Back to Journals » International Journal of Nanomedicine » Volume 17
Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment
Authors Yang Y, Zheng X, Chen L, Gong X, Yang H, Duan X, Zhu Y
Received 21 December 2021
Accepted for publication 20 April 2022
Published 6 May 2022 Volume 2022:17 Pages 2041—2067
DOI https://doi.org/10.2147/IJN.S355142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Yan Yang,1,* Xi Zheng,1,* Lu Chen,1 Xuefeng Gong,2 Hao Yang,2 Xingmei Duan,1 Yuxuan Zhu1
1Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Academy of Medical Science & Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China; 2POWERCHINA Chengdu Engineering Corporation Limited, Chengdu, 611130, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuxuan Zhu, Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Academy of Medical Science & Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China, Email [email protected]
Abstract: Cancer is the second leading cause of death in the world, behind only cardiovascular diseases, and is one of the most serious diseases threatening human health nowadays. Cancer patients’ lives are being extended by the use of contemporary medical technologies, such as surgery, radiotherapy, and chemotherapy. However, these treatments are not always effective in extending cancer patients’ lives. Simultaneously, these approaches are often accompanied with a series of negative consequences, such as the occurrence of adverse effects and an increased risk of relapse. As a result, the development of a novel cancer-eradication strategy is still required. The emergence of nanomedicine as a promising technology brings a new avenue for the circumvention of limitations of conventional cancer therapies. Gold nanoparticles (AuNPs), in particular, have garnered extensive attention due to their many specific advantages, including customizable size and shape, multiple and useful physicochemical properties, and ease of functionalization. Based on these characteristics, many therapeutic and diagnostic applications of AuNPs have been exploited, particularly for malignant tumors, such as drug and nucleic acid delivery, photodynamic therapy, photothermal therapy, and X-ray-based computed tomography imaging. To leverage the potential of AuNPs, these applications demand a comprehensive and in-depth overview. As a result, we discussed current achievements in AuNPs in anticancer applications in a more methodical manner in this review. Also addressed in depth are the present status of clinical trials, as well as the difficulties that may be encountered when translating some basic findings into the clinic, in order to serve as a reference for future studies.
Keywords: gold nanoparticles, cancer, drug delivery, photothermal therapy, photodynamic therapy
Introduction
In recent decades, a growing number of people are suffering from cancer. According to the data from GLOBOCAN 2020, there were about 19.3 million new cancer cases and 10.0 million cancer-related deaths occurred around the world.1 The number of new cases and death cases from cancer is predicted to rise to 22 million and 13 million annually, respectively, by 2030.2 Currently, there are many effective therapies against cancer in clinic, among which surgery, radiotherapy (RT) and chemotherapy are the most leading treatment modalities.3 Although surgery is usually considered the most effective therapy at the early stage of disease, it is difficult to achieve satisfactory results at the later stage of cancer because cancerous cells have spread throughout the body.4 Even some patients may undergo cancer recurrence after the operation and have short survival time.5 Similar to surgery, RT is also barely efficient for metastatic tumors and may result in the recurrence of cancer. Chemotherapy is regarded as the most valuable approach for most patients with metastatic and late staged cancer, since chemotherapeutic drugs can reach every organ in the body via the bloodstream.6 However, the widespread distribution of chemotherapeutic drugs might also lead to additional effects on normal tissues. Since chemotherapeutic drugs are not limited to cancer cells, large dosage and repeated administration are required for ideal results, which also leads to undesired side effects.7 These drawbacks, in combination with drug resistance development and poor aqueous solubility of drugs have emerged as significant problems restricting the clinical utilization of chemotherapeutic medications currently available.8 Therefore, there is an urgent need for new strategies and devices to treat and diagnose cancer more efficiently and accurately.
Various drug delivery systems have been developed during the last few decades to enhance the anti-tumor efficacy of chemotherapy drugs. It is feasible to deliver medications selectively into the target locations using a specialized delivery vector, hence reducing the likelihood of adverse effects. The use of nanotechnology in drug delivery systems gave reason to believe that this goal would be reached in the near future. For instance, the Food and Drug Administration (FDA) of the United States approved albumin-bound paclitaxel (nab-paclitaxel), a solvent-free nanoformulation of paclitaxel as a powerful therapy in patients with metastatic breast cancer in 2005. In the United States, the nab-paclitaxel has been approved for the treatment of many solid tumors, including breast cancer, non-small-cell lung cancer, and pancreatic cancer.9 More investigations for the treatment of other solid tumors based on the nab-paclitaxel are ongoing. Because of their unique physicochemical features, nanoparticles (NPs) have garnered a great deal of interest in recent decades and were believed to be one of the most promising drug delivery vectors. Given that solid tumors have wider vascular cell gaps (200 nm-1.2 µm in size) than normal tissues (<10 nm in size),10,11 the NPs loading drugs may readily cross leaky tumor vascular walls and then be accumulated into tumor areas, as a result of the enhanced permeability and retention (EPR) effect.12–14 More important, surface modification of NPs by introducing targeting molecules that bind to overexpressed receptors or antigens on the target cells also favors the increase in intratumoral accumulation of NPs. Both the passive (the EPR effect) and active (conjugating targeting molecules) approaches can help decrease the accumulation of available chemotherapy drugs at non-target sites, reducing unwanted side effects. Therefore, the use of nanoparticles as transport carriers brings a potential avenue for the circumvention of limitations of conventional cancer therapies.
Noble metal nanoparticles, especially gold nanoparticles (AuNPs), have drawn more and more attention in medical field, with particular emphasis on oncology in recent studies.15 First of all, AuNPs have multiple geometric shapes and sizes (Figure 1), which can be controlled through simple synthetic approaches.16–18 As well, many physical and chemical properties of AuNPs, such as catalytic ability, melting point, electric conductivity and color, can be controlled by changing their shape, size and even surrounding environment.19 Such design flexibility for ideal performance and a specific application is difficult to be achieved in organic nanoparticles. Moreover, AuNPs have unique size- and shape-dependent optical nature, owing to the coherent oscillation of free electrons, so-called surface plasma resonance (SPR) effect.20 Due to the optical property, AuNPs have been developed as ideal nano-objects, such as biosensors, imaging agents and photothermal agents for medical diagnosis, imaging and treatment, which is relatively infrequent for the other inorganic nanomaterials. Meanwhile, large surface area and high surface activity of AuNPs endow the ability of functionalization and high loading amounts. AuNPs can directly or indirectly conjugate and interact with various molecules, including drugs, nucleic acids (DNA or RNA), proteins or peptides, antibodies, targeting ligands, and other molecules (Figure 2).21 The coupling possibility and diversity largely improve their biological activities and broaden the range of their potential anticancer applications. Furthermore, AuNPs have been found to be relatively stable in physiological medium because of the modification of amphiphilic materials,22 and biocompatible due to inert nature of metallic gold.23 All of these features have rendered AuNPs increasingly popular nano-vectors in oncology.
 |
Figure 1 Different sizes and shapes of AuNPs. The sizes and shapes of AuNPs can be controlled through simple synthetic methods. |
This review focuses on various widely utilized AuNPs applications in cancer treatment and diagnostics, including drug and nucleic acid delivery, photodynamic therapy (PDT), photothermal therapy (PTT), and X-ray computed tomography (CT) imaging, among others. In addition, possible combination therapy for full tumor suppression should be investigated further. Apart from preclinical studies and data, current breakthroughs in AuNPs in clinical trials were also thoroughly reviewed and discussed in detail. Additionally, also discussed in detail are the existing obstacles that are preventing AuNPs from receiving FDA approval, with the goal of providing a theoretical contribution to the clinical translation of AuNPs.
AuNPs in Cancer Therapy
Due to their unique optical properties and conjugating variety, AuNPs show great potential in cancer therapy. In this section, we will discuss in detail the therapeutic applications of gold nanoparticles for malignant tumors, including drug and nucleic acid delivery, PDT, PTT and possible combination therapies. Currently, these applications are still under development.
AuNPs in Drug/Nucleic Acid Delivery
It has been proven that AuNPs have several fundamental properties when serving as drug delivery carriers. According to the EPR effect, the nanoscale particles can preferentially accumulate at tumor sites. In addition, surface functionalization can be easily achieved since AuNPs have negative surface charges and strong covalent affinity for thiol, amino and carboxyl. It means that AuNPs can bind with various molecules, including drugs, nucleic acids, antibodies and targeting ligands, which shows potential for active targeted delivery. These features render AuNPs promising candidates as drug carriers. Herein, we will present the utilization of AuNPs with regard to drug delivery and nucleic acid delivery.
Drug Delivery
In 2004, Paciotti and co-workers first reported the use of colloidal gold as delivery vectors.24 They conjugated tumor necrosis factor (TNF) onto the surface of AuNPs, with the aim of delivering the TNF to the tumor tissue growing in mice. It was shown that the AuNP-TNF conjugate actually possessed higher tumor accumulation and consequent lower toxicity in healthy organs when compared to native TNF.24 Thereafter, the use of AuNPs as delivery tools has been explored deeply. Nowadays, it has been reported that AuNPs had the ability to deliver multiple types of antitumor molecules (Table 1), including synthetic compounds,25 phytochemicals,26,27 therapeutic peptides28,29 and coordination compounds.30,31 These antitumor molecules have cytotoxic or regulating effects on cancer cells but some drawbacks such as low solubility, short half‑life, the development of drug resistance and weak tumor selectivity limit their practical applications. One of the effective approaches is to conjugate the anticancer molecules to nanoparticles, particularly AuNPs with a “hard” core.
 |
Table 1 Examples of Gold Nanoparticles for Various Anticancer Drug Delivery |
Doxorubicin (DOX) is one of the most frequently used anticancer drugs, but it is highly likely to induce drug resistance in tumor cells. In some studies, DOX could conjugate with stabilizer-modified AuNPs through either non-covalent or covalent interactions.32–34 Studies suggested that the connection favored the intracellular accumulation of the DOX in drug-resistant cancer cells, demonstrating the possibility of bypassing drug resistance in the case of conjugation (Figure 3).34 The mechanism by which drug resistance could be avoided by nanoparticle-mediated conjugation may be related to different internalization mechanisms. The internalization mechanism of free DOX is different compared with the conjugated DOX that enter cells by endocytosis approach, avoiding P glycoprotein related drug resistance, as it was suggested by Wojcik et al32 5-fluorouracil (5-FU) is another powerful antineoplastic drug, whose highly polar nature limits its topical use in the treatment of skin cancer. Delivery of 5-FU by cetyltrimethylammonium bromide (CTAB)-stabilized AuNPs could gain about 2-fold higher skin permeability compared with the free 5-FU formulation and achieve 6.8- and 18.4-fold lower tumor volume compared with the negative group (Figure 4).35 It indicated that attaching hydrophilic drugs to AuNPs can contribute to an increase in the skin permeability and consequent drug efficacy against skin cancer. This may have something to do with the use of stabilizer CTAB with positive electricity.36 It is worth noting that stabilizers or spacers seem not to be indispensable in the structure of the conjugates. Some drugs with specific groups can directly link with AuNPs, such as methotrexate (MTX) with carboxylic groups.37 Even the simplest conjugate (MTX-AuNPs) also showed enhanced cytotoxic effect against cancer cells, while free MTX exhibited reduced anticancer effect at equal doses.37,38
 |
Figure 3 Intracellular distribution of free DOX and Au-SS-DOX in HepG2-R cells. Confocal images of cells treated with (A) free DOX and (B) Au-SS-DOX showing distribution of DOX-derived fluorescence (red). (C) Intracellular DOX fluorescence intensity in HepG2-R cells after exposure to free DOX and Au-SS-DOX for 24 hours. Reprinted from Nanomedicine, 8(2), Gu YJ, Cheng J, Man CW, Wong WT, Cheng SH. Gold-doxorubicin nanoconjugates for overcoming multidrug resistance. 204–211, copyright 2012, with permission from Elsevier.34 Abbreviations: DOX, doxorubicin; SS, disulfide linkage. |
 |
Figure 4 Schematic diagram for AuNPs synthesis and 5-FU loading using CTAB, and growth curves of A431 tumors in C57BL/6 mice. Reprinted with permission from Safwat MA, Soliman GM, Sayed D, Attia MA. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol Pharm. 2018;15(6):2194–220.35 Abbreviations: 5-FU, 5-fluorouracil; CTAB, cetyltrimethylammonium bromide; GNPs, gold nanoparticles. |
It is known that the selectivity of drugs for specific tissues and organs has become a major challenge in recent years. The development of drug delivery systems may be a crucial solution to overcome this problem. AuNPs are one of the most popular drug delivery platforms, based on their negative surface and high affinity with many functional groups, especially thiol. For active targeting, the most classic and valuable approach is still introducing targeting ligands onto the surface of AuNPs.39 The working principle of this approach lies in taking advantage of the affinity of certain substrates for specific receptors. As a fact, compared with normal cells, cancer cells with a higher metabolic rate can over-express certain receptors that attract and contact substances to achieve their metabolite.40 The delivery carrier carrying drugs must have a selective affinity for cancer cells, so it is highly possible for drugs loaded to be selectively transported to the cancer cells with a desired dose. Based on this, several targeting molecules have been chosen, such as transferrin (Tf),41 pectin (PEC),42 hyaluronic acid (HA),43 folic acid (FA),44,45 and galactose (Gal).46 Additionally, less stable oligonucleotide fragments can also be used as targeting ligands. It has been reported that by using targeting element nucleolin aptamer (AS1411), a guanosine-rich single-stranded DNA (ssDNA), both DOX and aptamer against Forkhead box M1 (FOXM1 Apt) were selectively co-delivered into cancer cells.47 In return, AuNPs could also protect unstable nucleic acid fragments against degradation by nucleases.48,49 Besides, some therapeutic drugs that belong to monoclonal antibodies (mAbs) are inherently targeting, such as cetuximab (C225),50 trastuzumab (Tmab).51 The conjugation between mAbs and AuNPs is more important for cancer treatment compared to the mAb-based monotherapy. Except for those effects mediated by mAbs alone, the conjugation can lead to other cytotoxic effects, such as oxidative stress and autophagy caused by AuNPs,52 which can largely increase the overall efficacy. By loading another therapeutic drug, not only targeted delivery of the drug but also a combined antibody/drug effect were achieved.53 These investigations demonstrated that the AuNPs modified with targeting ligands are largely useful for the targeted delivery of anticancer drugs.
