Back to Journals » Infection and Drug Resistance » Volume 17
Multidrug-Resistant Proteus mirabilis Infections and Clinical Outcome at Tertiary Hospital in Riyadh, Saudi Arabia
Authors Hafiz TA , Alghamdi GS, Alkudmani ZS , Alyami AS, AlMazyed A, Alhumaidan OS, Mubaraki MA, Alotaibi FE
Received 15 November 2023
Accepted for publication 2 February 2024
Published 14 February 2024 Volume 2024:17 Pages 571—581
DOI https://doi.org/10.2147/IDR.S448335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Taghreed A Hafiz,1 Ghadi S Alghamdi,1 Zeina S Alkudmani,1 Ahmed S Alyami,2 Abeer AlMazyed,2 Ohoud S Alhumaidan,1 Murad A Mubaraki,1 Fawzia E Alotaibi3
1Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, 12372, Saudi Arabia; 2Pathology and Clinical Laboratory Medicine, King Fahad Medical City, Riyadh, 11525, Saudi Arabia; 3Pathology Department, College of Medicine, King Saud University, Riyadh, 12372, Saudi Arabia
Correspondence: Taghreed A Hafiz, Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, 12372, Saudi Arabia, Email [email protected]
Background: Proteus mirabilis (P. mirabilis) is known to cause various infections, most commonly urinary tract infections, and is a threat to hospitalized patients, especially in long-stay departments that utilize invasive devices. This study aims to fill the knowledge gap regarding P. mirabilis epidemiology and antimicrobial resistance in Saudi Arabia. It investigates epidemiological patterns, resistance characteristics, and clinical outcomes among P. mirabilis patients at King Fahad Medical City in Riyadh from 2019 to 2021.
Methods: A total of 598 P. mirabilis isolated from diverse clinical specimens, including the clinical information of 78 intensive care unit (ICU) patients, were included in the current study. The Phoenix BD instrument was used for complete identification and sensitivity testing of Proteus spp. Demographic, clinical, and outcome data were reported and compared using statistical analysis.
Results: Pan-drug-resistant isolates were identified in 2019 (n = 6), although multi- and extensively drug-resistant isolate frequencies were greatest among all patients in 2019. The highest susceptibility levels were observed for piperacillin-tazobactam, carbapenems, and cephalosporins antibiotics. In contrast, Cephalothin, trimethoprim-sulfamethoxazole, and ampicillin had the lowest susceptibilities. Urine infections with a positive culture of P. mirabilis were significantly higher in females and non-ICU patients (p < 0.001), but respiratory infections were significantly higher in ICU patients (p < 0.001). Moreover, ICU patients infected with P. mirabilis and undergoing renal dialysis have a 7.2-fold (P 0.034) higher risk of death than those not receiving dialysis.
Conclusion: Hospitalized patients are at risk of fatal consequences due to P. mirabilis infection. It is crucial to conduct further investigation to fully understand the severity of this issue and take necessary measures to prevent it.
