Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Multidimensional Frailty Instruments Can Predict Acute Exacerbations Within One Year in Patients with Stable Chronic Obstructive Pulmonary Disease: A Retrospective Longitudinal Study
Authors Wei L , Li P, Liu X, Wang Y, Tang Z, Zhao H, Yu L, Li K, Li J, Du M, Chen X, Zheng X, Zheng Y, Luo Y, Chen J, Jiang X, Chen X, Long H
Received 16 November 2023
Accepted for publication 24 March 2024
Published 4 April 2024 Volume 2024:19 Pages 859—871
DOI https://doi.org/10.2147/COPD.S448294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Lujie Wei,1,* Pingyang Li,2,* Xiaofeng Liu,1 Yuxia Wang,1 Zhengping Tang,1 Hang Zhao,1 Lu Yu,3 Kaixiu Li,1 Jianping Li,1 Min Du,4 Xinzhu Chen,2 Xin Zheng,2 Yixiong Zheng,5 Yao Luo,2 Jing Chen,5 Xiamin Jiang,6 Xiaobing Chen,7 Huaicong Long1
1Geriatric Intensive Care Unit, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan, People’s Republic of China; 2Medical College, University of Electronic Science and Technology of China, Chengdu, Sichuan, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Nanbu County People’s Hospital, Nanbu, Sichuan, People’s Republic of China; 5College of Medicine and Life Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 6Clinical College, North Sichuan Medical College, Nanchong, Sichuan, People’s Republic of China; 7Department of Respiratory and Critical Care Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huaicong Long, Geriatric Intensive Care Unit, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan, 610000, People’s Republic of China, Tel +86 18981838105, Fax +86-28-8779 5585, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is closely associated with frailty, and prevention of acute exacerbations is important for disease management. Moreover, COPD patients with frailty experience a higher risk of acute exacerbations. However, the frailty instruments that can better predict acute exacerbations remain unclear.
Purpose: (1) To explore the factors influencing frailty and acute exacerbations in stable COPD patients, and (2) quantify the ability of multidimensional frailty instruments to predict acute exacerbations within 1 year.
Patients and methods: In this retrospective longitudinal study, stable COPD patients were recruited from the outpatient department of Sichuan Provincial People’s Hospital from July 2022 to June 2023. COPD patients reviewed their frailty one year ago and their acute exacerbations within one year using face-to-face interviews with a self-developed frailty questionnaire. Frailty status was assessed using the Frailty Index (FI), frailty questionnaire (FRAIL), and Clinical Frailty Scale (CFS). One-way logistic regression was used to explore the factors influencing frailty and acute exacerbations. Multivariate logistic regression was used to establish a prediction model for acute exacerbations, and the accuracy of the three frailty instruments was compared by measuring the area under the receiver operating characteristic curve (AUC).
Results: A total of 120 individuals were included. Frailty incidence estimates using FI, FRAIL, and CFS were 23.3%, 11.7%, and 15.8%, respectively. The three frailty instruments showed consistency in COPD assessments (P< 0.05). After adjusting for covariates, frailty reflected by the FI and CFS score remained an independent risk factor for acute exacerbations. The CFS score was the best predictor of acute exacerbations (AUC, 0.764 (0.663– 0.866); sensitivity, 57.9%; specificity, 80.0%). Moreover, the combination of CFS plus FRAIL scores was a better predictor of acute exacerbations (AUC, 0.792 (0.693– 0.891); sensitivity, 86.3%; specificity, 60.0%).
Conclusion: Multidimensional frailty assessments could improve the identification of COPD patients at high risk of acute exacerbations and facilitate targeted interventions to reduce acute exacerbations in these patients.