Nucleic Acid Delivery
Gene therapy is an ideal method to prevent and treat cancer by using foreign DNA and RNA. Compared to small-molecule drugs, nucleic acid drugs are largely labile. On the one hand, the nucleic acid drugs are vulnerable to various environmental risks, such as enzymatic, chemical and physical degradation during gene manipulation and gene transfection.54 On the other hand, such drugsas biologic agents are prone to immunogenicity and are consumed readily by innate immune cells. Therefore, ideal delivery vehicles are required to deliver such drugs into cells, to prevent nucleic acid drugs from degradation and have a better transfection effect.55 Currently, viral vector systems are very popular for gene delivery but can activate the host’s immune response, which reduces efficiency of future gene therapy.56 Delivery of nucleic acids via non-viral vectors systems, such as gold nanoparticles, can avoid this problem. In comparison to viral vectors, surface design of AuNPs is more flexible, which aids in functionalization and biocompatibility in the body.56 Moreover, AuNPs can protect nucleic acid from nuclease degradation and physical damage54 and show more than 99% cellular uptake in spite of surface negative charge.57
Based on the properties above, AuNPs conjugated with nucleic acid can be used for gene silencing therapy in tumor model. For instance, Tunc et al embedded morpholino antisense oligonucleotides into a DNA-tile-AuNPs structure for treatment of breast cancer. They found that the DNA-tile-AuNPs structure delivered morpholinos and silenced the expression of HER2 and ERα gene in breast cancer cells more effectively than the liposome-based system.58 Besides, due to the photothermal effect of AuNPs, the conjugate has the ability to become a dual functional delivery nanoplatform that achieves simultaneously gene silencing and photothermal therapy.59 The complex still has a good photothermal effect even after nucleic acid functionalization. The composite significantly inhibited tumor growth without overt side effects for major organs after laser exposure.60 Furthermore, AuNPs can load simultaneously gene and chemotherapy drugs to achieve a synergistic effect. Huang et al prepared a multifunctional nanoplatform based on AuNPs, which co-delivered microRNA-122 and DOX, achieving triple therapy (photothermal therapy, chemotherapy and gene therapy). With the aid of polyethylene glycol (PEG) and HA, this delivery system could selectively target hepatoma carcinoma cells without toxicity to the main organs and showed a better antitumor effect than any single therapy.61
The release of DNA from the gold nanocomplex can be triggered by exogenous light. Upon laser irradiation, the heat generated by AuNPs through the photothermal effect is transmitted to the ambient DNA molecules. When the temperature reaches the threshold, the chemical linkages break, thus leading to DNA release.62 Interestingly, the specific DNA release mechanisms induced by continuous wave (CW) versus pulsed lasers are different. Upon CW laser irradiation, high temperature results in dehybridization between double-stranded DNA (dsDNA) and release of nonthiolated ssDNA, while upon pulsed laser illumination, the entire DNA molecules are liberated through Au-S bond cleavage (Figure 5).63 The discrepancy in release mechanism makes cell mortality rate different. In a work, an anticancer drug docetaxel (DTX) was inset into complementary dsDNA that was first attached to gold nanoshells (silica core) through the Au–thiol bond for the treatment of breast cancer. The CW laser-induced DTX release caused a significant increase in breast cancer cell death, while the pulsed laser-induced drug release resulted in unobvious cell death (Figure 6).64 Accordingly, AuNPs can be used as a promising genetic drug delivery vector, achieving multifunctional anti-cancer therapy.
 |
Figure 5 NIR light-induced DNA release. CW irradiation results in dehybridization between dsDNA and release of nonthiolated ssDNA, while pulsed irradiation results in Au−S bond cleavage and release of entire DNA. Reprinted with permission from Goodman AM, Hogan NJ, Gottheim S, Li C, Clare SE, Halas NJ. Understanding Resonant Light-Triggered DNA Release from Plasmonic Nanoparticles. ACS Nano. 2017;11(1):171–179.63 Abbreviation: hv, irradiation with light. |
 |
Figure 6 Comparison of cell viability after DTX release from a DNA host without (blue) and with (Orange) CW and pulsed lasers in (A) MDA-MB-231 and (B) RAW 264.7 cells. *P<0.05, **P<0.01, and ***P<0.001. Reprinted with permission from Goodman AM, Neumann O, Norregaard K, et al. Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc Natl Acad Sci U S A. 2017;114(47):12419–12424. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.64 Abbreviations: CW, continuous wave; DTX, docetaxel; NS, nanoshell. |
AuNPs in Photodynamic Therapy
More than 100 years ago, photodynamic therapy has been first proposed. Nowadays, it has developed into a relatively safe and effective therapeutic modality, especially for skin diseases.65 The PDT includes three important elements: a photosensitizer (PS), light with an appropriate wavelength and molecular oxygen (O2). The original PS molecule can be activated easily by light to transform into an excited singlet state. However, the unstable excited singlet PS easily loses spare energy in the form of heat production or emission of light to turn into an excited triplet state. Compared to the excited singlet state, the excited triplet state is more stable, so that it has sufficient time to undergo photochemical reactions in the presence of O2 to produce reactive oxygen species (ROS) such as singlet oxygen (1O2).66 The overproduction of ROS triggers oxidative stress, causing cell death by cell apoptosis or necrosis. The PDT takes advantage of this working principle to damage diseased cells. However, most of the PSs are low water-soluble and tend to aggregate in physiological media, largely affecting the quantum yield of 1O2. Another drawback of PSs is their non-specific biodistribution in vivo, which may cause unwanted systematic side effects. Hence, to obtain the ideal PDT result, it is crucial to ensure the stability and tumor selectivity of the PS molecules in vivo.
Conjugating the PSs on the AuNP surface is an effective method to overcome the above drawbacks. It has been reported that incorporating hydrophobic porphyrin derivatives to the AuNPs that were first modified with hydrophilic PEG chains rendered it possible to obtain a higher aqueous solubility.67,68 Meanwhile, the water-soluble, porphyrin-containing conjugate was shown to produce higher 1O2 compared to free porphyrin derivatives, thus achieving increased PDT results in cell model.67,68 A similar effect could also be achieved in the case of conjugation of AuNPs with meso-tetrahydroxyphenylchlorin (mTHPC), a hydrophobic second-generation photosensitizer.69 Modifying hydrophilic molecules onto the AuNPs bound to hydrophobic drugs is of great significance to improve the solubility and stability. This strategy has enabled higher death rates of cancer cells compared to free PS molecules. For some water-soluble second-generation PSssuch as methylene blue (MB)70 and 5-aminolevulinic acid (5-ALA),71 they fortunately bypass the problem associated with solubility of first-generation PSs. However, the hydrophilic nature limits the ability to cross lipophilic cell membranes, consequently decreasing the intracellular accumulation of the water-soluble PSs and PDT efficacy. It was confirmed that the 5-ALA when bound to AuNPs grafted with targeting moiety Arg-Gly-Asp (RGD) peptide, could significantly increase its intracellular amount and exhibit the stronger photodynamic cytotoxicity than free 5-ALA.72 This result can be attributed to the integrin (specific receptor of the RGD peptide) mediated endocytosis effect, which thus causes the increase in intracellular 5-ALA amount and PDT efficacy. Different from the 5-ALA, the MB can easily cross the cell membrane in spite of the hydrophilicity due to the special structure of plane benzene ring.73
Targeted delivery of PS molecules using AuNPs for PDT is an important strategy for the selective treatment of malignant tumors. Dixit and co-workers constructed a nanocomplex (Tfpep-coated PEGylated AuNPs) to selectively deliver photodynamic prodrug phthalocyanine 4 (Pc 4) to glioblastoma cells. Interestingly, they found that the efficiency of Pc 4 delivery and PDT was superior over untargeted (AuNPs-Pc 4) NPs, confirming the potential of the Tfpep-AuNPs for targeted delivery to glioblastoma cells with high Tf receptor expression level (Figure 7).74 In other studies, authors used the same PEGylated Au nanostructures to load Pc 4. By conjugating the targeting molecules to AuNPs, selective delivery of the Pc 4 into the cancer cells was achieved.75,76 Savarimuthu et al synthesized AuNPs conjugated photosensitizer protoporphyrin IX (PpIX) equipped with FA, which is efficiently taken up by Vero and HeLa cells due to the presence of the FA and thus exhibit superior phototoxicity compared to the Au-PpIX electrostatic and covalent complexes.77 In 2018, Calavia et al prepared Pc-AuNPs with lactose as a Pc-releasing nanosystem, where the targeting ability of the lactose towards galectin-1 receptors overexpressed on breast cancer cells was assessed in vitro. It was shown that the lactose-Pc-AuNPs possessed higher cytotoxicity in comparison with the control Pc-sPEG-AuNPs.78 In several studies, small AuNPs (<10 nm) and photosensitizer verteporfin are co-encapsulated into different organic nanocarriers via different methods, whose surfaces then were modified with triphenylphosphonium, a cationic mitochondrial targeting moiety.79,80 Upon X-ray radiation, the nanocomplexes generated cytotoxic 1O2 within the mitochondria, leading to the alteration of mitochondrial membrane potential and increase in the level of cell apoptosis, but with greatly reduced radiation doses and radiation side effects.79,80 In conclusion, AuNPs bound to the PS and targeting warheads have great application prospects in terms of targeted PDT.