Keywords: Proteus mirabilis, ICU, nosocomial infection, multidrug-resistant, extensively drug-resistant, pan-drug-resistant, risk factors, mortality
Introduction
The Gram-negative bacterium Proteus mirabilis is known for its urease activity and swarming motility.1,2 It a component of of the normal flora in the intestinal tracts of humans and animals and is widespread in the environment.3 Associated with various infections, P. mirabilis can lead to severe and persistent respiratory, skin, eye, wound, and gastrointestinal infections.4 Notably, it is responsible for 90% of Proteus infections and is classified as community-acquired infection.5 P. mirabilis, the third most prevalent cause of urinary tract infections (UTIs) and the second most common cause of catheter-associated urinary tract infections (CAUTIs) in long-term catheterized patients, is responsible for 12% of complicated UTIs.1 Elderly patients undergoing long-term catheterization have the highest P. mirabilis CAUTI incidence rates.4 Patients who contract an infection in the hospital, have a history of recurrent infections, urinary tract structural abnormalities, or a urethral catheter are more likely to develop Proteus infections.5 Furthermore, P. mirabilis can form complex biofilms containing polysaccharides between sessile cells, increasing the severity of the infection. Biofilm formation has been attributed to the severity and dissemination of Proteus infections.6
Most P. mirabilis isolates in the past were susceptible to standard antibiotic classes. However, recent studies indicate that antibiotic resistance is increasing among P. mirabilis isolates in different countries.7,8 Like many other members of the Enterobacteriaceae family, P. mirabilis harbors plasmids and integrons that code for antimicrobial resistance.9 The prevalence of multidrug-resistant (MDR) strains of P. mirabilis in some settings may be relatively high owing to extended-spectrum beta-lactamase (ESBL), ampC-type cephalosporinases and carbapenemases production.10–12 Resistant P. mirabilis isolates have been associated with nosocomial outbreaks, and ESBL-producing P. mirabilis has been linked to nosocomial outbreaks in neonatal ICUs in India.13 A Chinese neurology department reported a nosocomial outbreak caused by a carbapenem-resistant P. mirabilis clone producing New Delhi metallo-β -lactamase 1.14 Additionally, in Ethiopia15 and Nigeria,16 P. mirabilis has been linked to several nosocomial infection outbreaks.
A study conducted in Saudi Arabia has reported the prevalence of nosocomial pathogens. During the study period, Proteus spp. Infections were extremely low, with insignificant decreases towards the end of 2019 (3.2% vs 2.2%). Resistance rates increased for MDR Proteus (4.9% vs 6.9%), and aminoglycoside resistance was shown to be the most prevalent (51.1%). Furthermore, Proteus spp. demonstrated lower resistance to all antimicrobial classes except for carbapenems.17
There is a shortage of data regarding the epidemiology of P. mirabilis and its antimicrobial resistance in Saudi Arabia. Therefore, the study aimed to investigate the epidemiological pattern, resistance characteristics, and clinical outcomes of patients with P. mirabilis at King Fahad Medical City, Riyadh, Saudi Arabia, from 2019 to 2021.
Materials and Methods
Study Design and Data Collection
A retrospective analysis was conducted at King Fahad Medical City (KFMC), which has a capacity of 1200 beds in Riyadh, Saudi Arabia, from January 2019 to December 2021. In total, 598 P. mirabilis isolates from various clinical samples were studied. This study also included the clinical histories of 78 patients in the ICU. Samples were collected from various sources, including urine (mid-stream urine, indwelling catheter, and in and out catheter), blood (central and peripheral lines), respiratory (sputum and endotracheal), and miscellaneous (wound and ulcer, abscess, body fluid, tissue, and swab). The data collection criteria are: (A) Age divided into pediatric and adult categories. The age groups were divided into four categories: pediatrics (ages 0 to 18 years), young adults (ages 19 to 44 years), adults (ages 45 to 64 years), and geriatrics (age > 65 years). (B) The ward or clinic where the patient was admitted, including the emergency department, ICU, outpatient clinic, and ward. (C) Sample source and site; and (D) bacterial resistance pattern. Any growth that was not P. mirabilis was excluded. In addition, the clinical history of pediatric and adult patients admitted to the ICU was obtained from the KFMC database. The clinical history of the patients in the ICU included the following criteria: (1) exposure to carbapenem, other antibiotics, or both in the past 14 to 30 days; (2) renal dialysis at isolation or not; (3) on mechanical ventilation or not; (4) chronic diseases such as diabetes, hypertension, renal disease, or malignancy; (5) clinical symptoms such as fever, gastrointestinal symptoms, or respiratory symptoms; (6) the presence of a wound or urinary tract infection; (7) bacteremia or septicemia; and (8) clinical outcomes for the patient and additional notes if present. On multiple occasions, more than one isolate was recovered from the same patient at different P. mirabilis infection sites and was considered a different isolate.