Keywords: chronic obstructive pulmonary disease, frailty, acute exacerbations
Introduction
Frailty is a syndrome characterized by reduced physiological reserves and increased vulnerability to disease stress.1,2 Patients with chronic obstructive pulmonary disease (COPD) are twice as likely to be frail as those without COPD,3 with increasing evidence suggesting a relationship between frailty and chronic respiratory diseases. Frailty may play a role in the development of certain chronic diseases; conversely, chronic diseases may increase the risk of frailty.4 COPD patients with frailty experience a higher risk of acute exacerbations, hospitalization, and mortality.5 Acute exacerbations of COPD frequently occur and place substantial utilization and cost burdens on the healthcare system,6 reflecting the high social importance of these exacerbations. Since identification of high-risk patients can facilitate targeted interventions to diminish the progression of COPD,7 interest in the study of frailty in patients with COPD has increased recently. A good frailty tool should be able to accurately identify frailty and predict adverse consequences.8
According to a recent review, various frailty assessment tools have been used in combination with relevant clinical practices to predict progression in patients with COPD.9 The Frailty Index (FI) was constructed on the basis of a mathematical model of the accumulation of deficits, where a deficit can be any symptom, sign, disease, or disability that accumulates with age and is associated with adverse events.10 In contrast, the frailty questionnaire scale (FRAIL) is a simple, self-reported questionnaire based on the phenotype model.11 The FRAIL score is an independent risk factor for predicting adverse outcomes in specific populations.12 The Clinical Frailty Scale (CFS) is a global synthetic scale that allows screening for frailty using routine data from the comprehensive geriatric assessments.13 The CFS score is a comprehensive measure of frailty that shows a favorable correlation with the FI and has been validated across different clinical settings.10,14 Several studies have used frailty instruments to predict clinical outcomes in patients with COPD.3,8
Nevertheless, the frailty tools that can better measure acute exacerbations within 1 year in patients with stable COPD have not been adequately assessed in clinical trials. Since appropriate assessments of frailty may potentially help physicians identify patients at higher risk of acute exacerbations and thereby establish early interventions and treatments to mitigate or reverse frailty, we used multidimensional frailty instruments to assess stable COPD patients with the aim of (1) exploring the factors influencing frailty and acute exacerbations in COPD patients; and (2) evaluating the ability of multidimensional frailty to predict the identification of acute exacerbations within 1 year in stable COPD patients.
Methods
Study Design and Participants
This was a retrospective longitudinal study. Over a 12-month period from July 2022, patients with stable COPD were recruited at the outpatient department of the Respiratory and Critical Care Medicine Clinic of Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China. Inclusion criteria were as follows: stable COPD was defined as (1) COPD diagnosis for over a year based on the 2022 GOLD (Global Initiative for Chronic Obstructive Lung Disease) guideline and (2) no change in medication for at least three months. Potential participants were excluded on the basis of the following criteria: (1) non-COPD lung disease (eg, asthma, diffuse parenchymal lung disease, tuberculosis, and other limiting lung diseases); (2) inability to cooperate in completing a frailty assessment; and (3) inability to answer self-reported questions. The research protocol was based on the principles of the Helsinki Declaration and approved by the Research Ethics Committee of Sichuan Academy of the Medical Sciences and Sichuan Provincial People’s Hospital (Lun Audit [Research] No. 52–1 of 2022). All participants provided written informed consent.
Data Collection
The investigators underwent uniform and strict standardized training and passed an examination before they were allowed to enter the data collection session, which helped reduce reporting bias attributable to limited expertise and the influence of participant subjectivity. The investigator helped the patients with COPD to review their frailty one year ago and their acute exacerbations within one year with the help of a self- developed questionnaire during face-to-face interviews, which generally took 20 to 30 minutes. The questionnaire consisted of the following sections: sociodemographic characteristics (age, sex, height, weight, marital status, education level, monthly income level, and smoking), chronic diseases, maximum grip force, calf circumference, exercise habits, GOLD classification, lung function (peak expiratory flow [PEF], forced expiratory volume in one second/forced vital capacity [FEV1/FVC], and predicted FEV1 percentage [FEV1%pred]), modified Medical Research Council scale (mMRC) scores, COPD Assessment Test (CAT) scores, inhalation situation one year ago, and acute exacerbations in the past year. This study categorized the participant groups into ABE groups according to the 2023 GOLD guidelines.15 Acute exacerbations were defined as acute events characterized by worsening of the patient’s respiratory symptoms beyond normal day-to-day variations and leading to a change in treatment. Additionally, exercise habits were defined as the lifestyle habit of exercising more than three times a week for more than 30 minutes each time.16
Frailty Assessments
Frailty Index
Using a cumulative deficit model, Rockwood et al created the FI, a measure of the cumulative burden of a number of symptoms, diseases, disorders, and disabilities. Individuals with higher FI values are considered to be more frail.17 We obtained the data for 45 FI-related variables (Supplementary Table 1) based on our standardized procedures for FI calculation.18 The FI was calculated using these coded variables by dividing the number of items with deficits by 45 items, yielding a score ranging from 0 (the lowest possible frailty burden) to 1 (the highest possible frailty burden). Using the Rockwood team’s grading methodology,19 the patients were categorized as showing no frailty (FI < 0.1), mild frailty (0.1 ≤ FI < 0.2), or moderate/severe frailty (FI ≥ 0.2).
