM/CS when selecting carbapenem. For patients without severe symptoms, our hospital commonly used ABPC/SBT at insufficient doses and dosages, thereby often requiring an intervention by the AST. AST should confirm whether appropriate doses are being administered for routinely used antimicrobials in the hospital.
Naturally, the ineffective use in definitive therapy was identified as a significant factor for 30-day mortality (OR, 3.65). Its cause, unfortunately, could be the oversight of culture results for multidrug-resistant bacteria and carbapenem-resistant enterococci. While there has been improvement over the years, there have been cases where the opinions of the AST were not heeded or overlooked by the attending physician.
De-escalation is an important aspect of ASP.30,31 However, while broadly recommended, its definition remains unclear, with little guidance available on best evidence-based practices for de-escalation.32 We categorized the antimicrobials often used in Japan as “very broad”, “moderately broad”, or “narrow”, according to spectrum (Table 1). This classification was determined comprehensively, considering the spectrum score from the study by Madaras-Kelly,22 the antimicrobial rankings from two de-escalation studies,22,33 and our hospital antibiogram. We think that the classification should rely on the susceptibility rates of each antimicrobial in each country, region, and hospital. The antimicrobial ranking from Weiss’ study33 showed that carbapenems were ranked higher than PIPC/TAZ and fourth-generation cephalosporins according to not only spectrum but also resistance-promoting potential. However, carbapenems and PIPC/TAZ would offer similar spectrum coverage, except for extended spectrum β-lactamase and AmpC β-lactamase. In 2020, the Japan Nosocomial Infection Surveillance reported that the detection rates for third-generation cephalosporin-resistant E. coli and Klebsiella species were 3.39% and 0.65%, respectively, in Japanese hospitals.34 Therefore, we are hesitant to define a switch from carbapenems to PIPC/TAZ (or fourth-generation cephalosporins) as a complete de-escalation, suggesting the need for repeated de-escalation from PIPC/TAZ.
De-escalation is supported by the results of numerous observational studies.25,35 However, the International Guidelines for Management of Sepsis and Septic Shock 2021 suggested that daily assessment for antimicrobial de-escalation over using fixed durations of therapy without daily reassessment for de-escalation was only a weak recommendation (very low quality of evidence).36 Although our results showed a significant negative association between de-escalation and mortality (OR, 0.23), most of the de-escalation studies on mortality in patients with sepsis have been observational, with concerns that de-escalation has been practiced primarily in patients with an improving condition; hence, caution is required when interpreting reported short-term improvements.32,37
In year-on-year comparisons, the 30-day mortality rate decreased, in line with the increase in multiple blood culture sampling and de-escalation and the decrease in ineffective use in empiric therapy and underdosing and ineffective use in definitive therapy. These results are consistent with the Model 2 results in Table 3.
Study Limitations
The aim of this study is to evaluate the appropriateness of empiric and definitive therapy by focusing on cases where bacteria believed to be non-contaminants were identified in the initial blood culture conducted after the suspicion of sepsis. Regarding the submission of blood cultures, we examined whether the initial blood culture was submitted as a single set or two or more sets. Therefore, the number of repeated blood cultures performed after the initiation of empiric treatment was not included in the analysis. The study does not include many cases where sepsis was diagnosed based on factors such as the SOFA score, yet bacterial identification could not be achieved.
Second, this study was conducted only in our hospital; thus, some biases in terms of antimicrobial use could be unavoidable.
Third, our study could not analyze the duration of administration, given that our hospital is a university hospital with several immunocompromised patients. Hence, this study included many such cases for which antimicrobials had been administered for a long period before the onset of bacteremia and after the improvement of vital signs, symptoms, and laboratory data.
Finally, in this study, we specifically investigated the presence or absence of malignant diseases, including cancers and blood disorders such as leukemia, concerning underlying diseases. Due to the frequent difficulty in distinguishing whether the direct cause of death is attributable to a malignant disease or an infectious disease, cases of death within 30 days due to underlying diseases (malignant diseases) would be also included in this study. Indeed, the presence of malignant disease was identified as a factor for 30-day mortality (OR, 2.15).