 |
Figure 7 Tfpep-Au NPs-Pc 4 accumulate in orthotopic brain tumors in vivo. (A) Representative in vivo fluorescence hotmap images of a mouse implanted with an orthotopic glioma injected with Tfpep-Au NPs-Pc 4 over time. Bar graph quantifies uptake of Tfpep-Au NPs-Pc 4 versus Au NPs-Pc 4. (B) Mice were analyzed for free Pc 4 fluorescence (RFU) 6 hours post injection. (C) Brains of glioma mice were excised and examined for ex vivo fluorescence (hotmap) after 6 hours. (D) Fluorescence imaging 24 hours post injection of ex vivo organs from mice injected with Au NPs-Pc 4 or Tfpep-Au NPs-Pc 4. Reprinted with permission from Royal Society of Chemistry. Dixit S, Novak T, Miller K, Zhu Y, Kenney ME, Broome AM. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale. 2015;7(5):1782–1790.74 Abbreviations: Au NPs, gold nanoparticles; Pc 4, phthalocyanine 4; Tfpep, transferrin. |
AuNPs in Photothermal Therapy and in Combined Therapy
Beyond PDT, photothermal therapy is another attractive light-activated application of AuNPs for cancer treatment. It is reported that AuNPs have the ability to absorb light to produce localized high temperatures (>43 °C), based on the SPR effect of free electrons, ultimately resulting in thermal damage for cancer cells.81–83 Importantly, AuNPs can change their absorption and scattering cross sections and spectrums (from visible to near-infrared (NIR) region) by changing their size, shapes and adjacent environment.84,85 For instance, the transition efficiency of rod-shaped AuNPs can be tuned by changing their aspect ratio of length to diameter.86 A similar effect can also be achieved in shell-shaped AuNPs by changing the relative ratio of core radius and shell thickness.87,88 All of these characteristics endow AuNPs with outstanding advantages over other nanomaterials that are also used as photothermal agents. However, PTT using visible light is usually limited to superficial tumors due to their short wavelengths and consequent weak tissue penetration ability. By contrast, the NIR region is more important since the long wavelengths allow deeper light penetration into living tissues with less tissue damage.55,89
Hyperthermia involves cancer cell death through two mechanisms, namely cell apoptosis and necrosis. Generally, hyperthermia induces cell apoptosis by irreversible mitochondrial damage and ROS overproduction.90 Some investigations, however, have found that the hyperthermia can also induce cell necrosis by cell membrane damage and protein denaturation.91,92 Markovic et al thought that the mechanisms of thermal damage of cancer cells were apparently related to induced oxidative stress and depolarization of mitochondrial membrane, eventually resulting in mixed apoptotic/necrotic cancer cell death.93 In fact, using a single PTT usually cannot completely eradicate the tumor owing to the intratumoral uneven and uncontrollable heat distribution; the surviving cancer cells fast develop resistance to thermal stress, jointly leading to tumor metastasis and recurrence.94,95 To further improve the efficacy of cancer treatment, PTT has been developed to combine with other therapies, such as chemotherapy, PDT and RT.11,96,97
With regard to the combination of PTT and chemotherapy, many types of gold nanocomplexes exhibited strikingly enhanced cytotoxicity and tumor inhibition effect in comparison with a single application of PTT or chemotherapy.98–101 A good example is the AuNPs modified with aptamer AS1411 and hairpin DNA loading with DOX, which were used for targeted and synergistic chemo-PTT.102 Upon 808 nm NIR laser illumination (1 or 2 W/cm2), the viability of colon cancer cells was found to dramatically decrease when compared to separate application of PTT and chemotherapy (Figure 8).102 The NIR laser not only generated the photothermal effect of gold nanomaterials, but also accelerated the release of chemotherapeutic drugs from AuNP-based nanocomplexes, enhancing the synergistic treatment effect at the target site.103 In addition, a high concentration of glutathione (GSH)104 and a weak acidic condition105 are also responsible for a controlled release of the loaded drugs. Importantly, the combination therapy effectively avoided side effects caused by high doses of chemotherapeutic agents.106 Even under sub-therapeutic doses, combined therapy also shows a better antitumor efficacy.107,108 The synergistic effect between hyperthermia and drugs may be attributed to the following mechanisms. First of all, high temperatures can delay the repair pathways of DNA damage induced by chemotherapeutic agents, stabilizing chemotherapy-caused DNA damage response.109 Moreover, hyperthermia can increase the permeability of tumor vasculature and blood flow, which increases the accumulation of chemotherapeutic drugs within tumor tissue.110,111 Furthermore, hyperthermia can inhibit the expression of drug resistance-related transporters, mainly P-glycoprotein (P-gp) through high expression of heat shock factor-1 (HSF-1) trimers, and then provide the possibility of overcoming multidrug resistance (MDR).112 The gold nanocomplex in combination with drugs can merge dual therapeutic functions simultaneously under laser irradiation, thus giving rise to synergistic therapeutic effects.
 |
Figure 8 CCK-8 assay of different formulations. (A & B) Cytotoxicity and IC50 of free DOX, AAHD-NPs. (C) The cell viability of SW480 or L929 cell treated with different NPs. (D) Cytotoxicity of chemotherapy and PTT effect by a 808 nm laser irradiation (1 W/cm2 or 2 W/cm2) for 10 min. *P < 0.05. **P < 0.01. Reprinted from Biomed Pharmacother, 130, Zhang Y, Zhou L, Tan J, Liu J, Shan X, Ma Y. Laser-triggered collaborative chemophotothermal effect of gold nanoparticles for targeted colon cancer therapy. 2020;10492. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.102 Abbreviations: AAH, gold nanoparticles modified with AS1411 and DNA; AAHD, gold nanoparticles modified with AS1411 and DNA loading with doxorubicin; APHD, gold nanoparticles modified with DNA loading with doxorubicin; DOX, doxorubicin; IC50, half-inhibitory concentration; NPs, nanoparticles; PTT, photothermal therapy. |
Another interesting aspect is that PTT can be combined with PDT to achieve a synergistic anticancer treatment. As mentioned above, PTT alone cannot completely annihilate tumors. Likewise, a single PDT may do not attain a satisfactory result. For one thing, the PDT treatment usually uses light with limited wavelengths (<700 nm), which are difficult to penetrate deep tumor tissue.113 For another thing, ample 1O2 generation requires sufficient oxygen supply within tissue, but the hypoxic state of tumor microenvironment often makes the PSs ineffective in the area.114 Fortunately, it has been corroborated that a combination of PTT and PDT could complement one another to provide better therapeutic effects than a single PTT or PDT.115–117 The reason may be that hyperthermia caused by PTT can increase oxygen amount within tumor tissue via boosting blood flow to improve the generation of 1O2, and in turn 1O2 generation caused by PDT can enhance cytotoxicity induced by PTT.118 However, combined PTT/PDT treatment often requires two kinds of different wavelength lights, which causes complexity of treatment and limits clinical transformation. Hence, it is necessary to develop a novel PTT and PDT agent that can be activated by a single wavelength light. For this purpose, Liu’s group designed captopril-stabilized Au nanoclusters (Au25(Capt)18) as the PTT and PDT agent and chose 808 nm NIR light as the single light source. They found the Au25(Capt)18 had a quick temperature increasing rate (within 5 min) and continuous 1O2 generation (at least 1 hour) under NIR laser exposure (Figure 9).118 The results suggested that modified AuNPs can be used as a single agent which performs concurrently PTT and PDT under single wavelength light irradiation.
 |
Figure 9 Photothermal and photodynamic effects of Au25(Capt)18. (A) Thermal images of Au25(Capt)18 aqueous solution at different Au concentrations under laser irradiation (808 nm, 2 W/cm2, 5 min); also, DI water under the same condition was set as the control. (B) Temperature curves in (A). (C) Temperature changes in (A). (D) Photostability evaluation by measuring the temperature curves of Au25(Capt)18 aqueous solutions after 4 heating–cooling cycles under repeated laser irradiation (2 W/cm2, 5 min for each). (E) Generation of 1O2 by Au25(Capt)18 via recording the fluorescence emission spectra at 530 nm under laser irradiation at different time points (1 W/cm2, 1–70 min). (F) Fluorescence intensities with irradiation time increasing in (E); (G) Generation of 1O2 by Au25(Capt)18 via recording the fluorescence intensities at 530 nm under laser irradiation with different power densities for 5 min. Reprinted with permission from Royal Society of Chemistry, Liu P, Yang W, Shi L, et al. Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J Materials Chem B. 2019;7(44):6924–6933.118 Abbreviation: SOSG, singlet oxygen sensor green. |
Furthermore, a collaborative effect also can be achieved by the application of both PTT and RT together. The combination of PTT and RT promotes an increase in cancer cell lethality, even at a low nanoparticle concentration.97 Additionally, heating the tumors with NIR laser to 48 °C before RT could reduce the X-ray dose required to control the tumor, thus protecting adjacent normal tissues from ionizing radiation-associated damage.119 On the other hand, using mild PTT after RT was able to inhibit the self-repair of damaged DNA induced by RT by down-regulating the expression of DNA repair proteins, thus improving radiosensitive efficiency.120 Accordingly, the dual PTT/RT therapy was indeed more effective than the use of PTT alone or RT alone.
Triple therapy seems more necessary for complete tumor inhibition, as evidenced by the fact that its therapeutic efficacy is obviously superior to that of any monotherapy or dual therapy. Although various dual therapeutic therapies (chemotherapy/PTT, PTT/PDT, PTT/RT) based on AuNPs have been vigorously developed, the triple therapy of AuNP-based nanoplatforms is still in an infancy stage. Recently, several multifunctional nanoplatforms based on copper sulfide nanoparticles,121,122 bismuth sulfide nanoparticles,123 and especial AuNPs have been developed for triple-modality cancer therapy.124–126 The outcomes of these studies demonstrated the great potential of triple therapy to eradicate tumors compared to monotherapy and dual therapy. In 2018, Xu et al reported a pH-responsive DOX and 5-ALA co-loaded gold nanorods (AuNRs) for enhanced breast cancer treatment through combined chemo/photothermal/photodynamic therapy.127 This complex nanosystem was constructed by first decorating AuNRs with mercaptopropionylhydrazide (MPH) and thiol-terminated monomethoxy PEG (mPEG-SH) via Au-S bonds, and subsequently linking onto MPH molecules with chemotherapeutic agent DOX and pro-photosensitizer 5-ALA through acid-liable hydrazone bonds, termed asAuNRs-MPH−ALA/DOX-PEG. The cumulative release amounts of DOX and 5-ALA from AuNRs-MPH−ALA/DOX-PEG were higher in phosphate buffer solution (PBS) at 37 °C at pH 5.0 than at pH 7.4 (eg 58% vs 3% of DOX, 71% vs 6% of 5-ALAwithin one day). In vitro and in vivo studies showed that the resulting GNRs-MPH−ALA/DOX-PEG could kill human breast cancer MCF-7 cells and inhibit tumor growth without overt side effects more efficiently, respectively, through a superadditive antitumor effect.127 Previously, a novel multifunctional nanoplatform based on gold nanocages (AuNCs) with NIR stimuli-responsive DOX and photosensitizer indocyanine green (ICG) release was also developed for simultaneous chemo/photothermal/photodynamic therapy of breast cancer.128 In another work, Xu et al reported the preparation and in vitro and in vivo evaluation of a new nanocomposite based on HA modified AuNCs (AuNCs-HA) forsynergetic RT/PTT/PDT of breast cancer. In agreement with results of reports on chemotherapy/PTT/PDT, the AuNCs-HA exhibited complete tumor growth inhibition, compared to each monotherapy or dual therapy.129 These studies provide new avenues for triple therapy of malignant tumors, which represents a feasible and simple strategy for constructing ideal cancer-aimed nanoplatforms that integrate multiple treatment functionalities.
AuNPs in Cancer Imaging
Imaging technologies can provide anatomical information of tissues with accuracy and specificity, which is indispensable for clinicians to diagnose diseases. Many studies have reported AuNPs have promising potential applications in cancer detection, especially in the fields of CT imaging. Therefore, AuNPs-based CT imaging technology will be elaborated in detail in this part.
Nowadays, CT imaging has become a widely used diagnostic tool due to its high efficiency and fairly low cost. The X-ray-based CT technology can take advantage of differences in X-ray attenuation to reconstruct 3D images of subject tissues.130 Tissues with different densities will present different X-ray attenuation, particularly between healthy and diseased tissues.131,132 Thus imaging and distinguishing the interface between two neighboring tissues or imaging soft tissues in contact with physiological fluids such as blood is very challenging unless using contrast agents.133 The most commonly used CT contrast agent currently remains iodine-based compounds, such as iohexol.39 However, short imaging times caused by rapid renal clearance and side effects at iodine concentrations required for imaging limit their use.133,134 Hence, it is necessary to develop novel materials as CT contrast agents. This need has driven the development of AuNPs as promising alternatives. Due to higher atomic number and electron density of gold (79 and 19.32 g/cm3), AuNPs exhibit a higher X-ray absorption coefficient than iodine-based contrast agents.133 Importantly, AuNPs have prolonged vascular retention time due to high molecular weight and increased intratumoral accumulation for enhanced CT imaging.135,136 These favorable characterizations, in combination with good biocompatibility make the AuNPs an ideal contrast candidate for CT imaging.
In a study, PEGylated hollow AuNPs (mPEG@HAuNPs) with a diameter of 63.4 nm were chosen as contrast agents for CT imaging studies in vivo. The results showed a sustained contrast enhancement at the tumor area in the mPEG@HAuNPs group. Comparatively, no inherent contrast created from the same doses of iohexol (0.6 mg/g) at the tumor site was observed at 12 h post-injection.137 Moreover, AuNPs can be multi-functionalized through modification on their surface, taking advantage of the interaction between sulfur and gold atoms. By exploiting this characteristic, it is possible to link multiple targeting moieties on the surface, which can contribute to the selectivity of the contrast agents towards specific tissues. For instance, the FA was conjugated onto AuNPs through a cysteamine (Cys) linking, to target against human nasopharyngeal KB cancer cells. The authors found that the FA-Cys-AuNPs conjugates led to a 2.03-times increase in the attenuation of X-ray intensity in comparison to AuNPs alone (Figure 10).138 Likewise, Beik et al also conjugated the FA to AuNPs for targeted CT imaging of KB cells. They found the FA-modified AuNPs had benefits of image contrast enhancement as well as radiation dose reduction.139 Hence, the AuNPs modified with FA can be used not only for targeted CT imaging to detect cancer cells overexpressing FA receptors but also for X-ray-based RT where RT needs to increase the radiation dose of X-rays.