P. mirabilis Identification and Antimicrobial Susceptibility Testing
This study included only patients whose isolates were positively identified as P. mirabilis. The Phoenix BD instrument (Becton Dickinson Diagnostic Systems, Sparks, MD, USA) was used for complete identification and sensitivity testing of Proteus spp. The following antibiotics were tested for antimicrobial sensitivity (AST): ampicillin (AMP), amoxicillin-clavulanate (AMC), piperacillin-tazobactam (TZP), cephalothin (CEF), cefepime (FEP), cefoxitin (FOX), cefuroxime (CXM), cefotaxime (CTX), ceftriaxone (CRO), ceftazidime (CAZ), imipenem (IPM), meropenem (MEM), ertapenem (ETP), ciprofloxacin (CIP), tigecycline (TGC), gentamicin (GEN), amikacin (AMK), levofloxacin (LVX) and trimethoprim-sulfamethoxazole (SXT). The results were interpreted and reported according to the 32nd Edition of the CLSI-M100 document and classified as susceptible, intermediate, and resistant. The AST results were classified into four categories: susceptible, multi-drug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR). MDR isolates were identified as resistant to one or more antibiotics from three or more antibiotic classes. XDR isolates were those resistant to at least one antimicrobial agent in all categories except for ≤2 categories, whereas PDR isolates were defined as resistant to all antimicrobial agents.
Statistical Analysis
Descriptive and inferential statistical methods were performed using the GraphPad Prism software, version 9.3.1. Regarding the inferential statistics, the demographic and clinical characteristics were analyzed by sex using 2×2 contingency table analyses and Fisher’s exact test to compare data of patients in the ICU and patients not in the ICU. The results were considered significant if the P-value was ≤0.05. Simple frequencies (n) and percentages (%) were calculated for each variable. Moreover, data were analyzed to identify risk factors associated with mortality among ICU patients with P. mirabilis infections. The odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated to assess the strength of associations. Multivariate logistic regression analysis was conducted to adjust for potential confounding variables. Variables with a univariate p-value ≤ 0.05 were included in the multivariate model.
Ethical Consideration
The KFMC review board committee approved this project after review by the local ethical research committee (IRB Registration Number with KACST, KSA: H-01-R‐012). Patient consent was not required as the data had been properly anonymized prior to access. This follows the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice guidelines and institutional review board Log Number: 21–426E ethical code.
Results
Clinical and Demographic Characteristics of Patients with P. Mirabilis Infection
Identified from various clinical samples, including urine, blood, sputum, endotracheal secretions, and other body tissues and fluids, a total of 598 P. mirabilis isolates were documented. Most P. mirabilis isolates were obtained from urine specimens (54.51%), of which 61.65% were from mid-stream urine, and 38.34% were collected from a catheter. Isolates from miscellaneous samples followed with 29.09% of which, 74.14% were obtained from wounds and ulcers, 6.32% were drawn from abscesses, 4.60% from the body fluid, 13.79% from tissue, 0.57% from a nasal swab, and 0.57% from a rectal swab. The fewest isolates were from blood (8.69%, 5.77%, and 94.23% from the central and peripheral lines, respectively) and respiratory samples (7.69%, 82.60%, and 17.39% from sputum and endotracheal tubes, respectively).
The proportion of P. mirabilis isolated from urine samples was higher in females (53.37%) than in males. A higher P. mirabilis infection rate was reported in males (53.94%) than in females. P. mirabilis infection was the highest in geriatric patients aged between 65 and 84 years (35.95%), followed by young adults aged 19 to 44 years (27.75%), adults between 45 and 64 years (20.56%), and pediatric patients aged 0 to 18 years (15.71%). The proportion of P. mirabilis infection based on the hospital department was highest in the emergency department (38.62%) and lowest in the ICU (13.04%).