Conclusion
The 30-day mortality rate of patients with sepsis was associated with multiple blood culture sampling, ineffective use of antimicrobials in empiric therapy, both underdosing and ineffective use in definitive therapy, and de-escalation practice. To improve the appropriateness of using antimicrobials in the future, the AST should actively intervene along with their educational efforts.
Abbreviations
AST, Antimicrobial Stewardship Team; ICT, Infection Control Team; ASP, Antimicrobial Stewardship Programs; PK/PD, pharmacokinetic/pharmacodynamic; MIC, minimum inhibitory concentration; SOFA, Sequential Organ Failure Assessment; CLSI, Clinical and Laboratory Standards Institute; TA%, target attainment percentage; %TAM, percentage time above the minimum inhibitory concentration; AUC, area under the curve; Cpeak, peak serum concentration; VIF, variance inflation factor; R2, coefficient of determination; CCr, creatinine clearance; J-SSCG, Japanese Clinical Practice Guidelines for the Management of Sepsis and Septic Shock.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, Saito N, upon reasonable request.
Ethics and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hirosaki University Graduate School of Medicine (approval number 2021-078). This study protocol was approved by the Institutional Review Board (IRB). The hospital patient data we used in the study were anonymized to parameters agreed by the governance of Hirosaki University Graduate School of Medicine. As the study comprised solely of retrospective data collected by Hirosaki University Hospital, a waiver of informed consent was obtained from the IRB. Caretakers, legal guardians, and relatives of the patients included in this study were given the opportunity to opt out of the study at any time.
Acknowledgments
We gratefully acknowledge Ms. Kitsu A (laboratory technician) and Ms. Yasuda A (nurse in the ICT) for cooperating with works of ICT and AST in Hirosaki University Hospital.
Funding
The authors received no specific funding for this work.
Disclosure
None of the authors has any conflicts of interest to declare.
References
1. Campion M, Scully G. Antibiotic use in the intensive care unit: optimization and De-Escalation. J Intensive Care Med. 2018;33:647–655. doi:10.1177/0885066618762747
2. CDC. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. Available from: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html.
3. Seok H, Jeon JH, Park DW. Antimicrobial Therapy and antimicrobial stewardship in sepsis. Infect Chemother. 2020;52:19–30. doi:10.3947/ic.2020.52.1.19
4. Ambrose PG, Grasela DM. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2000;38:151–157. doi:10.1016/s0732-8893(00)00185-1
5. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–774. doi:10.1001/jama.2016.0288
6. Oda K. Development of software for antimicrobial PK/PD simulation incorporating Montecarlo simulation based on Microsoft® Office Excel. Iryo Yakugaku. 2011;37:335–344. doi:10.5649/jjphcs.37.335
7. Yamada T, Minami K, Oda K, et al. Probability of target attainment of oral antimicrobials for Escherichia coli and Klebsiella pneumoniae based on Monte Carlo simulations. Diagn Microbiol Infect Dis. 2022;103:115662. doi:10.1016/j.diagmicrobio.2022.115662
8. Yamada T, Ooi Y, Oda K, et al. Observational study to determine the optimal dose of daptomycin based on pharmacokinetic/pharmacodynamic analysis. J Infect Chemother. 2020;26:379–384. doi:10.1016/j.jiac.2019.11.002
9. Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis. 2003;36(Supplement 1):S42–50. doi:10.1086/344653
10. Lacy MK, Lu W, Xu X, et al. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob Agents Chemother. 1999;43:672–677. doi:10.1128/AAC.43.3.672
11. Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi:10.1128/AAC.37.5.1073
12. Moise-Broder PA, Forrest A, Birmingham MC, et al. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi:10.2165/00003088-200443130-00005