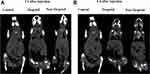 |
Figure 10 Coronal CT images of nude mice after intravenous injection of AuNPs and FA-Cys-AuNPs at 3 hours (A) and 6 hours (B) (blue arrows indicate the tumor site and red arrows indicate other sites). The injection of the AuNPs (either non-targeted or targeted) leads to an enhanced CT contrast of the tumor area, and 3 h post injection shows a maximum CT enhancement of the tumor area. Reprinted from Int J Biochem Cell Biol, 114, Khademi S, Sarkar S, Shakeri-Zadeh A, et al. Targeted gold nanoparticles enable molecular CT imaging of head and neck cancer: an in vivo study. 105554, Copyright 2019, with permission from Elsevier.138 |
Multimode imaging is essential for clinicians to identify diseased tissues as it can offer more accurate and ample imaging information than single imaging modalities. By combining other contrast-enhancing agents with gold nanomaterials, it is possible to exploit novel systems that provide contrast for CT imaging along with other imaging approaches, such as magnetic resonance imaging (MRI)140 and NIR fluorescence imaging.141 Ge’s group developed a PEG modified nano-system (named as PEG-LnAu NPs) incorporating 10 nm AuNPs and lanthanide ions (Gd3+ and Yb3+) for CT/MR dual mode imaging. The multifunctional nanoplatform (PEG-LnAu NPs) showed good performance in both MRI and CT imaging and could be used as a positive-contrast agent for dual mode imaging.142 Kuhn’s team reported a versatile nano-system loading two imaging enhancing elements (gold and magnetic iron oxide NPs) for tri-modal (MR, CT and intravascular ultrasound (IVUS)) imaging. In vitro results showed that the nanoprobe has concentration-dependent contrast-enhancing ability for MR, CT and IVUS imaging, without toxicity to cells even at a maximum concentration of 100 μg/mL.143 Moreover, the use of FA modified AuNPs loaded with other imaging materials for targeted tumor dual mode imaging has also been successfully reported.141,144
AuNPs in Clinical Trials
As described above, AuNPs do show potentially useful properties in many preclinical studies. Nevertheless, there are few examples associated with AuNPs being under clinical trials, and, till now, no AuNP-containing formulations are used successfully in clinical practices. Table 2 exhibits a list of clinical trials of AuNPs for disease treatment and diagnosis to date.
 |
Table 2 List of Clinical Trials of Gold Nanoparticles for Disease Treatment and Diagnosis154 |
Aurimune (CYT-6091) is the most typical example of AuNPs used in clinical testing for cancer treatment. The nanocomplex is a PEGylated 27-nm AuNP containing recombinant human tumor necrosis factor alpha (rhTNFα). TNFα is a potent anticancer agent, but its extreme side effects largely limit its clinical use.145 Here, by conjugating the rhTNFα onto the colloidal AuNPs to deliver the rhTNFα to tumor sites, it is favorable to ameliorate the systemic toxicity of TNFα.24 Moreover, the PEG coating is introduced to the surface of colloidal gold particles, prolonging circulation time in blood and accumulation of colloidal gold particles in the tumor sites. Nowadays, its Phase I clinical trial (NCT00356980) has been successfully completed and published, where clinical safety profile of this drug was demonstrated. The trial results showed that the CYT-6091 at the dose tested (50–600 μg/m2) was safely tolerated and a maximum tolerated dose was not reached even at the highest dose (600 μg/m2). No severe side effects occurred except that first two patients experienced controllable fevers. As for efficacy, although it was not the overarching objective of this study, one patient underwent a partial response and other four patients displayed stable disease.146 The encouraging results obtained here have initiated the Phase II clinical study to further demonstrate its clinical efficacy.
Auroshell is another example of AuNPs under clinical trials for cancer treatment. Auroshell is a silica-gold core shell nanoparticle with a total diameter of 144–150 nm, which is developed by Nanospectra Sciences Inc to thermally ablate the localized tumors.147 In Aurotherapy, the Auroshell particles accumulate in the tumor tissues via the EPR effect and then are activated by an external NIR laser, without effect to adjacent normal tissues. Therefore, photothermal cancer therapy is an effective and highly selective approach with minimal side effects. These efforts build on many previous testing.148–150 Preclinical biosafety of the Auroshell particles has been assessed in a series of vitro and vivo studies, where the particles were well tolerated and toxicity was not observed after intravenous infusion.151 Furthermore, an initial clinical trial based on the Auroshell particles was performed in 22 patients with prostate cancer, whose results also suggested excellent tolerability in human beings. It was found that only 2 adverse events (an itching and a burning sensation of the epigastrium) were judged to be associated with the particle infusion.152 After confirming safety profile of the Auroshell particles, researchers further assessed the feasibility and safety of the AuroShell-mediated laser ablation in combination with MR/ultrasound fusion imaging in 16 low- or intermediate-grade prostate cancer men.153 Corresponding with previous findings, a patient suffered from transient epigastric pain during the particle infusion. Except for this, no other adverse events occurred. With respect to treatment efficacy, the tumor ablation zones were free in 62.5% of patients at 3 months and 87.5% of patients at 12 months.153 These clinical results revealed that the AuroShell-directed PTT is a relatively safe and feasible modality for the targeted treatment of prostate tumors. Now this company is recruiting participants with prostate cancer for another clinical study (NCT04240639), with the aim of the focal ablation of prostate tissue via Auroshell particle directed laser irradiation.154
Besides, several other gold nanoparticle-based agents have also completed preliminary clinical studies or are under clinical testing. Khoobchandani et al investigated an intervention of green nanotechnology in preclinical and clinical studies for human metastatic breast cancer therapy. In this work, authors have succeeded in clinical translation, from cells, animals to humans, of AuNP-based Nano-Ayurvedic drug: Nano Swarna Bhasma, which is composed of AuNPs and mangiferin (a phytochemical).155 Another pilot clinical trial (NCT01420588) related to AuNPs has been performed and published. Researchers developed a gold nanomaterial-based nanosensor and assessed the feasibility of a new avenue involving a breath test with the sensor for identifying gastric cancer from other gastric diseases.156 The completion of the pilot study has provided great confidence in the success of an ongoing multicenter clinical trial.
Moreover, due to the excellent photothermal conversion efficiency, AuNPs show great promise in other fields other than oncology. Pang et al developed a hydrogel eye patch that was composed of AuNR core and palladium shell and assessed safety and effectiveness in animal and human tests. It was found that the hydrogel eye patches could heat up spontaneously in response to various visible light, thus producing more tears to relieve the dry eye and moisturize the eyelid skin.157 In another example, the safety and feasibility of two delivery approaches for treatment of coronary atherosclerosis were evaluated (NCT01270139). The first is a PTT method using silica-gold NPs and the second is a magnetic navigation approach with delivery of silica-gold iron bearing NPs. The obtained results showed that both delivery approaches have an acceptable level of safety for clinical practice.158
Challenges of AuNPs in Clinical Applications
Although the preclinical and initial clinical studies are encouraging, there are still several important issues that need to be fully clarified prior to clinical use of AuNPs. It is reported that toxicity is the most important problem amongst them. Despite several studies indicating that AuNPs were relatively low toxic due to chemical inertness of metal gold,159–162 the toxicity produced by AuNPs have been demonstrated in multiple cell and animal models. Many factors, no doubt, are able to largely affect their biodistribution in vivo and eventual toxicity, such as fundamental features of particles (eg, particle size, shape, surface charge, and coating), experimental conditions (eg, cell and animal model tested, assessed duration), administration scheme (eg, administration route, dose, time and times) and so forth (Figure 11). Thereby, the results may be diverse and even contradictory sometimes. This, along with the heterogeneity among individual tissues and cells, makes it intricate and challenging to utterly comprehend their interplay with the living organisms. Hence, toxicity profile of AuNPs and other reasons decelerating clinical translation of the AuNPs will be described fully in this part.
Particle size has been reported to impact toxicity of AuNPs, wherein smaller particles were observed to be more toxic than the larger ones.163,164 This may be attributed to the fact that small nanoparticles cross the cell membrane and the nucleus pore more easily, thus favoring the intracellular ROS generation and DNA damage.165,166 However, at a more early time point, Chen et al found that AuNPs of 8–37 nm induced severe disease in BALB/C mice after the AuNPs were injected intraperitoneally, while AuNPs of 3, 5, 50, and 100 nm did not exhibit deleterious effects.167 The results may be related to urinary elimination and excretion since particles smaller than 5.5 nm can be removed rapidly and efficiently through urinary system from the body.168
Particle shape is equally thought to be an important factor in affecting AuNP toxicity. Comparative toxicity analysis among various shaped AuNPs has already been established. Nevertheless, opinions differ in the shape effect of nanoparticles on cells. In the view of Patibandla et al, AuNRs have more deleterious effects on zebrafish than spherical AuNPs.169 They attributed the toxicity of AuNRs to CTAB coating, which is an essential but toxic surfactant for the synthesis of AuNRs.170 Thus, the toxicity of AuNRs can be improved by coating them with alternative biocompatible materials, such as phosphatidylcholine and PEG,171,172 underlining the impact of surface coating materials on toxicity. However, Tarantola et al pointed out that spherical AuNPs are more toxic than rod-shaped particles due to the larger surface area ratio of spherical particles and thus higher intracellular gold content.173 In other studies, it was observed that non-spherical (star/flower-shaped) AuNPs had relatively stronger toxicity than spherical AuNPs.174,175 They attributed this outcome to the larger specific surface area presented by non-spherical AuNPs than spherical AuNPs. Higher is the internalization, more is the harmful substances carried into cells and severer is the cell damage. However, in another study, spherical and rod-shaped nanoparticles were observed to be more toxic than star-, flower- and prism-shaped AuNPs.176
It is equally important to consider the impact of surface charge on the toxicity of AuNPs. It was reported that positively charged particles were more toxic than negative or neutral counterparts.177,178 The toxicity of cationic AuNPs may be as a result of the presence of electrostatic interaction of positive NPs with a negative cell surface, thereby increasing cellular uptake or membrane disruption. The outcome is either beneficial or unintended, which depends on the research purpose and concrete application. Cho et al provided a different example of the effect of cationic AuNPs on two different cells. They found that a positively charged AuNP-dendron conjugate (PCD-AuNP) is no toxic towards two cells used even at the highest dose tested (40 μg/mL), but there is a visible attachment of the PCD-AuNPs on the monkey kidney Vero cell surface.179 The specific mechanism requires to be further researched and elucidated.
Furthermore, Bahamonde et al found that mice and rats responded differently to PEGylated AuNPs exposure, where mice experienced robust macrophage response and no fatality was seen, whereas abundant rats suddenly died within hours of administration.180 The results indicated AuNPs have species-specific differences in terms of toxicity, even amongst closely related groups. Ginzburg et al observed a synergistic increase in toxicity of PEG-stabilized AuNPs in the presence of the surfactant, while the components separately were no toxic.181 These results demonstrated the importance of identifying strategies for choosing safe NP/additive combinations. Additionally, Schwartz et al reported that 10-nm AuNPs had potential nephrotoxicity when were co-administrated with cisplatin, paraquat and 5-aminosalicylic acid due to their interactions with drugs, whereas AuNPs themselves were not hepatotoxic or nephrotoxic when alone administered.182 It is very clear that the dose at which particles are administered is critical for toxicity, since many drugs that are beneficial at low doses may exhibit harm at high doses. The administration route and assessed duration are also crucially important for toxicity.183–185 However, these conditions are diverse in different studies, and they are not always fully reported in the literature. Therefore, it is suggested that authors reported the employed conditions in as much detail as possible, which helps readers to comparatively analyze.