Antibiotic Susceptibility of P. mirabilis Isolates
Antimicrobial susceptibility profiles were obtained for 598 P. mirabilis isolates (Figure 1). The susceptibility was highest for TZP (piperacillin-tazobactam), followed by ETP (ertapenem), MEM (meropenem), FOX (cefoxitin), IPM (imipenem), AMK (amikacin), CFP (cefepime), CTZ (ceftazidime), CTX (cefotaxime) and CRO (ceftriaxone), LVX (levofloxacin), GEN (gentamicin), and CIP (ciprofloxacin). The lowest susceptibilities were observed for CEF (cephalothin), TMP-SMX (trimethoprim-sulfamethoxazole), and AMP (ampicillin). Additionally, P. mirabilis was highly resistant to TGC (tigecycline) as P. mirabilis is naturally resistant to several antibiotics, including tigecycline and colistin.
 |
Figure 1 Antimicrobial susceptibility test results for P. mirabilis isolates (n = 598). |
During the three-year study period, the number of MDR, XDR, and PDR isolates decreased (Figure 2). MDR isolates were 29.77% in 2019, dropping to 13.04% in 2020 and 2.84% in 2021. In addition, XDR isolates were 15.21% in 2019, 8.53% in 2020, and 5.18% in 2021. PDR isolates accounted for 1% of all cases in 2019, but there were no PDR isolates in 2020 or 2021.
 |
Figure 2 Resistance phenotypes of P. mirabilis isolates. |
Comparison of Specimen Types and Antibiotic Susceptibility Between Male and Female Patients
A comparison of male and female infection types and susceptibility patterns was evaluated (Table 1). Overall, UTIs caused by P. mirabilis were higher in females (P <0.001), whereas P. mirabilis infections isolated from wounds as Miscellaneous specimens were higher in males (P <0.001). MDR P. mirabilis infection was associated with female patients (P 0.023), whereas XDR P. mirabilis infection (P 0.001) and PDR P. mirabilis infection (P 0.033) were associated with male patients.
 |
Table 1 Comparison of Infection Type and Antibiotic Susceptibility Between Males and Females |
Comparison of Infection and Antibiotic Susceptibility Among ICU and Non-ICU Patients
Infection types with a positive culture of P. mirabilis and antibiotic susceptibility patterns were compared between ICU and non-ICU patients from 2019 to 2021 (Table 2). Overall, respiratory infections were more common in ICU patients than non-ICU patients (P <0.001). In contrast, non-ICU patients had higher UTIs (P <0.001). MDR P. mirabilis isolates were more prevalent among non-ICU patients (P 0.039), while susceptible isolates were more common among ICU patients (P 0.023).
 |
Table 2 Comparison of Infection Type and Antibiotic Susceptibility Between ICU and Non-ICU Patie. Comparison of Infection Type and Antibiotic Susceptibility Between ICU and Non-ICU Patient |
Clinical Outcomes and Factors Associated with Mortality of ICU Patients with P. Mirabilis Infection
The mortality of ICU patients was calculated regarding age, type of infection, comorbidities, and antimicrobial susceptibility (Table 3). Regarding patients’ health conditions, the highest mortality rate was in patients with septic shock, followed by patients who had pre-exposure to antibiotics, malignancies, bacteremia, septicemia, and renal dialysis. P. mirabilis infections (UTI, CAUTI, blood, or respiratory) did not affect the mortality rate of ICU patients. Moreover, 81.48% (P <0.0001) of ICU patients infected with a susceptible strain had a better outcome with a high survival rate. Generally, the significant predictors of mortality among ICU patients were septicemia, septic shock, bacteremia, malignancy, pre-exposure to antibiotics, and renal dialysis in isolation. Furthermore, the presence of XDR P. mirabilis isolates was a significant predictor of death (OR: 3.150 (1.129–8.791)), but the presence of Susceptible P. mirabilis isolates was not (OR: 0.172 (0.056–0.527)).
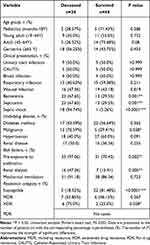 |
Table 3 Comparison of Demographic and Clinical Characteristics Among Deceased and Survived ICU Patients Infected with P. mirabilis |
As expected, the probability of death among ICU patients infected with P. mirabilis and suffering from septic shock is 105.6 times (P 0.002) higher than the odds of not having septic shock. Similarly, the probabilities of mortality among ICU patients infected with P. mirabilis and receiving renal dialysis are 7.2 times (P 0.034) more significant than those not. While bacteremia, malignancy, pre-exposure to antibiotics, XDR infection, and susceptible isolates were associated with mortality risk on univariate analysis, these factors did not retain statistical significance when adjusted for other variables in the multivariate model (Table 4).