Frailty Questionnaire
FRAIL, which was proposed by the International Association of Nutrition and Aging, consists of five components (fatigue, resistance, ambulation, illness, and weight loss).11,20 Fatigue was measured by querying the participant how often they had felt fatigued in the past four weeks, scoring one point for answering “all of the time” or “the majority of the time.” Resistance was estimated by a question asking if the participant had difficulty walking up ten steps alone without rest and assistance, while ambulation was measured by asking if the participant had difficulty walking several hundred yards alone without aids; one point was awarded for “yes” answers to these questions. Illness was scored 1 for participants who reported suffering from five or more illnesses. Loss of weight was scored 1 for participants with a weight decline of 5% or greater within the past 1 year based on self-report. Patients were grouped as (1) frail if they met three or more criteria; (2) pre-frail if they met one or two criteria; and (3) robust if they did not meet any criteria.
Clinical Frailty Scale
The CFS is a 9-point global assessment tool that summarizes an older adult’s overall fitness or frailty level.10 The CFS is a practical meaningful instrument for assessing frailty and provides predictive information comparable to other established tools about the need for an institution. The CFS scores show a bimodal distribution, with peaks at 3 (“well, with treated comorbid disease”) and at 6 (“moderately frail”). Therefore, based on previous research, the CFS scores assigned by a trained physician according to functional performance and activities of daily living were categorized as CFS score ≤ 3; 4 ≤ CFS score ≤ 5; and CFS score ≥ 6.
Statistical Analysis
The quantitative variables were presented as mean, standard deviation (SD) or median, interquartile range (IQR), while qualitative variables were described as frequencies and percentages. Participants were categorized as follows: non-frail, mildly frail (0.1 ≤ FI < 0.2, 1 ≤ FRAIL ≤ 2, 4 ≤ CFS ≤ 5), and frail, based on each of the three frailty instruments. The consistency between the instruments was assessed using the Kappa statistic. Mosaic plots were presented to illustrate the frequency of frailty status using the three frailty instruments. We used analysis of variance or the Kruskal–Wallis test after verification of normality using the Kolmogorov–Smirnov test to examine differences in baseline characteristics in relation to the frailty categories. For comparing categorical characteristics between patients who showed acute exacerbations and those who did not, differences between qualitative variables were analyzed using the Pearson χ2 or Fisher’s exact test if the numbers were low. For continuous variables, between-group differences were assessed using the independent t-test or Kruskal–Wallis H-tests (if non-normally distributed data). A violin plot was utilized to visually compare the three frailty levels in relation to CAT and mMRC scores. We used a receiver operator characteristic (ROC) curve to assess the unadjusted predictive properties of the three frailty instruments for acute exacerbations within 1 year. Individual frailty instruments’ area under the curve (AUC) values were compared to determine statistical significance. An AUC value > 0.70 was considered to indicate an excellent discriminatory value. Associations between the frailty status assessed by the three instruments and frailty were determined using Poisson regression or logistic regression by estimating the odds ratio adjusted by age, educational level, exercise habit, and CAT score. All statistical analyses and plotting were performed using SPSS version 25.0 (IBM, Armonk, NY, USA) and Origin Pro version 2021 software. P values < 0.05 were considered indicative of statistical significance.
Results
General Characteristics and the Factors Influencing Frailty
The study population consisted of 120 patients with stable COPD, including 85 (70.8%) male and 35 (29.2%) female patients, with an average age of 68.34 ± 8.76 years. The estimated frailty incidence was 23.3% with FI, 11.7% with the FRAIL score, and 15.8% with the CFS score. Frailty, as defined by all instruments, was associated with older age, lower body mass index (BMI), exercise habits, smoking, maximum grip force, calf circumference, pulmonary function (PEF, FEV1/FVC, and FEV1%pred), more symptoms (CAT or mMRC score), acute exacerbations, and inhalation status (Table 1). We observed mild consistency among the three instruments (Kappa values ranged from 0.237 to 0.348), whereas FI and FRAIL showed better consistency (Kappa = 0.348) (Figure 1). Figure 2 shows the distribution of CAT and mMRC scores for different frailty statuses stratified using the three frailty instruments. The CAT and mMRC scores of the three frail groups were significantly different (P < 0.05; Supplementary Tables 2 and 3).