Although plentiful researchers have been attempting to figure out the toxicity profile of AuNPs in vivo, it is still difficult to draw consistent and meaningful conclusions from these reports owing to the variations in many parameters and conditions.186 As a result, the contradictory results were obtained in different studies with different experimental designs. Moreover, many studies merely considered toxicity evaluation for particles, without involving the possible toxicity mechanisms.187 Thereby there is an urgent demand for deeper studies to reveal the potential toxicity mechanism of AuNPs in vivo, not only at the cellular level but also at the molecular level. Furthermore, most results are built on short-term observation data, and long-term data are relatively scarce. Thus further long-term in vivo investigations should be implemented for more representative data. Lastly, no standardized method that is universally appropriate for toxicity testing of various types of AuNPs to date is also a matter of concern.188 The lack of a unified detection method and evaluation standard leads to the presence of conflicting results and varying interpretations. Therefore, it is recommended to establish uniform standards and methods for toxicological testing of nanoparticles, to facilitate comparisons of data among different studies and clinical translation of AuNPs.
Last but not least, it is worth considering the trade-off between underlying benefit and cost of production when designing new multifunctional formulations. The addition of new functionality, such as targeting and imaging contrast enhancement not only means an increase in patient survival time and available clinical benefit, but also additional cost and complexity of synthesis and purification.189 The production and conjugation of some ligands for targeting is greatly expensive and inefficient.190 Even though the cost related to adding imaging contrast materials may be relatively lower than adding targeting ligands, the costs would be likely to be shrunk partly and the benefits more profound, if the nanomaterial itself had both therapeutic and imaging abilities, such as AuNPs.189 Hence, the functionality of the materials themselves and design cost are also major concerns when constructing novel formulations.
Conclusion and Outlooks
Cancer is still a largely complex and serious disease. In addition to exploiting new therapeutic drugs, taking full advantage of available chemotherapy drugs is another feasible strategy. At present, the use of nanotechnology has had a crucial impact on many fields of science, including medicine, biology, physics and chemistry. Due to unique pathophysiological phenomenon of solid tumors and unique physicochemical properties of AuNPs, the use of AuNP-based delivery systems provided the potential for improved cancer treatment and diagnosis. In previous studies, AuNPs have been used as delivery carriers for various agents, especially cytotoxic drugs, unstable nucleic acid drugs, and hydrophobic/hydrophilic photosensitizers for PDT. In addition, AuNPs have been used for cancer phototherapy, such as PTT by using their light absorption properties. To complement their respective disadvantages and further improve the efficacy of cancer treatment, the AuNPs have been developed as a promising nanoplatform that integrates multiple treatment functionalities, like chemotherapy/PTT, PTT/PDT, PTT/RT, chemotherapy/PTT/PDT, and RT/PTT/PDT. In preclinical research, AuNPs have also been used as biological imaging contrast agents, particularly CT imaging, which helps clinicians recognize tumor conditions and choose applicable therapeutic strategies.
In addition to positive preclinical research, a series of AuNP-based nanoproducts (NCT00356980, NCT00848042, NCT01270139, NCT01246336, NCT02755870, NCT03020017, etc) have been used in clinical trials. These results achieved in the clinical studies are mostly positive and very encouraging. However, most of them are limited to early phase 1 or phase 1 clinical studies, and to date, no AuNP-based products have actually entered the late phase of the clinical process or have been successfully on the market. Some clinical trials are recruiting, withdrawn, and even terminated. Compared to other nano-formulations, like liposomes and polymeric micelles, the number of AuNP-based products in clinical trials is still relatively small. When basic preclinical studies are being conducted, more studies are required to devote to pushing basic experimental studies of AuNPs towards clinicalization.
Although the results achieved from many studies are very encouraging, there are several critical issues that deserve to be taken seriously into account. One important problem is that toxicity of AuNPs requires to be addressed properly. In spite of chemical inertness, gold is, after all, a noble metal and has inherently chemical toxicity to a certain degree. Introducing functional moieties such as stabilizing materials and biocompatible materials seems to reduce toxicity of AuNP core to some extent, but it is worth noting that a number of surface modifications may also cause unwanted side effects. Except for the nanoparticle core, some toxicity may be attributed to these modifying materials.Further research should be required to quantify the trade-off between treatment/diagnosis benefit and toxicity after functional modifications. It is equally important to consider whether and how the functional substances affect the biodistribution and consequent side effects. Similarly, if we want to avoid the plague of immunogenicity in vivo, surface modification technology requires to be further optimized. Another possible effect on biosafety is biodistribution of AuNPs, because AuNPs can accumulate in the spleen and liver, and other sites, hence showing toxic effects in these organs.There is need for further knowledge to understand the biodistribution profile of AuNPs.
Moreover, molecular interactions between gold nanoparticles with targets were scarcely reported. Such interactions include not only therapeutic/diagnostic effects but also toxicological aspects. A full understanding of nanoparticle-target interactions and potential mechanisms is greatly important in nanotechnology for improved cancer management.Furthermore, the specific mechanism that AuNPs are internalized by target cells also needs to be fully studied in diversified cell models. A comprehensive understanding of the intake mechanism is crucial for improved intracellular drug amount and eventual therapeutic effect. Although most of the current literature attributes the internalization mechanism of nanoparticles to endocytosis, not all AuNPs are suitable for this case. In agreement with the toxicity, the uptake mechanism of AuNPs by cells may depend on a variety of factors, such as particle size, shape, surface charge, coating, cell model tested and so on. However, so far there is no unified conclusion and explanation about the internalization mechanism of AuNPs in the academic circle.
For biomedical applications, especially in clinical practice, a requirement for the production of AuNPs with long-time stability is essential. Possible aggregation of AuNPs during preparation and use processes may pose further threats to the lives of patients. To avoid this, improved and reproducible manufacturing technologies are indispensable for large-scale production of AuNPs with high stability. In addition, there is still a need for adequately cost-effective AuNP-based systems. Adding additional functions means not only the increase in clinical benefit, but also elevated cost and complexity of synthesis and purification. The high cost places a financial burden on the cancer patients themselves and their families. Hence, in future work, more studies should be performed to develop newly budget-friendly functional AuNP-based products.
Taking all aspects into account, there is still a long way and there is more work to be done before AuNP-based products are successfully on the market. Nevertheless, existing evidence on potential of nanogold in cancer research field allows us to believe that we can develop more efficient and accurate cancer therapies based on AuNPs in the future. We also believe that, in the future, AuNP-based systems will play a more important role in early tumor diagnosis and tumor treatment in different stages, with the hope of circumventing the common drawbacks of currently available tumor therapies in clinic.
Abbreviations
1O2, singlet oxygen; 5-ALA, 5-aminolevulinic acid; 5-FU, 5-fluorouracil; AAH, gold nanoparticles modified with AS1411 and DNA; AAHD, gold nanoparticles modified with AS1411 and DNA loading with doxorubicin; anti-EGFR D-11, monoclonal antibody D-11 against epidermal growth factor receptor; APHD, gold nanoparticles modified with DNA loading with doxorubicin; Au25(Capt)18, captopril-stabilized Au nanoclusters; AuNCs, gold nanocages; AuNPs, gold nanoparticles; AuNRs, gold nanorods; BSA, bovine serum albumin; C225, cetuximab; CALNN, Cys-Ala-Leu-Asn-Asn; CD24, cluster of differentiation 24; COVID-19, Coronavirus Disease 2019; CT, computed tomography; CTAB, cetyltrimethylammonium bromide; CW, continuous wave; Cys, cysteamine; DOX, doxorubicin; dsDNA, double-stranded DNA; DTX, docetaxel; EPR, enhanced permeability and retention; FA, folic acid; FDA, Food and Drug Administration; FOXM1 Apt, Forkhead box M1; Gal, galactose; GBM, Glioblastoma; GC, gastric cancer; GFLGC, Gly-Phe-Leu-Gly-Cys; GNP, gold nanoparticles; GSH, glutathione; HA, hyaluronic acid; HSF-1, heat shock factor-1; hv, irradiation with light; Hv, irradiation with light; IC50, half-inhibitory concentration; ICG, indocyanine green; IVUS, intravascul arultrasound; K, kaempferol; LIN, linalool; mAbs, monoclonal antibodies; MB, methylene blue; MDR, multidrug resistance; mPEG@HAuNPs, PEGylated hollow AuNPs; mPEG-SH, thiol-terminated monomethoxy polyethylene glycol; MPH, mercaptopropionylhydrazide; MRI, magnetic resonance imaging; mTHPC, meso-tetrahydroxyphenylchlorin; MTX, methotrexate; NIR, near-infrared; NPs, nanoparticles; NS, nanoshell; O2, oxygen; P1, Boc-L-DP-L-OMe; PAH, pulmonary arterial hypertension; PBS, phosphate buffer solution; Pc 4, phthalocyanine 4; PCD-AuNP, AuNP-dendron conjugate; PDT, photodynamic therapy; PEC, pectin; PEG, polyethylene glycol; PepGNP, gold nanoparticle mounted with peptides; P-gp, P-glycoprotein; PpIX, protoporphyrin IX; PS, photosensitizer; PTT, photothermal therapy; RGD, Arg-Gly-Asp; rhTNFα, recombinant human tumor necrosis factor alpha; ROS, oxygen species; RT, radiotherapy; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SOSG, singlet oxygen sensor green; SPR, surface plasma resonance; ssDNA, single-stranded DNA; Tf, transferrin; Tfpep, transferrin; Tmab, trastuzumab; TNF, tumor necrosis factor; TS265, CoCl(H2O) (phendione)2][BF4]; ZnD, [Zn(DION)2]Cl2.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81603050), the Foundation of Sichuan Provincial People’s Hospital (No. 2021QN14), Research Program of Science and Technology Department of Sichuan Province (2021YJ0189), the Central Universities Foundation of University of Electronic Science and Technology of China (No. ZYGX2019J107), Key Research and Development Program of Science and Technology Department of Sichuan Province (2019YFS0514, 2020YFS0570) and the National Key Specialty Construction Project of Clinical Pharmacy (No. 30305030698).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: a review. Scand J Public Health. 2018;46(1):27–36. doi:10.1177/1403494817715400
3. Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2016;24(3):179–191. doi:10.3109/1061186X.2015.1051049
4. Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi:10.1016/j.ejpb.2015.03.018
5. Burstein HJ, Krilov L, Aragon-Ching JB, et al. Clinical Cancer Advances 2017: annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol. 2017;35(12):1341–1367. doi:10.1200/JCO.2016.71.5292
6. Chabner BA, Roberts TG. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72. doi:10.1038/nrc1529
7. Bharti S, Kaur G, Jain S, Gupta S, Tripathi SK. Characteristics and mechanism associated with drug conjugated inorganic nanoparticles. J Drug Target. 2019;27(8):813–829. doi:10.1080/1061186X.2018.1561888
8. Banstola A, Emami F, Jeong J-H, Yook S. Current Applications of Gold Nanoparticles for Medical Imaging and as Treatment Agents for Managing Pancreatic Cancer. Macromolecular Res. 2018;26(11):955–964. doi:10.1007/s13233-018-6139-4
9. Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767–3777. doi:10.2147/DDDT.S88023
10. Wong AD, Ye M, Ulmschneider MB, Searson PC. Quantitative Analysis of the Enhanced Permeation and Retention (EPR) Effect. PLoS One. 2015;10(5):e0123461. doi:10.1371/journal.pone.0123461
11. Kim H, Nguyen VP, Manivasagan P, et al. Doxorubicin-fucoidan-gold nanoparticles composite for dual-chemo-photothermal treatment on eye tumors. Oncotarget. 2017;8(69):113719–113733. doi:10.18632/oncotarget.23092
12. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.
13. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. doi:10.1016/j.addr.2010.03.011
14. Wu J. The Enhanced Permeability and Retention (EPR) Effect: the Significance of the Concept and Methods to Enhance Its Application. J Pers Med. 2021;11(8):771. doi:10.3390/jpm11080771
15. Arvizo RR, Bhattacharyya S, Kudgus RA, Giri K, Bhattacharya R, Mukherjee P. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chem Soc Rev. 2012;41(7):2943–2970. doi:10.1039/c2cs15355f
16. Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110(32):15700–15707. doi:10.1021/jp061667w
17. Li S, Zhang L, Wang T, Li L, Wang C, Su Z. The facile synthesis of hollow Au nanoflowers for synergistic chemo-photothermal cancer therapy. Chem Commun (Camb). 2015;51(76):14338–14341. doi:10.1039/C5CC05676D
18. Perezjuste J, Pastorizasantos I, Lizmarzan L, Mulvaney P. Gold nanorods: synthesis, characterization and applications. Coord Chem Rev. 2005;249(17–18):1870–1901. doi:10.1016/j.ccr.2005.01.030
19. Alex S, Tiwari A. Functionalized Gold Nanoparticles: synthesis, Properties and Applications–A Review. J Nanosci Nanotechnol. 2015;15(3):1869–1894. doi:10.1166/jnn.2015.9718
20. Paris JL, Baeza A, Vallet-Regi M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin Drug Deliv. 2019;16(10):1095–1112. doi:10.1080/17425247.2019.1662786
21. Ramalingam V. Multifunctionality of gold nanoparticles: plausible and convincing properties. Adv Colloid Interface Sci. 2019;271:101989. doi:10.1016/j.cis.2019.101989
22. Kong FY, Zhang JW, Li RF, Wang ZX, Wang WJ, Wang W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules. 2017;22(9):1445. doi:10.3390/molecules22091445
23. Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019;144:57–77. doi:10.1016/j.addr.2019.07.010
24. Paciotti GF, Myer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–183. doi:10.1080/10717540490433895
25. Chinnaiyan SK, Soloman AM, Perumal RK, Gopinath A, Balaraman M. 5 Fluorouracil‐loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET Nanobiotechnol. 2019;13(8):824–828. doi:10.1049/iet-nbt.2019.0007
26. Srinivas Raghavan B, Kondath S, Anantanarayanan R, Rajaram R. Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochemistry. 2015;50(11):1966–1976. doi:10.1016/j.procbio.2015.08.003
27. Jabir MS, Taha AA, Sahib UI, Taqi ZJ, Al-Shammari AM, Salman AS. Novel of nano delivery system for Linalool loaded on gold nanoparticles conjugated with CALNN peptide for application in drug uptake and induction of cell death on breast cancer cell line. Mater Sci Eng C Mater Biol Appl. 2019;94:949–964. doi:10.1016/j.msec.2018.10.014
28. Kapur A, Medina SH, Wang W, Palui G, Schneider JP, Mattoussi H. Intracellular Delivery of Gold Nanocolloids Promoted by a Chemically Conjugated Anticancer Peptide. ACS Omega. 2018;3(10):12754–12762. doi:10.1021/acsomega.8b02276
29. Banerjee K, Ravishankar Rai V, Umashankar M. Effect of peptide-conjugated nanoparticles on cell lines. Prog Biomater. 2019;8(1):11–21. doi:10.1007/s40204-019-0106-9
30. Fernandes AR, Jesus J, Martins P, et al. Multifunctional gold-nanoparticles: a nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J Control Release. 2017;245:52–61. doi:10.1016/j.jconrel.2016.11.021
31. Pedrosa P, Corvo ML, Ferreira-Silva M, et al. Targeting Cancer Resistance via Multifunctional Gold Nanoparticles. Int J Mol Sci. 2019;20(21):5510. doi:10.3390/ijms20215510
32. Wojcik M, Lewandowski W, Krol M, et al. Enhancing anti-tumor efficacy of Doxorubicin by non-covalent conjugation to gold nanoparticles - in vitro studies on feline fibrosarcoma cell lines. PLoS One. 2015;10(4):e0124955. doi:10.1371/journal.pone.0124955
33. Kumar K, Moitra P, Bashir M, Kondaiah P, Bhattacharya S. Natural tripeptide capped pH-sensitive gold nanoparticles for efficacious doxorubicin delivery both in vitro and in vivo. Nanoscale. 2020;12(2):1067–1074. doi:10.1039/C9NR08475D
34. Gu YJ, Cheng J, Man CW, Wong WT, Cheng SH. Gold-doxorubicin nanoconjugates for overcoming multidrug resistance. Nanomedicine. 2012;8(2):204–211. doi:10.1016/j.nano.2011.06.005
35. Safwat MA, Soliman GM, Sayed D, Attia MA. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol Pharm. 2018;15(6):2194–2205. doi:10.1021/acs.molpharmaceut.8b00047
36. Som I, Bhatia K, Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioallied Sci. 2012;4(1):2–9. doi:10.4103/0975-7406.92724
37. Alvarez-Gonzalez B, Rozalen M, Fernandez-Perales M, Alvarez MA, Sanchez-Polo M. Methotrexate Gold Nanocarriers: loading and Release Study: its Activity in Colon and Lung Cancer Cells. Molecules. 2020;25(24):6049. doi:10.3390/molecules25246049
38. Tran NTT, Wang T-H, Lin C-Y, Tai Y. Synthesis of methotrexate-conjugated gold nanoparticles with enhanced cancer therapeutic effect. Biochem Eng J. 2013;78:175–180. doi:10.1016/j.bej.2013.04.017
39. Yu Y, Yang T, Sun T. New insights into the synthesis, toxicity and applications of gold nanoparticles in CT imaging and treatment of cancer. Nanomedicine. 2020;15(11):1127–1145. doi:10.2217/nnm-2019-0395
40. Llevot A, Astruc D. Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem Soc Rev. 2012;41(1):242–257. doi:10.1039/C1CS15080D
41. Ramesh R, Amreddy N, Muralidharan R, et al. Tumor-targeted and pH-controlled delivery of doxorubicin using gold nanorods for lung cancer therapy. Int J Nanomedicine;2015. 6773. doi:10.2147/IJN.S93237
42. Borker S, Pokharkar V. Engineering of pectin-capped gold nanoparticles for delivery of doxorubicin to hepatocarcinoma cells: an insight into mechanism of cellular uptake. Artif Cells Nanomed Biotechnol. 2018;46(sup2):826–835. doi:10.1080/21691401.2018.1470525
43. Gotov O, Battogtokh G, Ko YT. Docetaxel-Loaded Hyaluronic Acid-Cathepsin B-Cleavable-Peptide-Gold Nanoparticles for the Treatment of Cancer. Mol Pharm. 2018;15(10):4668–4676. doi:10.1021/acs.molpharmaceut.8b00640
44. Ngernyuang N, Seubwai W, Daduang S, Boonsiri P, Limpaiboon T, Daduang J. Targeted delivery of 5-fluorouracil to cholangiocarcinoma cells using folic acid as a targeting agent. Mater Sci Eng C Mater Biol Appl. 2016;60:411–415. doi:10.1016/j.msec.2015.11.062
45. Yücel O, Şengelen A, Emik S, Önay-Uçar E, Arda N, Gürdağ G. Folic acid-modified methotrexate-conjugated gold nanoparticles as nano-sized trojans for drug delivery to folate receptor-positive cancer cells. Nanotechnology. 2020;31(35):355101. doi:10.1088/1361-6528/ab9395
46. Gao -Y-Y, Chen H, Zhou -Y-Y, et al. Intraorgan Targeting of Gold Conjugates for Precise Liver Cancer Treatment. ACS Appl Mater Interfaces. 2017;9(37):31458–31468. doi:10.1021/acsami.7b08969
47. Khademi Z, Lavaee P, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM. Co-delivery of doxorubicin and aptamer against Forkhead box M1 using chitosan-gold nanoparticles coated with nucleolin aptamer for synergistic treatment of cancer cells. Carbohydr Polym. 2020;248:116735. doi:10.1016/j.carbpol.2020.116735
48. Chung CH, Kim JH, Jung J, Chung BH. Nuclease-resistant DNA aptamer on gold nanoparticles for the simultaneous detection of Pb2+ and Hg2+ in human serum. Biosens Bioelectron. 2013;41:827–832. doi:10.1016/j.bios.2012.10.026
49. Seferos DS, Prigodich AE, Giljohann DA, Patel PC, Mirkin CA. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009;9(1):308–311. doi:10.1021/nl802958f
50. Qian Y, Qiu M, Wu Q, et al. Enhanced cytotoxic activity of cetuximab in EGFR-positive lung cancer by conjugating with gold nanoparticles. Sci Rep. 2014;4(1):53.
51. Kubota T, Kuroda S, Kanaya N, et al. HER2-targeted gold nanoparticles potentially overcome resistance to trastuzumab in gastric cancer. Nanomedicine. 2018;14(6):1919–1929. doi:10.1016/j.nano.2018.05.019
52. Li JJ, Hartono D, Ong CN, Bay BH, Yung LY. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials. 2010;31(23):5996–6003. doi:10.1016/j.biomaterials.2010.04.014
53. Liszbinski RB, Romagnoli GG, Gorgulho CM, Basso CR, Pedrosa VA, Kaneno R. Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells. Materials. 2020;13(2):375. doi:10.3390/ma13020375
54. Han G, Martin CT, Rotello VM. Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem Biol Drug Des. 2006;67(1):78–82. doi:10.1111/j.1747-0285.2005.00324.x
55. Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B. Gold Nanoparticles in Cancer Treatment. Mol Pharm. 2019;16(1):1–23. doi:10.1021/acs.molpharmaceut.8b00810
56. Riley MK, Vermerris W. Recent Advances in Nanomaterials for Gene Delivery-A Review. Nanomaterials. 2017;7(5):94. doi:10.3390/nano7050094
57. Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312(5776):1027–1030. doi:10.1126/science.1125559
58. Tunc CU, Oztas DY, Uzunoglu D, Bayrak OF, Culha M. Silencing Breast Cancer Genes Using Morpholino Embedded DNA-Tile-AuNPs Nanostructures. Hum Gene Ther. 2019;30(12):1547–1558. doi:10.1089/hum.2019.119
59. Karimi S, Fouani MH, Moshaii A, Nikkhah M, Hosseinkhani S, Sheikhnejad R. Development of Dual Functional Nucleic Acid Delivery Nanosystem for DNA Induced Silencing of Bcl-2 Oncogene. Int J Nanomedicine. 2020;15:1693–1708. doi:10.2147/IJN.S236217
60. Liu Y, Xu M, Zhao Y, et al. Flower-like gold nanoparticles for enhanced photothermal anticancer therapy by the delivery of pooled siRNA to inhibit heat shock stress response. J Mater Chem B. 2019;7(4):586–597. doi:10.1039/C8TB02418A
61. Huang S, Liu Y, Xu X, et al. Triple therapy of hepatocellular carcinoma with microRNA-122 and doxorubicin co-loaded functionalized gold nanocages. J Mater Chem B. 2018;6(15):2217–2229. doi:10.1039/C8TB00224J
62. Lee K, Kim T, Kim YM, Yang K, Choi I, Roh YH. Multifunctional DNA Nanogels for Aptamer-Based Targeted Delivery and Stimuli-Triggered Release of Cancer Therapeutics. Macromol Rapid Commun. 2021;42(2):e2000457. doi:10.1002/marc.202000457
63. Goodman AM, Hogan NJ, Gottheim S, Li C, Clare SE, Halas NJ. Understanding Resonant Light-Triggered DNA Release from Plasmonic Nanoparticles. ACS Nano. 2017;11(1):171–179. doi:10.1021/acsnano.6b06510
64. Goodman AM, Neumann O, Norregaard K, et al. Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc Natl Acad Sci U S A. 2017;114(47):12419–12424. doi:10.1073/pnas.1713137114
65. Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic Therapy: a Clinical Consensus Guide. Dermatol Surg. 2016;42(7):804–827. doi:10.1097/DSS.0000000000000800
66. Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473(4):347–364. doi:10.1042/BJ20150942
67. Alea-Reyes ME, Soriano J, Mora-Espi I, et al. Amphiphilic gemini pyridinium-mediated incorporation of Zn(II)meso-tetrakis(4-carboxyphenyl)porphyrin into water-soluble gold nanoparticles for photodynamic therapy. Colloids Surf B Biointerfaces. 2017;158:602–609. doi:10.1016/j.colsurfb.2017.07.033
68. Penon O, Marin MJ, Russell DA, Perez-Garcia L. Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. J Colloid Interface Sci. 2017;496:100–110. doi:10.1016/j.jcis.2017.02.006
69. Haimov E, Weitman H, Polani S, Schori H, Zitoun D, Shefi O. meso-Tetrahydroxyphenylchlorin-Conjugated Gold Nanoparticles as a Tool To Improve Photodynamic Therapy. ACS Appl Mater Interfaces. 2018;10(3):2319–2327. doi:10.1021/acsami.7b16455
70. Jesus VPS, Raniero L, Lemes GM, Bhattacharjee TT, Caetano PC, Castilho ML. Nanoparticles of methylene blue enhance photodynamic therapy. Photodiagnosis Photodyn Ther. 2018;23:212–217. doi:10.1016/j.pdpdt.2018.06.011
71. Chi YF, Qin JJ, Li Z, Ge Q, Zeng WH. Enhanced anti-tumor efficacy of 5-aminolevulinic acid-gold nanoparticles-mediated photodynamic therapy in cutaneous squamous cell carcinoma cells. Braz J Med Biol Res. 2020;53(5):e8457. doi:10.1590/1414-431x20208457
72. Wu J, Lin Y, Li H, Jin Q, Ji J. Zwitterionic stealth peptide-capped 5-aminolevulinic acid prodrug nanoparticles for targeted photodynamic therapy. J Colloid Interface Sci. 2017;485:251–259. doi:10.1016/j.jcis.2016.09.012
73. Yu J, Hsu CH, Huang CC, Chang PY. Development of therapeutic Au-methylene blue nanoparticles for targeted photodynamic therapy of cervical cancer cells. ACS Appl Mater Interfaces. 2015;7(1):432–441. doi:10.1021/am5064298
74. Dixit S, Novak T, Miller K, Zhu Y, Kenney ME, Broome AM. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale. 2015;7(5):1782–1790. doi:10.1039/C4NR04853A
75. Mangadlao JD, Wang X, McCleese C, et al. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano. 2018;12(4):3714–3725. doi:10.1021/acsnano.8b00940
76. Meyers JD, Cheng Y, Broome A-M, et al. Peptide-Targeted Gold Nanoparticles for Photodynamic Therapy of Brain Cancer. Part Part Syst Charact. 2015;32(4):448–457. doi:10.1002/ppsc.201400119
77. Savarimuthu WP, Gananathan P, Rao AP, Manickam E, Singaravelu G. Protoporphyrin IX-Gold Nanoparticle Conjugates for Targeted Photodynamic Therapy–An In-Vitro Study. J Nanosci Nanotechnol. 2015;15(8):5577–5584. doi:10.1166/jnn.2015.10302
78. Garcia Calavia P, Chambrier I, Cook MJ, Haines AH, Field RA, Russell DA. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J Colloid Interface Sci. 2018;512:249–259. doi:10.1016/j.jcis.2017.10.030
79. Deng W, McKelvey KJ, Guller A, et al. Application of Mitochondrially Targeted Nanoconstructs to Neoadjuvant X-ray-Induced Photodynamic Therapy for Rectal Cancer. ACS Cent Sci. 2020;6(5):715–726. doi:10.1021/acscentsci.9b01121
80. Gu X, Shen C, Li H, Goldys EM, Deng W. X-ray induced photodynamic therapy (PDT) with a mitochondria-targeted liposome delivery system. J Nanobiotechnology. 2020;18(1):87. doi:10.1186/s12951-020-00644-z
81. Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155(3):344–357. doi:10.1016/j.jconrel.2011.06.004