 |
Table 4 Risk Factors Associated with Mortality Among ICU Patients with P. mirabilis (n=34 Deceased, n=44 Survived) |
Discussion
P. mirabilis clinical isolates are primarily responsible for UTIs.18,19 Most P. mirabilis isolates in our study were from urine samples, followed by samples mainly obtained from wounds, ulcers, and abscesses, similar to the findings of some studies.20–22 In contrast, the highest number of isolates were reported from pus, followed by urine samples in other studies.23–27 P. mirabilis infection rates were higher in female UTI patients in this study, although comparable infections were more common in geriatric patients aged 65-84. The exact frequency of P. mirabilis isolation in this age group was reported in other studies.25,28 This finding contradicts a study that reported Proteus isolates were obtained from male patients with UTIs aged 61 and 75 years old.27 In our study, respiratory P. mirabilis infections were the most common in the ICU, unlike UTIs or wound infections that are usually reported.27 Since the highest frequency was found in the emergency department, we could suggest that P. mirabilis is a community-acquired infection among the Saudi population. Wound infections caused by P. mirabilis were more frequent in males than females, which was consistent with the findings of another study.29
In this study, most P. mirabilis isolates were susceptible to imipenem, contradicting other studies.30,31 However, a recent meta-analysis reported similar imipenem susceptibility, and nitrofurantoin and colistin resistance was highest among the P. mirabilis isolates.32 Similarly, the highest resistance was reported to nitrofurantoin, while the lowest was for ceftriaxone.33 However, this study did not investigate nitrofurantoin and colistin resistance because KFMC stopped using colistin and nitrofurantoin in 2021 to treat P. mirabilis owing to its intrinsic resistance.34 Therefore, ceasing these antibiotics administration caused a surge in susceptible isolates in ICU and non-ICU patients. P. mirabilis isolates are intrinsically resistant to tigecycline,18 and this study showed high resistance to tigecycline, although some isolates were still susceptible. The highest MDR and XDR rates in isolates were observed in 2019 in all patients, but PDR isolates were only found in 2019. The lowest prevalence of MDR and XDR isolates was observed in 2021. After 2019, the resistance dropped significantly, possibly owing to the law prohibiting selling antibiotics without prescriptions by the Saudi Ministry of Health (MOH) and hospitals’ policies during and after the COVID-19 outbreak.
Mortality in ICU patients with P. mirabilis infection was associated with septic shock and renal dialysis. However, in a similar study, predisposing factors included indwelling urinary catheters, surgical intervention, intravascular catheters, respiratory assistance, and corticosteroid therapy.20 The discrepancy in the results may be explained by the application of infection control bundles to control nosocomial infections that have been applied in Saudi Arabian hospitals since 2019. Moreover, previous hospitalization in a nursing home and using urinary catheters were substantial risk factors for P. mirabilis infections due to ESBL-positive strains.35 In another study on bloodstream infections caused by P. mirabilis, the mortality rate was higher in patients with septic shock, inadequate initial antimicrobial therapy, and infections caused by an MDR strain.36 Additionally, our study suggests that Septic shock and dialysis were identified as statistically significant independent risk factors associated with increased mortality in ICU patients with P. mirabilis infection on multivariate analysis. This highlights their importance as predictors of poorer prognosis. Similarly, a study reported that bacteremia, UTIs, septic shock, and low body mass index are independent risk factors for mortality.37
In this study, bacteremia, malignancy, pre-exposure to antibiotics, XDR infection, and susceptible isolates were associated with mortality risk on univariate analysis. However, these factors did not retain statistical significance when adjusted for other variables in the multivariate model. A study from Japan found that pre-administration of antimicrobial agents was not a risk factor among 14 patients who had pneumonia caused by P. mirabilis.38
Antimicrobial resistance is a growing problem in hospitals and the ICU, and stewardship is an important strategy to combat it. The overuse and misuse of antibiotics can lead to the development of resistant strains of bacteria, making infections more difficult to treat. The importance of appropriate antimicrobial therapy was highlighted in a study on bloodstream infections caused by P. mirabilis, where inadequate initial antimicrobial therapy was identified as a risk factor for mortality.36
The observed demographic trends, with higher infection rates among females and non-ICU patients, provide valuable insights for tailoring antimicrobial stewardship efforts. Targeted interventions can be implemented to address these specific patient populations effectively. Moreover, the significantly elevated mortality risk among ICU patients infected with P. mirabilis and undergoing renal dialysis emphasizes the need for a meticulous approach to antibiotic selection and dosage adjustment in this vulnerable group. This underscores the critical role of antimicrobial stewardship programs in optimizing patient outcomes.