13. Yasuhara M, Iga T, Zenda H, et al. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit. 1998;20:139–148. doi:10.1097/00007691-199804000-00003
14. Ogawa R, Kobayashi S, Sasaki Y, et al. Population pharmacokinetic and pharmacodynamic analyses of teicoplanin in Japanese patients with systemic MRSA infection. Int J Clin Pharmacol Ther. 2013;51:357–366. doi:10.5414/CP201739
15. Assandri A, Bernareggi A. Binding of teicoplanin to human serum albumin. Eur J Clin Pharmacol. 1987;33:191–195. doi:10.1007/BF00544566
16. Sasaki T, Takane H, Ogawa K, et al. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob Agents Chemother. 2011;55:1867–1873. doi:10.1128/AAC.01185-10
17. Tsuji Y, Hiraki Y, Matsumoto K, et al. Pharmacokinetics and protein binding of linezolid in cerebrospinal fluid and serum in a case of post-neurosurgical bacterial meningitis. Scand J Infect Dis. 2011;43:982–985. doi:10.3109/00365548.2011.600327
18. Brier ME, Stalker DJ, Aronoff GR, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother. 2003;47:2775–2780. doi:10.1128/AAC.47.9.2775-2780.2003
19. Xuan D, Nicolau DP, Nightingale CH. Population pharmacokinetics of gentamicin in hospitalized patients receiving once-daily dosing. Int J Antimicrob Agents. 2004;23:291–295. doi:10.1016/j.ijantimicag.2003.07.010
20. Ng PK. Determining aminoglycoside dosage and blood levels using a programmable calculator. Am J Hosp Pharm. 1980;37:225–231.
21. Kirby WM, Clarke JT, Libke RD, et al. Clinical pharmacology of amikacin and kanamycin. J Infect Dis. 1976;134:S312–5. doi:10.1093/infdis/135.supplement_2.s312
22. Madaras-Kelly K, Jones M, Remington R, et al. Development of an antibiotic spectrum score based on Veterans Affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35:1103–1113. doi:10.1086/677633
23. Imaeda T, Nakada TA, Takahashi N, et al. Trends in the incidence and outcome of sepsis using data from a Japanese nationwide medical claims database-The Japan Sepsis Alliance (JaSA) study group. Crit Care. 2021;25:338. doi:10.1186/s13054-021-03762-8
24. Zitek T, Bourne M, Raber J, et al. Blood culture results and overtreatment associated with the use of a 1-hour sepsis bundle. J Emerg Med. 2020;59:629–636. doi:10.1016/j.jemermed.2020.06.055
25. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40:32–40. doi:10.1007/s00134-013-3077-7
26. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi:10.1086/421946
27. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi:10.1097/CCM.0000000000002255
28. Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008;4:1065–1074. doi:10.1517/17425255.4.8.1065
29. Wong VK, Wright HT, Ross LA, et al. Imipenem/cilastatin treatment of bacterial meningitis in children. Pediatr Infect Dis J. 1991;10:122–125. doi:10.1097/00006454-199102000-00009
30. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis. 2016;62:e51–77. doi:10.1093/cid/ciw118
31. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: a prospective interventional study. Med Intensiva. 2018;42:266–273. doi:10.1016/j.medin.2017.07.002
32. Tabah A, Bassetti M, Kollef MH, et al. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020;46:245–265. doi:10.1007/s00134-019-05866-w
33. Weiss E, Zahar JR, Lesprit P, et al. Elaboration of a consensual definition of de-escalation allowing a ranking of beta-lactams. Clin Microbiol Infect. 2015;21:649.e1–10. doi:10.1016/j.cmi.2015.03.013
34. Japan nosocomial infection surveillance (JANIS). Annual Report of Public Information for January to December 2020. Available from: https://janis.mhlw.go.jp/report/open_report/2020/3/1/ken_Open_Report_202000_200over.pdf.
35. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014;40:1399–1408. doi:10.1007/s00134-014-3411-8
36. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063–143. doi:10.1097/CCM.0000000000005337
37. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62:1009–1017. doi:10.1093/cid/civ1199
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