 |
Table 1 General Demographics and Clinical Characteristics of the Frailty Status Assessed by FI, FRAIL, and CFS in the COPD Patients |
 |
Figure 1 Mosaic plot representing the frequency of frailty status when evaluated by (A) FI and FRAIL, (B) FI and CFS, and (C) FRAIL and CFS. |
The Predictive Role of Frailty in Acute Exacerbations of COPD
Table 2 shows that education level, exercise habits, FEV1/FVC, CAT, mMRC, GOLD classification, and inhalation status were potential factors for acute exacerbations within one year in patients with COPD. After adjusting for covariates (age, educational level, exercise habit, and CAT score) in the final multivariate model, FI and CFS score remained independent risk factors for one-year acute exacerbations in patients with COPD (Table 3).
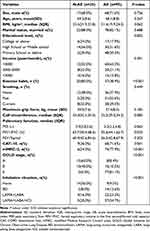 |
Table 2 General Characteristics Stratified by the Occurrence of Exacerbation Within 1 Year |
 |
Table 3 Multivariate Logistic Regression of Factors Predicting Acute Exacerbations Within 1 Year |
Comparison of the Three Frailty Instruments in Predicting Acute Exacerbations of COPD
For FI, a cut-off value of 0.106 showed a sensitivity of 74.7% and specificity of 56.0%. For the FRAIL score, a cut-off value of 1.500 showed a sensitivity of 29.5% and specificity of 96.0%. For the CFS score, a cut-off value of 4.500 showed a sensitivity of 57.9% and a specificity of 80.0% (Supplementary Table 4). Frailty evaluated by the CFS showed moderate performance in predicting 1-year acute exacerbations [AUC (95% CI): 0.764 (0.663–0.866)]. Furthermore, the AUC value of the CFS scores improved from 0.764 to 0.792 when combined with FRAIL (Figure 3).
Discussion
This retrospective longitudinal study showed a high incidence of frailty, as assessed by the FI, FRAIL score, and CFS score, in patients with stable COPD. Moreover, the three frailty instruments showed mild consistency. Our analysis revealed that frailty and acute exacerbations in patients with stable COPD shared some common influences. Notably, the FI and CFS instruments performed comparably in predicting 1-year acute exacerbations. Our findings add to the existing evidence showing that multidimensional frailty instruments can predict acute exacerbations within 1 year in patients with COPD. Therefore, proactive identification of frailty may facilitate risk stratification for acute exacerbations and help identify candidates for targeted interventions.
The high incidence of frailty in patients with COPD likely represents complex inter-relationships between features of both frailty and COPD.21 Patients with COPD usually experience dyspnea, muscle atrophy, and exercise intolerance, creating a vicious cycle. For example, impaired lung function limits exercise capacity in patients with COPD, and prolonged reductions in activity can lead to wasting atrophy of skeletal muscles and exercise intolerance, further exacerbating the dyspnea that manifests as a result of progressively limited activity.22,23 Thus, the prevalence of frailty in patients with COPD is unsurprising. A review of 27 studies found that an estimated 19% were frail, with 56% meeting the criteria for pre-frailty,3 which is consistent with our results. Moreover, the reported incidence of frailty among patients with COPD ranges from 6.6 to 75.5%, depending on the study population and the frailty assessment tools.24,25 In our study, the FI identified the highest percentage of frail patients with stable COPD. This is likely attributable to the multidimensional nature of the instrument, which evaluates comorbidities, function, and activities of daily living. In particular, patients with COPD in our study showed multidimensionally significant functional impairment. Thus, the fact that FI, which is primarily guided by the cumulative deficit model, evaluated a much higher rate of frailty, is not surprising. Notably, assessments using the three frailty instruments showed mild consistency, while better consistency was observed between FI and FRAIL. The results of the present study were similar to those reported by Zhang et al.26 However, the effect of this consistency was better in their study than ours, probably since their study only categorized patients with COPD into frail and non-frail groups, whereas our study categorized more patients into the pre-frail and mild frailty groups.