Therefore, considering these findings, hospitals should bolster their antimicrobial stewardship efforts by implementing strategies to monitor and respond to emerging resistance patterns. This may include routine susceptibility testing, regular review of prescribing practices, and education initiatives for healthcare providers. Additionally, comprehensive infection control measures should be revised, such as rigorous adherence to device protocols and stringent hand hygiene practices.
A few limitations were encountered in this research that should be considered in future studies. Clinical data were too limited to make inferences about the clinical outcomes of ICU patients. Future research should examine patient outcomes inside and outside the ICU to better understand septic shock. Moreover, data from a single tertiary institution were used, implying that a large-scale, multicenter, effective monitoring system in Riyadh and other Saudi Arabian cities with a larger sample size is required to understand P. mirabilis clinical consequences.
Conclusion
The study’s findings underscore a strategic approach to combatting P. mirabilis infections, emphasizing the prioritization of piperacillin-tazobactam, carbapenems, and cephalosporins for effective treatment. Caution is warranted regarding Cephalothin, trimethoprim-sulfamethoxazole, and ampicillin, given their lower susceptibilities. Of particular concern is the significantly heightened mortality risk observed among ICU patients with P. mirabilis infections undergoing renal dialysis, necessitating increased vigilance and targeted interventions for this vulnerable population. To address these challenges, hospitals must intensify their antimicrobial stewardship initiatives and implement comprehensive education programs for healthcare professionals. These measures are essential in preserving the effectiveness of antibiotics and mitigating the potentially devastating consequences of P. mirabilis infections in hospitalized patients.
Acknowledgments
The authors thank the Deputyship for Research and Innovation, ‘’Ministry of Education’’ in Saudi Arabia through project no. (IFKSUOR3–218–3) for funding this project. The authors thank Prince Naif Health Research Center, Investigator Support Unit for the language editing service provided.
Funding
The Deputyship for Research and Innovation, ''Ministry of Education'' in Saudi Arabia through project NO. (IFKSUOR3-218-3).
Disclosure
All authors declare that they have no competing interests for this work.
References
1. Armbruster CE, Smith SN, Johnson AO., et al. The pathogenic potential of Proteus mirabilis is enhanced by other uropathogens during polymicrobial urinary tract infection. Infec andim. 2017;85(2):e00808–16.
2. Armbruster CE, Mobley HL, Pearson MM. Pathogenesis of Proteus mirabilis infection. EcoSalPlus. 2018;8(1):128.
3. Algammal AM, Hashem HR, Alfifi KJ, et al. atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci Rep. 2021;11(1):1–15. doi:10.1038/s41598-021-88861-w
4. Schaffer JN, Pearson MM. Proteus mirabilis and urinary tract infections. Microbiol Spectr. 2015;3(5):UTI–0017–2013. doi:10.1128/microbiolspec.UTI-0017-2013
5. Jamil RT, Foris LA, Snowden J. Proteus Mirabilis Infections. Treasure Island, FL, USA: StatPearls; 2019.
6. Nucleo E, Fugazza G, Migliavacca R, et al. Differences in biofilm formation and aggregative adherence between β-lactam susceptible and β-lactamases producing P. mirabilis clinical isolates. The Newmicrobiologica. 2010;33(1):37.