82. Zhang Y, Zhan X, Xiong J, et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci Rep. 2018;8(1):562.
83. Ahmad R, Fu J, He N, Li S. Advanced Gold Nanomaterials for Photothermal Therapy of Cancer. J Nanosci Nanotechnol. 2016;16(1):67–80. doi:10.1166/jnn.2016.10770
84. Lee SA, Link S. Chemical Interface Damping of Surface Plasmon Resonances. Acc Chem Res. 2021;54(8):1950–1960. doi:10.1021/acs.accounts.0c00872
85. Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110(14):7238–7248. doi:10.1021/jp057170o
86. Murphy CJ, Sau TK, Gole AM, et al. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B. 2005;109(29):13857–13870. doi:10.1021/jp0516846
87. Terentyuk GS, Maslyakova GN, Suleymanova LV, et al. Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. Journal of Biomedical Optics. 2009;14(2):021016. doi:10.1117/1.3122371
88. Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5(4):709–711. doi:10.1021/nl050127s
89. Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int J Mol Sci. 2018;19(7):1979. doi:10.3390/ijms19071979
90. Tong L, Cheng JX. Gold nanorod-mediated photothermolysis induces apoptosis of macrophages via damage of mitochondria. Nanomedicine. 2009;4(3):265–276. doi:10.2217/nnm.09.4
91. Koller MR, Hanania EG, Stevens J, et al. High-throughput laser-mediated in situ cell purification with high purity and yield. Cytometry A. 2004;61(2):153–161. doi:10.1002/cyto.a.20079
92. Feyh J, Gutmann R, Leunig A, et al. MRI-guided laser interstitial thermal therapy (LITT) of head and neck tumors: progress with a new method. J Clin Laser Med Surg. 1996;14(6):361–366. doi:10.1089/clm.1996.14.361
93. Markovic ZM, Harhaji-Trajkovic LM, Todorovic-Markovic BM, et al. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials. 2011;32(4):1121–1129. doi:10.1016/j.biomaterials.2010.10.030
94. Cai X, Jia X, Gao W, et al. A Versatile Nanotheranostic Agent for Efficient Dual-Mode Imaging Guided Synergistic Chemo-Thermal Tumor Therapy. Adv Funct Mater. 2015;25(17):2520–2529. doi:10.1002/adfm.201403991
95. Li L, Chen C, Liu H, et al. Multifunctional Carbon-Silica Nanocapsules with Gold Core for Synergistic Photothermal and Chemo-Cancer Therapy under the Guidance of Bimodal Imaging. Adv Funct Mater. 2016;26(24):4252–4261. doi:10.1002/adfm.201600985
96. Keyvan Rad J, Mahdavian AR, Khoei S, Shirvalilou S. Enhanced Photogeneration of Reactive Oxygen Species and Targeted Photothermal Therapy of C6 Glioma Brain Cancer Cells by Folate-Conjugated Gold-Photoactive Polymer Nanoparticles. ACS Appl Mater Interfaces. 2018;10(23):19483–19493. doi:10.1021/acsami.8b05252
97. Neshastehriz A, Tabei M, Maleki S, Eynali S, Shakeri-Zadeh A. Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6 MV X-ray on mouth epidermal carcinoma cells. J Photochem Photobiol B. 2017;172:52–60. doi:10.1016/j.jphotobiol.2017.05.012
98. Mirrahimi M, Abed Z, Beik J, et al. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol Res. 2019;143:178–185. doi:10.1016/j.phrs.2019.01.005
99. Mendes R, Pedrosa P, Lima JC, Fernandes AR, Baptista PV. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of Gold Nanoparticles. Sci Rep. 2017;7(1):10872. doi:10.1038/s41598-017-11491-8
100. Wang B, Wu S, Lin Z, et al. A personalized and long-acting local therapeutic platform combining photothermal therapy and chemotherapy for the treatment of multidrug-resistant colon tumor. Int J Nanomedicine. 2018;13:8411–8427. doi:10.2147/IJN.S184728
101. Wang L, Pei J, Cong Z, et al. Development of anisamide-targeted PEGylated gold nanorods to deliver epirubicin for chemo-photothermal therapy in tumor-bearing mice. Int J Nanomedicine. 2019;14:1817–1833. doi:10.2147/IJN.S192520
102. Zhang Y, Zhou L, Tan J, Liu J, Shan X, Ma Y. Laser-triggered collaborative chemophotothermal effect of gold nanoparticles for targeted colon cancer therapy. Biomed Pharmacother. 2020;130:110492. doi:10.1016/j.biopha.2020.110492
103. Banstola A, Poudel K, Emami F, et al. Localized therapy using anti-PD-L1 anchored and NIR-responsive hollow gold nanoshell (HGNS) loaded with doxorubicin (DOX) for the treatment of locally advanced melanoma. Nanomedicine. 2021;33:102349. doi:10.1016/j.nano.2020.102349
104. Zhao Q, Yang Y, Wang H, Lei W, Liu Y, Wang S. Gold nanoparticles modified hollow carbon system for dual-responsive release and chemo-photothermal synergistic therapy of tumor. J Colloid Interface Sci. 2019;554:239–249. doi:10.1016/j.jcis.2019.07.005
105. Huang J, Xu Z, Jiang Y, et al. Metal organic framework-coated gold nanorod as an on-demand drug delivery platform for chemo-photothermal cancer therapy. J Nanobiotechnology. 2021;19(1):219. doi:10.1186/s12951-021-00961-x
106. Ebrahim HM, El-Rouby MN, Morsy ME, Said MM, Ezz MK. The Synergistic Cytotoxic Effect of Laser-Irradiated Gold Nanoparticles and Sorafenib Against the Growth of a Human Hepatocellular Carcinoma Cell Line. Asian Pac J Cancer Prev. 2019;20(11):3369–3376. doi:10.31557/APJCP.2019.20.11.3369
107. Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074. doi:10.1038/s41467-018-03473-9
108. Poursalehi Z, Salehi R, Samadi N, Rasta SH, Mansoori B, Majdi H. A simple strategy for chemo-photothermal ablation of breast cancer cells by novel smart gold nanoparticles. Photodiagnosis Photodyn Ther. 2019;28:25–37. doi:10.1016/j.pdpdt.2019.08.019
109. Schaaf L, Schwab M, Ulmer C, et al. Hyperthermia Synergizes with Chemotherapy by Inhibiting PARP1-Dependent DNA Replication Arrest. Cancer Res. 2016;76(10):2868–2875. doi:10.1158/0008-5472.CAN-15-2908
110. Masunaga S-I. Tumor Microenvironment and Hyperthermia. Hyperthermic Oncol Bench Bedside. 2016;1:151–169.
111. Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev. 2010;62(3):339–345. doi:10.1016/j.addr.2009.11.006
112. Wang L, Lin X, Wang J, et al. Novel Insights into Combating Cancer Chemotherapy Resistance Using a Plasmonic Nanocarrier: enhancing Drug Sensitiveness and Accumulation Simultaneously with Localized Mild Photothermal Stimulus of Femtosecond Pulsed Laser. Adv Funct Mater. 2014;24(27):4229–4239. doi:10.1002/adfm.201400015
113. Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11(9):1034–1044. doi:10.1634/theoncologist.11-9-1034
114. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi:10.1038/nrc1071
115. Riley RS, O’Sullivan RK, Potocny AM, Rosenthal J, Day ES. Evaluating Nanoshells and a Potent Biladiene Photosensitizer for Dual Photothermal and Photodynamic Therapy of Triple Negative Breast Cancer Cells. Nanomaterials. 2018;8(9):658. doi:10.3390/nano8090658
116. Zhang L, Yang XQ, Wei JS, Li X, Wang H, Zhao YD. Intelligent gold nanostars for in vivo CT imaging and catalase-enhanced synergistic photodynamic & photothermal tumor therapy. Theranostics. 2019;9(19):5424–5442. doi:10.7150/thno.33015
117. Wang B, Wang JH, Liu Q, et al. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials. 2014;35(6):1954–1966. doi:10.1016/j.biomaterials.2013.11.066
118. Liu P, Yang W, Shi L, et al. Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J Materials Chem B. 2019;7(44):6924–6933. doi:10.1039/C9TB01573F
119. Hainfeld JF, Lin L, Slatkin DN, Avraham Dilmanian F, Vadas TM, Smilowitz HM. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine. 2014;10(8):1609–1617. doi:10.1016/j.nano.2014.05.006
120. Zhang Y, Liu J, Yu Y, et al. Enhanced radiotherapy using photothermal therapy based on dual-sensitizer of gold nanoparticles with acid-induced aggregation. Nanomedicine. 2020;29:56.