Acute exacerbations of COPD and frailty share some risk factors (eg, age and smoking) and pathophysiological mechanisms, including chronic inflammation, immune system dysfunction, and impaired neuroendocrine regulation.3 We found some aspects of the same, but not all, risk factors for frailty and acute exacerbation in patients with COPD. Consistent with the study by Lahousse,27 we observed an association between frailty (assessed with any of the three instruments) and pulmonary function (PEF and FEV1/FVC). Lara’s study showed that frailty was correlated with higher mMRC and CAT scores, and a CAT/mMRC combination ([CAT/8] + MRC) score ≥ 5.5 was highly associated with frailty.28 Similarly, our frailty instruments, apart from FRAIL, powerfully illustrated the subjective symptoms (CAT/mMRC score) and high frequency of exacerbations in COPD patients. Like previous studies investigating frailty in COPD patients,25,29 the COPD patients with frailty in our study showed more symptoms and less physical activity, factors that have been associated with higher acute exacerbations. Sedentary behavior has also been reported to be independently associated with frailty.30 Shortness of breath leads to inactivity,31 which causes deficits in muscle strength, mass, and quality.32 Conversely, the loss of muscle endurance and strength are components of frailty. Thus, a lack of exercise habits alone may be a helpful marker of frailty and a decreased physiologic reserve. Notably, no differences were observed in the age of COPD patients with different frailty statuses in our study. This finding, consistent with those reported by Elsa.33 and Kennedy,8 emphasizes that the frailty syndrome cannot be attributed to age or comorbidity.
More than 70 instruments for clinical evaluation of frailty are available at present,34 with no consensus regarding the gold standard instrument for assessment of frailty. The choice of a particular frailty assessment tool depends on the purpose, setting, time available, and characteristics of the interviewees.35 However, frailty evaluation is a feasible technique for prognostic assessments of acute exacerbations in patients with COPD. Our study used three of the more commonly available frailty instruments to assess patients with stable COPD. After adjusting for covariates in the final multivariate model, the FI and CFS score remained independent risk factors for one-year acute exacerbations in patients with COPD. These results were consistent with those obtained previously.5,29 Frailty evaluation is a valuable tool for predicting acute exacerbations in patients with COPD. The benefit of FI is that it enables more thorough evaluation of an individual’s overall condition, but it is time-constrained because the evaluation is difficult. The FRAIL is a simple questionnaire that a physician or healthcare professional can rapidly administer. It also conveniently allows relatively low-cost surveys by phone or self-administered questionnaires over long intervals. However, like other studies,29 our study showed that frailty reflected by the FRAIL score was not associated with the key COPD outcome of exacerbations. This may be because the boundaries of the FRAIL grading are not very clear or the sample size is relatively small. Therefore, the differences between the frailty assessment tools may be reflected not only in the incidence of frailty in COPD patients, but also in the prediction of acute exacerbations. The CFS relies upon a health professional’s evaluation of an individual’s frailty status using the descriptors as guidance, and is utterly dependent on an individual’s ability to perform the activities of daily living. The ease of use of the CFS, which can be readily utilized in a clinical setting, is one of the strengths that make it superior to previously developed tools. Notably, the CFS score was the best predictor of acute exacerbations among the three instrumental measurements in our study, which is consistent with the actual clinical situation.
Few studies have compared the association of frailty with COPD outcomes such as exacerbations by using heterogeneous definitions of frailty.27 In our study, the three frailty assessment tools showed similar mild predictive ability for acute exacerbations within 1 year. Our study simultaneously highlighted the usefulness of the CFS for predicting acute exacerbations in COPD patients since the CFS is a global measure of physical performance. Indeed, studies have shown tight correlations between validated frailty measures.36 This study corroborates previously reported information regarding the relationship between frailty and COPD. The CFS and FRAIL assess frailty, and generalized tools for summarizing multidimensional information with questionnaires and measurements may better meet clinical needs. Multidimensional frailty assessments may identify COPD patients who could benefit the most from clinical interventions.
The strengths of this study include the fact that we provided new data, including the association between frailty and acute exacerbations COPD within 1 year, which encouraged frailty assessments at the beginning of COPD management. Additionally, we used different frailty combinations to predict acute exacerbations in patients with COPD, which allowed more accurate prediction. Early assessment and diagnosis of multidimensional frailty may help physicians identify patients at higher risk of experiencing acute exacerbations and establish early interventions and treatments to mitigate or reverse the frailty condition.