7. Luzzaro F, Perilli M, Amicosante G, et al. Properties of multidrug-resistant, ESBL-producing Proteus mirabilis isolates and possible role of β-lactam/β-lactamase inhibitor combinations. Int J Antimicrob Agents. 2001;17(2):131–135. doi:10.1016/S0924-8579(00)00325-3
8. Yang Q, Zhang H, Cheng J, et al. In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and proteus mirabilis producing extended-spectrum β-lactamases in China. Int J Antimicrob Agents. 2015;45(5):485–490. doi:10.1016/j.ijantimicag.2014.11.012
9. Hall RM, Collis CM. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist Updates. 1998;1(2):109–119. doi:10.1016/S1368-7646(98)80026-5
10. Empel J, Baraniak A, Literacka E, et al. Molecular survey of β-lactamases conferring resistance to newer β-lactams in Enterobacteriaceae isolates from polish hospitals. Antimicrob Agen Chem. 2008;52(7):2449–2454. doi:10.1128/AAC.00043-08
11. Luzzaro F, Brigante G, D’Andrea MM, et al. Spread of multidrug-resistant Proteus mirabilis isolates producing an AmpC-type β-lactamase: epidemiology and clinical management. Int J Antimicrob Agents. 2009;33(4):328–333. doi:10.1016/j.ijantimicag.2008.09.007
12. D’Andrea MM, Literacka E, Zioga A, et al. Evolution and spread of a multidrug-resistant Proteus mirabilis clone with chromosomal AmpC-type cephalosporinases in Europe. Antimicrob Agen Chem. 2011;55(6):2735–2742. doi:10.1128/AAC.01736-10
13. Lv P, Hao G, Cao Y, Cui L, Wang G, Sun S. Detection of carbapenem resistance of proteus mirabilis strains isolated from foxes, raccoons and minks in China. Biology. 2022;11(2):292. doi:10.3390/biology11020292
14. Yang L, He H, Chen Q, et al. Nosocomial outbreak of carbapenemase-producing proteus mirabilis with two novel salmonella genomic island 1 variants carrying different bla NDM–1 gene copies in China. Front Microbiol. 2022;12(12):800938. doi:10.3389/fmicb.2021.800938
15. Gebre-Sealsssie S. Antimicrobial resistance patterns of clinical bacterial isolates in southwestern Ethiopia. Ethiopian Med J. 2007;45(4):363–370.
16. Chukwu BF, Okafor HU, Ikefuna AN. Asymptomatic bacteriuria in children with sickle cell anemia at the University of Nigeria teaching hospital, Enugu, South East, Nigeria. Italian J pediatrI. 2011;37(1):1–5. doi:10.1186/1824-7288-37-45
17. Mutair AA, Alhumaid S, Alawi ZA, et al. Five-year resistance trends in pathogens causing healthcare-associated infections at a multi-hospital healthcare system in Saudi Arabia, 2015–2019. J Global Antimicrob Resist. 2021;25(25):142–150. doi:10.1016/j.jgar.2021.03.009
18. Kanzari L, Ferjani S, Saidani M, et al. First report of extensively-drug-resistant Proteus mirabilis isolate carrying plasmid-mediated blaNDM-1 in a Tunisian intensive care unit. Int J Antimicrob Agents. 2018;52(6):906–909. doi:10.1016/j.ijantimicag.2018.06.009
19. Canton R, Akova M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18(5):413–431. doi:10.1111/j.1469-0691.2012.03821.x
20. De Champs C, Bonnet R, Sirot D, Chanal C, Sirot J. Clinical relevance of proteus mirabilis in hospital patients: a two year survey. J Antimicrob Chemother. 2000;45(4):537–539. doi:10.1093/jac/45.4.537
21. Al-Bassam WW, Al-Kazaz AK. The isolation and characterization of Proteus mirabilis from different clinical samples. J Biotechnol Res Cen. 2013;7(2):24–30. doi:10.24126/jobrc.2013.7.2.261
22. Jabur MH, AL-Saedi EA, Trad JK. Isolation of Proteus mirabilis and Proteus vulgaris from different clinical sources and study of some virulence factors. J of Bab Univ Pure Applied Sci. 2013;21(1):43–48.