121. Poudel K, Banstola A, Tran TH, et al. Hyaluronic acid wreathed, trio-stimuli receptive and on-demand triggerable nanoconstruct for anchored combinatorial cancer therapy. Carbohydr Polym. 2020;249:116815. doi:10.1016/j.carbpol.2020.116815
122. Poudel K, Banstola A, Gautam M, et al. Macrophage-Membrane-Camouflaged Disintegrable and Excretable Nanoconstruct for Deep Tumor Penetration. ACS Appl Mater Interfaces. 2020;12(51):56767–56781. doi:10.1021/acsami.0c17235
123. Poudel K, Banstola A, Gautam M, et al. Redox/photo dual-responsive, self-targeted, and photosensitizer-laden bismuth sulfide nanourchins for combination therapy in cancer. Nanoscale. 2021;13(2):1231–1247. doi:10.1039/D0NR07736D
124. Tan H, Hou N, Liu Y, et al. CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomedicine. 2020;27:102192. doi:10.1016/j.nano.2020.102192
125. Wang Q, Zhang X, Sun Y, et al. Gold-caged copolymer nanoparticles as multimodal synergistic photodynamic/photothermal/chemotherapy platform against lethality androgen-resistant prostate cancer. Biomaterials. 2019;212:73–86. doi:10.1016/j.biomaterials.2019.05.009
126. Chang Y, Cheng Y, Feng Y, et al. Resonance Energy Transfer-Promoted Photothermal and Photodynamic Performance of Gold-Copper Sulfide Yolk-Shell Nanoparticles for Chemophototherapy of Cancer. Nano Lett. 2018;18(2):886–897. doi:10.1021/acs.nanolett.7b04162
127. Xu W, Qian J, Hou G, et al. PEGylated hydrazided gold nanorods for pH-triggered chemo/photodynamic/photothermal triple therapy of breast cancer. Acta Biomater. 2018;82:171–183. doi:10.1016/j.actbio.2018.10.019
128. Yu Y, Zhang Z, Wang Y, et al. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomater. 2017;59:170–180. doi:10.1016/j.actbio.2017.06.026
129. Xu X, Chong Y, Liu X, et al. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomater. 2019;84:328–338. doi:10.1016/j.actbio.2018.11.043
130. Bouche M, Hsu JC, Dong YC, Kim J, Taing K, Cormode DP. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjug Chem. 2020;31(2):303–314. doi:10.1021/acs.bioconjchem.9b00669
131. Hu X, Zhang Y, Ding T, Liu J, Zhao H. Multifunctional Gold Nanoparticles: a Novel Nanomaterial for Various Medical Applications and Biological Activities. Front Bioeng Biotechnol. 2020;8:990. doi:10.3389/fbioe.2020.00990
132. Meir R, Popovtzer R. Cell tracking using gold nanoparticles and computed tomography imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(2). doi:10.1002/wnan.1480
133. Lusic H, Grinstaff MW. X-ray-computed tomography contrast agents. Chem Rev. 2013;113(3):1641–1666. doi:10.1021/cr200358s
134. Silvestri A, Zambelli V, Ferretti AM, Salerno D, Bellani G, Polito L. Design of functionalized gold nanoparticle probes for computed tomography imaging. Contrast Media Mol Imaging. 2016;11(5):405–414. doi:10.1002/cmmi.1704
135. Yin D, Li X, Ma Y, Liu Z. Targeted cancer imaging and photothermal therapy via monosaccharide-imprinted gold nanorods. Chem Commun (Camb). 2017;53(50):6716–6719. doi:10.1039/C7CC02247F
136. Ghaziyani MF, Pourhassan Moghaddam M, Shahbazi-Gahrouei D, et al. Anti-CD24 bio Modified PEGylated Gold Nanoparticles as Targeted Computed Tomography Contrast Agent. Adv Pharm Bull. 2018;8(4):599–607. doi:10.15171/apb.2018.068
137. Wang R, Deng J, He D, et al. PEGylated hollow gold nanoparticles for combined X-ray radiation and photothermal therapy in vitro and enhanced CT imaging in vivo. Nanomedicine. 2019;16:195–205. doi:10.1016/j.nano.2018.12.005
138. Khademi S, Sarkar S, Shakeri-Zadeh A, et al. Targeted gold nanoparticles enable molecular CT imaging of head and neck cancer: an in vivo study. Int J Biochem Cell Biol. 2019;114:105554. doi:10.1016/j.biocel.2019.06.002
139. Beik J, Jafariyan M, Montazerabadi A, et al. The benefits of folic acid-modified gold nanoparticles in CT-based molecular imaging: radiation dose reduction and image contrast enhancement. Artif Cells Nanomed Biotechnol. 2018;46(8):1993–2001. doi:10.1080/21691401.2017.1408019
140. Cai J, Miao YQ, Li L, Fan HM. Facile Preparation of Gold-Decorated Fe(3)O(4) Nanoparticles for CT and MR Dual-Modal Imaging. Int J Mol Sci. 2018;19(12):4049. doi:10.3390/ijms19124049
141. Tang Y, Shi H, Cheng D, et al. pH-Activatable tumor-targeting gold nanoprobe for near-infrared fluorescence/CT dual-modal imaging in vivo. Colloids Surf B Biointerfaces. 2019;179:56–65. doi:10.1016/j.colsurfb.2019.03.049
142. Ge X, Song ZM, Sun L, et al. Lanthanide (Gd(3+) and Yb(3+)) functionalized gold nanoparticles for in vivo imaging and therapy. Biomaterials. 2016;108:35–43. doi:10.1016/j.biomaterials.2016.08.051
143. Kuhn J, Papanastasiou G, Tai CW, et al. Tri-modal imaging of gold-dotted magnetic nanoparticles for magnetic resonance imaging, computed tomography and intravascular ultrasound: an in vitro study. Nanomedicine. 2020;15(25):2433–2445. doi:10.2217/nnm-2020-0236
144. Zhou B, Xiong Z, Wang P, et al. Targeted tumor dual mode CT/MR imaging using multifunctional polyethylenimine-entrapped gold nanoparticles loaded with gadolinium. Drug Deliv. 2018;25(1):178–186. doi:10.1080/10717544.2017.1422299
145. Min Y, Caster JM, Eblan MJ, Wang AZ. Clinical Translation of Nanomedicine. Chem Rev. 2015;115(19):11147–11190. doi:10.1021/acs.chemrev.5b00116
146. Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16(24):6139–6149. doi:10.1158/1078-0432.CCR-10-0978
147. Schwartz JA, Shetty AM, Price RE, et al. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69(4):1659–1667. doi:10.1158/0008-5472.CAN-08-2535
148. O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209(2):171–176. doi:10.1016/j.canlet.2004.02.004
149. Stern JM, Stanfield J, Kabbani W, Hsieh JT, Cadeddu JA. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol. 2008;179(2):748–753. doi:10.1016/j.juro.2007.09.018
150. Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–1851. doi:10.1021/ar800150g
151. Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP. Evaluation of the toxicity of intravenous delivery of auroshell particles (gold-silica nanoshells). Int J Toxicol. 2012;31(6):584–594. doi:10.1177/1091581812465969
152. Stern JM, Kibanov Solomonov VV, Sazykina E, Schwartz JA, Gad SC, Goodrich GP. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int J Toxicol. 2016;35(1):38–46. doi:10.1177/1091581815600170
153. Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A. 2019;116(37):18590–18596. doi:10.1073/pnas.1906929116
154. ClinicalTrials.gov. 2021. Available from: https://clinicaltrials.gov/.
155. Khoobchandani M, Katti KK, Karikachery AR, et al. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine - Pre-Clinical and Pilot Human Clinical Investigations. Int J Nanomedicine. 2020;15:181–197. doi:10.2147/IJN.S219042
156. Xu ZQ, Broza YY, Ionsecu R, et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br J Cancer. 2013;108(4):941–950. doi:10.1038/bjc.2013.44
157. Pang Y, Wei C, Li R, et al. Photothermal conversion hydrogel based mini-eye patch for relieving dry eye with long-term use of the light-emitting screen. Int J Nanomedicine. 2019;14:5125–5133. doi:10.2147/IJN.S192407
158. Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7(17):8003–8015. doi:10.1039/C5NR01050K
159. Rambanapasi C, Zeevaart JR, Buntting H, et al. Bioaccumulation and Subchronic Toxicity of 14 nm Gold Nanoparticles in Rats. Molecules. 2016;21(6):763. doi:10.3390/molecules21060763
160. Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393(4):649–655. doi:10.1016/j.bbrc.2010.02.046
161. Glazer ES, Zhu C, Hamir AN, Borne A, Thompson CS, Curley SA. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology. 2011;5(4):459–468. doi:10.3109/17435390.2010.516026
162. Dam DH, Culver KS, Kandela I, et al. Biodistribution and in vivo toxicity of aptamer-loaded gold nanostars. Nanomedicine. 2015;11(3):671–679. doi:10.1016/j.nano.2014.10.005
163. Enea M, Pereira E, Silva DD, et al. Study of the intestinal uptake and permeability of gold nanoparticles using both in vitro and in vivo approaches. Nanotechnology. 2020;31(19):195102. doi:10.1088/1361-6528/ab6dfb
164. Li X, Hu Z, Ma J, et al. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf B Biointerfaces. 2018;167:260–266. doi:10.1016/j.colsurfb.2018.04.005
165. Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, Bettmer J, Llopis J, Sanchez-Gonzalez C. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine. 2018;14(1):1–12. doi:10.1016/j.nano.2017.08.011
166. Engstrom AM, Faase RA, Marquart GW, Baio JE, Mackiewicz MR, Harper SL. Size-Dependent Interactions of Lipid-Coated Gold Nanoparticles: developing a Better Mechanistic Understanding Through Model Cell Membranes and in vivo Toxicity. Int J Nanomedicine. 2020;15:4091–4104. doi:10.2147/IJN.S249622
167. Chen YS, Hung YC, Liau I, Huang GS. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale Res Lett. 2009;4(8):858–864. doi:10.1007/s11671-009-9334-6
168. Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi:10.1038/nbt1340
169. Patibandla S, Zhang Y, Tohari AM, et al. Comparative analysis of the toxicity of gold nanoparticles in zebrafish. J Appl Toxicol. 2018;38(8):1153–1161. doi:10.1002/jat.3628
170. Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5(6):701–708. doi:10.1002/smll.200801546
171. Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S. Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir. 2006;22(1):2–5. doi:10.1021/la0520029
172. Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114(3):343–347. doi:10.1016/j.jconrel.2006.06.017
173. Tarantola M, Pietuch A, Schneider D, et al. Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology. 2011;5(2):254–268. doi:10.3109/17435390.2010.528847
174. Sultana S, Djaker N, Boca-Farcau S, et al. Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology. 2015;26(5):055101. doi:10.1088/0957-4484/26/5/055101
175. Liu K, He Z, Byrne HJ, Curtin JF, Tian F. Investigating the Role of Gold Nanoparticle Shape and Size in Their Toxicities to Fungi. Int J Environ Res Public Health. 2018;15(5):97.
176. Wozniak A, Malankowska A, Nowaczyk G, et al. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J Mater Sci Mater Med. 2017;28(6):92. doi:10.1007/s10856-017-5902-y
177. Vales G, Suhonen S, Siivola KM, Savolainen KM, Catalan J, Norppa H. Size, Surface Functionalization, and Genotoxicity of Gold Nanoparticles In Vitro. Nanomaterials. 2020;10(2):271. doi:10.3390/nano10020271
178. Feng ZV, Gunsolus IL, Qiu TA, et al. Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem Sci. 2015;6(9):5186–5196. doi:10.1039/C5SC00792E
179. Cho TJ, MacCuspie RI, Gigault J, Gorham JM, Elliott JT, Hackley VA. Highly stable positively charged dendron-encapsulated gold nanoparticles. Langmuir. 2014;30(13):3883–3893. doi:10.1021/la5002013
180. Bahamonde J, Brenseke B, Chan MY, Kent RD, Vikesland PJ, Prater MR. Gold Nanoparticle Toxicity in Mice and Rats: species Differences. Toxicol Pathol. 2018;46(4):431–443. doi:10.1177/0192623318770608
181. Ginzburg AL, Truong L, Tanguay RL, Hutchison JE. Synergistic Toxicity Produced by Mixtures of Biocompatible Gold Nanoparticles and Widely Used Surfactants. ACS Nano. 2018;12(6):5312–5322. doi:10.1021/acsnano.8b00036
182. Isoda K, Tanaka A, Fuzimori C, et al. Toxicity of Gold Nanoparticles in Mice due to Nanoparticle/Drug Interaction Induces Acute Kidney Damage. Nanoscale Res Lett. 2020;15(1):141. doi:10.1186/s11671-020-03371-4
183. Zhang XD, Wu HY, Wu D, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. doi:10.2147/IJN.S8428
184. Fraga S, Brandao A, Soares ME, et al. Short- and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomedicine. 2014;10(8):1757–1766. doi:10.1016/j.nano.2014.06.005
185. Daems N, Verlinden B, Van Hoecke K, et al. In Vivo Pharmacokinetics, Biodistribution and Toxicity of Antibody-Conjugated Gold Nanoparticles in Healthy Mice. J Biomed Nanotechnol. 2020;16(6):985–996. doi:10.1166/jbn.2020.2928
186. Adewale OB, Davids H, Cairncross L, Roux S. Toxicological Behavior of Gold Nanoparticles on Various Models: influence of Physicochemical Properties and Other Factors. Int J Toxicol. 2019;38(5):357–384. doi:10.1177/1091581819863130
187. Verissimo TV, Santos NT, Silva JR, Azevedo RB, Gomes AJ, Lunardi CN. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater Sci Eng C Mater Biol Appl. 2016;65:199–204. doi:10.1016/j.msec.2016.04.030
188. Hanna SK, Montoro Bustos AR, Peterson AW, et al. Agglomeration of Escherichia coli with Positively Charged Nanoparticles Can Lead to Artifacts in a Standard Caenorhabditis elegans Toxicity Assay. Environ Sci Technol. 2018;52(10):5968–5978. doi:10.1021/acs.est.7b06099
189. Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338(6109):903–910. doi:10.1126/science.1226338
190. Thorek DLJ, Elias E, Tsourkas A. Comparative Analysis of Nanoparticle-Antibody Conjugations: carbodiimide Versus Click Chemistry. Mol Imaging. 2009;8(4):
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