Despite these strengths, our study also had limitations that require consideration. (1) Due to the single-center approach for recruitment, extrapolation of our results to other areas should be performed with caution. (2) The study participants included patients with stable COPD, which limited the suitability of the findings for the overall COPD population. In the future, it might be necessary to design multicenter, large-sample prospective studies to explore the effect of frailty assessment on acute exacerbations in patients with COPD to increase the credibility and applicability of the findings.
Conclusions
In this retrospective longitudinal study, the three frailty assessment instruments differed in their efficacy in predicting acute exacerbations in patients with COPD. Multidimensional frailty assessments could improve the identification and accuracy of patients at a high risk of acute exacerbations. Future studies should examine whether interventions for frailty can reduce acute exacerbations in COPD.
Abbreviations
FI, Frailty Index; FRAIL, Frailty Questionnaire; CFS, Clinical Frailty Scale; BMI, Body mass index; PEF, Peak expiratory flow; FEV1/FVC, Forced the first second of expiratory volume/forced vital capacity; CAT, COPD Assessment Test; mMRC, modified Medical Research Council scale; GOLD, Global Initiative for Chronic Obstructive Lung Disease; BD, Bronchodilator; LAMA, Long-acting muscarinic antagonists; LABA, Long-acting beta-antagonists; ICS, Inhaled corticosteroids; SD, Standard deviation; IQR, Interquartile range.
Data Sharing Statement
All the data used and/or analyzed during the current study are available from in the Sichuan Provincial Geriatric Medical Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China upon reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Research Ethics Committee of Sichuan Academy of the Medical Sciences and Sichuan Provincial People’s Hospital (Lun Audit (Research) No. 52-1 of 2022). Informed consent was obtained from all the study participants before enrollment.
Consent for Publication
We have obtained consent for publication from all participants.
Acknowledgments
The Authors acknowledge the assistance of nurses in the geriatric intensive care unit of “Sichuan Provincial Geriatric Medical Center, Sichuan Provincial People’s Hospital” and the doctors and nurses of Shuangliu district Maoyuan Hospital for their great efforts and help for data collection. We thank all the participants of this study for their willingness to take part in our research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors had drafted or written, or substantially revised or critically reviewed the article. All authors had agreed on the journal to which the article will be submitted. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agreed to take responsibility and be accountable for the contents of the article.
Funding
This study was funded by Technology Innovation R&D Project Fund of Chengdu Science and Technology Bureau (2021-YF05-02142-SN), Sichuan Cadre Health Key Project (Chuan Gan Yan ZH2021-201), and Sichuan Cadre Health Project (Chuan Gan Yan 2021-202).
Disclosure
The authors report no conflicts of interest in this work.
References
1. AJEK HEO. Frailty: implications for clinical practice and public health. Lancet. 2019;10206(394):1365–1375. doi:10.1016/S0140-6736(19)31786-6
2. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;10206(394):1376–1386. doi:10.1016/S0140-6736(19)31785-4
3. Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154(1):21–40. doi:10.1016/j.chest.2018.02.014
4. Veronese N, Sigeirsdottir K, Eiriksdottir G, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility-Reykjavik study. Rejuvenation Res. 2017;20(6):517–524. doi:10.1089/rej.2016.1905
5. Luo J, Zhang D, Tang W, Dou LY, Sun Y. Impact of frailty on the risk of exacerbations and all-cause mortality in elderly patients with stable chronic obstructive pulmonary disease. Clin Interv Aging. 2021;16:593–601. doi:10.2147/CIA.S303852
6. Dhamane AD, Moretz C, Zhou Y, et al. COPD exacerbation frequency and its association with health care resource utilization and costs. Int J Chron Obstruct Pulmon Dis. 2015;10:2609–2618. doi:10.2147/COPD.S90148
7. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
8. Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16(2):217–224. doi:10.1513/AnnalsATS.201803-175OC
9. Tarazona-Santabalbina FJ, Naval E, De la Cámara-de Las Heras JM, Cunha-Pérez C, Viña J. Is frailty diagnosis important in patients with COPD? A narrative review of the literature. Int J Environ Res Public Health. 2023;20(3):1678. doi:10.3390/ijerph20031678