23. Feglo PK, Gbedema SY, Quay SNA, Adu-Sarkodie Y, Opoku-Okrah C. Occurrence, species distribution and antibiotic resistance of Proteus isolates: a case study at the Komfo Anokye Teaching Hospital (KATH) in Ghana. Int J Pharm Sci Res. 2010;1(9):347–352.
24. Bahashwan SA, El Shafey HM. Antimicrobial resistance patterns of Proteus isolates from clinical specimens. Eur Sci J. 2013;9(27):1.
25. Shenoy SM, Sinha R, Sinha DR. Antibiotic sensitivity pattern of clinical isolates of Proteus species with special reference to ESBL and Amp C production. Indian J Appl Res. 2013;3(3):293–294. doi:10.15373/2249555X/MAR2013/97
26. Leulmi Z, Kandouli C, Benlabed K, Lezzar A, Ilhem Mihoubi I. Prevalence and evaluation of resistance to antibiotics of genera proteus, morganella and providencia isolates in university hospital of Constantine, Algeria. Int J Adv Res. 2014;2(1):220–227.
27. Priya PS, Leela K. Phenotypic characterisation of proteus species isolated from different clinical samples with special reference to antibiotic resistance pattern in a tertiary care centre. J Clin Diagn Res. 2022;16(1):1.
28. Prasad RR, Shree V, Sagar S, Kumar S, Kumar P. Prevalence and antimicrobial susceptibility pattern of proteus species in clinical samples. Int J Curr Microbiol Appl Sci. 2016;10(5):4.
29. Zafar U, Taj MK, Nawaz I, Zafar A, Taj I. Characterization of proteus mirabilis isolated from patient wounds at bolan medical complex hospital, Quetta. Jundishapur J Microbiol. 2019;12(7). doi:10.5812/jjm.87963
30. Neuwirth C, Siébor E, Duez JM, Péchinot A, Kazmierczak A. Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin-binding proteins. J Antimicrob Chemother. 1995;36(2):335–342. doi:10.1093/jac/36.2.335
31. Oliveira de Araujo JF, Lopes da Silva AL, Acioly de Omena IC, Alvino V, Todaro AR, Bastos MLDA. Proteus mirabilis resistant to carbapenems isolated from a patient with a venous leg ulcer: a case report. J Wound Care. 2022;31(5):460–464. doi:10.12968/jowc.2022.31.5.460
32. Vaez H, Kalarestaghi H, Sahebkar A, Khademi F. Prevalence of antibiotic resistance of Proteus species in urinary tract infections in Iran: a systematic review and meta-analysis. Gene Rep. 2022;27:101632. doi:10.1016/j.genrep.2022.101632
33. Hussein EI, Al-Batayneh K, Masadeh MM, et al. Assessment of pathogenic potential, virulent genes profile, and antibiotic susceptibility of Proteus mirabilis from urinary tract infection. Int J Microbiol. 2020;2:1.
34. Sheele JM, Vallabhaneni M. Should nitrofurantoin be used to treat alkaline urinary tract infection? Emerg Med. 2018;50(7):142–144. doi:10.12788/emed.2018.0099
35. Endimiani A, Luzzaro F, Brigante G, et al. Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum β-Lactamases. Antimicrob Agen Chem. 2005;49(7):2598–2605. doi:10.1128/AAC.49.7.2598-2605.2005
36. Tumbarello M, Trecarichi EM, Fiori B, et al. Multidrug-resistant Proteus mirabilis bloodstream infections: risk factors and outcomes. Antimicrob Agen Chem. 2012;56(6):3224–3231. doi:10.1128/AAC.05966-11
37. Chen C, Chen Y, Lu P, Lin W, Chen T, Lin CY. Proteus mirabilis urinary tract infection and bacteremia: risk factors, clinical presentation, and outcomes. J Microbiol Immunol Infect. 2012;45(3):228–236. doi:10.1016/j.jmii.2011.11.007
38. Okimoto N, Hayashi T, Ishiga M, et al. Clinical features of Proteus mirabilis pneumonia. J Infect Chemother. 2010;16(5):364–366. doi:10.1007/s10156-010-0059-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