10. Rockwood K. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–495. doi:10.1503/cmaj.050051
11. Morley JE. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Biochem. 2012;16(7):601–608. doi:10.1007/s12603-012-0084-2
12. Li Y, Zou Y, Wang S, et al. A pilot study of the FRAIL scale on predicting outcomes in Chinese elderly people with type 2 diabetes. J Am Med Dir Assoc. 2015;16(8):714–717. doi:10.1016/j.jamda.2015.05.019
13. Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771–787. doi:10.1007/s12603-019-1273-z
14. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20(1):393. doi:10.1186/s12877-020-01801-7
15. Global Initiative for Chronic Obstructive Lung Disease. Gold. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [2023 report]; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
16. Thompson PD, Arena R, Riebe D, Pescatello LS; American COSM. American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12(4):215–217. doi:10.1249/JSR.0b013e31829a68cf
17. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi:10.1100/tsw.2001.58
18. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi:10.1186/1471-2318-8-24
19. Theou O, Blodgett JM, Godin J, Rockwood K. Association between sedentary time and mortality across levels of frailty. Can Med Assoc J. 2017;189(33):E1056–E1064. doi:10.1503/cmaj.161034
20. Abellan Van Kan GA, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–72. doi:10.1016/j.jamda.2007.11.005
21. Bousquet J, Dinh-Xuan AT, Similowski T, et al. Should we use gait speed in COPD, FEV1 in frailty and dyspnoea in both? Eur Respir J. 2016;48(2):315–319. doi:10.1183/13993003.00633-2016
22. Jaitovich A, Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. What we know and can do for our patients. Am J Respir Crit Care Med. 2018;198(2):175–186. doi:10.1164/rccm.201710-2140CI
23. Poberezhets V, Mostovoy Y, Demchuk H. Exacerbation of chronic obstructive pulmonary diseases as a risk factor of the skeletal muscle dysfunction. Lung India. 2019;36(3):188–192. doi:10.4103/lungindia.lungindia_185_18
24. Mittal N, Raj R, Islam EA, Nugent K. The frequency of frailty in ambulatory patients with chronic lung diseases. J Prim Care Community Health. 2016;7(1):10–15. doi:10.1177/2150131915603202
25. Limpawattana P, Putraveephong S, Inthasuwan P, Boonsawat W, Theerakulpisut D, Chindaprasirt J. Frailty syndrome in ambulatory patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1193–1198. doi:10.2147/COPD.S134233
26. Zhang D, Tang W, Dou LY, Luo J, Sun Y. Four different frailty models predict health outcomes in older patients with stable chronic obstructive pulmonary disease. BMC Geriatr. 2022;22(1):57. doi:10.1186/s12877-022-02750-z
27. Lahousse L, Ziere G, Verlinden VJA, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci. 2016;71(5):689–695. doi:10.1093/gerona/glv154
28. de Souza Dias L, Galvão Ferreira AC, Rodrigues Da Silva JL, Barreto Conte M, Fouad Rabahi M. Prevalence of frailty and evaluation of associated variables among COPD patients. Int J Chronic Obstr. 2020;15:1349–1356. doi:10.2147/COPD.S250299
29. Yee N, Locke ER, Pike KC, et al. Frailty in chronic obstructive pulmonary disease and risk of exacerbations and hospitalizations. Int J Chron Obstruct Pulmon Dis. 2020;15:1967–1976. doi:10.2147/COPD.S245505
30. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate–vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80(2):187–191. doi:10.1016/j.maturitas.2014.11.010
31. Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005–1011. doi:10.1016/j.rmed.2010.01.012
32. Roig M, Eng JJ, MacIntyre DL, Road JD, Reid WD. Deficits in muscle strength, mass, quality, and mobility in people with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2011;31(2):120–124. doi:10.1097/HCR.0b013e3181f68ae4
33. Naval E, González MC, Giraldós S, et al. Frailty assessment in a stable COPD cohort: is there a COPD-frail phenotype? Chronic Obstruct Pulm Dis. 2021;18(5):525–532. doi:10.1080/15412555.2021.1975670
34. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi:10.1016/j.arr.2015.12.003
35. Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L. Frailty and risk of adverse outcomes in hospitalized older adults: a comparison of different frailty measures. J Am Med Dir Assoc. 2017;18(7):637–638. doi:10.1016/j.jamda.2017.04.011
36. Jung HW, Baek JY, Jang IY, et al. Short physical performance battery as a crosswalk between frailty phenotype and deficit accumulation frailty index. J Gerontol a Biol Sci Med Sci. 2021;76(12):2249–2255. doi:10.1093/gerona/glab087
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


