Back to Journals » Journal of Pain Research » Volume 15
Multicenter, Double-Blinded, Randomized, Active-Sham Controlled Clinical Study Design to Assess the Safety and Effectiveness of a Novel High Frequency Electric Nerve Block System in the Treatment of Post-Amputation Pain (The QUEST Study)
Authors Kapural L , Syed Shah N, Fang ZP, Mekhail N
Received 28 December 2021
Accepted for publication 3 May 2022
Published 3 June 2022 Volume 2022:15 Pages 1623—1631
DOI https://doi.org/10.2147/JPR.S353674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Dawood Sayed
Leonardo Kapural,1 Nemath Syed Shah,2 Zi-Ping Fang,2 Nagy Mekhail3
1Carolinas Pain Institute, Winston-Salem, NC, USA; 2Neuros Medical, Inc., Willoughby Hills, OH, USA; 3Evidence-Based Pain Management Research, Department of Pain Management, Cleveland Clinic, Cleveland, OH, USA
Correspondence: Leonardo Kapural, Carolinas Pain Institute, 145 Kimel Park Drive, Winston-Salem, NC, 27023, USA, Tel +1 336-765-6181, Email [email protected]
Background: Chronic pain that follows amputation of a limb is reported as “one of the most severe pains in the human experience,” due to the magnitude of tissue injury and the multiple potential of pain generators at the local peripheral, spinal, and cortical levels. The Altius® System was developed to deliver high-frequency nerve block (HFNB) therapy via a cuff electrode applied to the peripheral nerve(s) and an implantable pulse generator. We report a novel clinical trial design for the first study of an active-implantable medical device in subjects with lower-limb post-amputation pain utilizing a multicenter, double-blinded, randomized, active-sham controlled clinical study protocol called QUEST, which is an ongoing investigational device exemption study to support United States Food and Drug Administration approval.
Methods: The study enrollment of 180 subjects was completed in September 2021. Subjects were randomized 1:1 to the treatment group or the active-sham control group for the 3-month primary effectiveness and safety endpoints. After month 3, the active-sham control program group crossed over to the treatment program group and all subjects continued to the 12-month study endpoint. Study effectiveness success is determined by a superiority test between responder rates in the treatment and control groups at 3 months. A responder is defined as someone who experiences a 50% or greater reduction in pain scores – after a 30-minute treatment session – for more than 50% of all pain episodes in which the treatment was used.
Discussion: The QUEST study design employs an active-sham control group to objectively assess the effectiveness of HFNB therapy. Additionally, the electronic diary repeated measures data collection in QUEST is expected to reduce the intra-subject variation typically observed in pain treatment studies. Finally, the longitudinal measurement of health-related quality of life and use of pain medication may, for example, show effectiveness in reducing opioid use over time.
Keywords: post-amputation pain, phantom pain, chronic pain, high frequency nerve block, double-blind method, randomized controlled trial, repeated measures
Introduction
In the United States, the prevalence of limb loss was 1.6 million in 2005, which is projected to increase to 3.6 million by 2050.1 Approximately 160,000 lower-limb amputations are performed annually.2 Amputation of a limb is reported as “one of the most severe pains in the human experience,” due to the magnitude of tissue injury and the multiple potential of pain generators at the local peripheral, spinal, and cortical levels.3
In general, post-amputation pain (PAP) is estimated to afflict approximately 30% to 80% of patients who undergo a major limb amputation.4,5 The loss of a body part can lead to painful neurologic sequelae that fall into two descriptive categories. Phantom limb pain (PLP) is the pain felt as if it were coming from a missing limb or body part that no longer exists. Residual limb pain (RLP) is pain or discomfort experienced in or at the stump of an amputated limb.6
Pharmacological treatments remain the first- and second-line therapies, although their safety and efficacy are completely inadequate.7,8 First-tier therapy options provided after post-amputation typically include non-steroidal anti-inflammatory (NSAIDS) drugs, membrane stabilizers and regional nerve blocks. These options can provide short-term pain relief in the acute phase of recovery from amputation. For example, nerve blocks using lidocaine and/or corticosteroids often result in immediate relief of PAP; however, the duration of pain relief is highly variable and temporary at best. Second-tier therapy options if the pain persists for months or longer post-amputation typically include the addition of opioids and gabapentinoids. The pain relief provided by these drugs is associated with significant, and for many patients, intolerable side effects.9
Despite the fact that pre-amputation and intraoperative preventative pharmacological and interventional measures may decrease the frequency of post-amputation pain,10–12chronic pain in the residual limbs is present in over 1 million amputees in the US. The majority of those patients maintain pain refractory to current pharmacological treatments.
Spinal cord stimulation (SCS), dorsal root ganglia (DRG) and peripheral nerve stimulation (PNS) devices have been shown to reduce post-amputation pain in case reports and small single-investigator studies.13–17 However, the effectiveness of these treatment options for chronic PAP is poor when compared to other neuropathic pain states.14 Studies evaluating SCS have reported variable success rates, with one study reporting initial pain relief in 42.6% of patients and chronic pain relief in 25% of patients. Alternatively, other studies have found that pain relief for long-term use of SCS is either poor or non-existent.15 PNS has also been shown to have the potential as an effective therapy for PAP. In a preliminary study evaluating PNS for post-amputation pain, 67% of participants treated with PNS sustained a 50% reduction of the average pain scores at 12 months.16 However, clinical use of PNS for chronic PAP has been limited in part due to the lack of large, randomized-controlled clinical trials demonstrating its safety and effectiveness specifically, in PAP.13 Taken together, currently marketed SCS and PNS devices are not accepted as mainstream treatments of PAP and are considered experimental treatment options for lower limb amputees with chronic PAP.
A final option for these patients is surgical ablation of the neuroma at the severed end of the nerve(s). Neuromas may occur in up to 80% of PAP patients.18,19 This typically manifests as pain in the residual limb. The long-term outcomes of peripheral nerve surgery are mixed, and studies have found that patients only experience a short duration of pain relief, with recurrence of the neuromas, and frequent return of PLP and RLP, re-developing months after initial resection.19–21
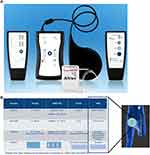
Recent bench research and preclinical studies have explored the use of a high frequency (≥5000 Hz) alternating current to block nerve conduction through the inactivation of sodium channels by sustained depolarization of the cell membrane. These studies have demonstrated that continuous application of an alternating current within the 5–50 kHz frequency range could result in a reliable and reversible conduction block in motor, sensory, and autonomic nerves.22–25 A novel implantable neuromodulation device has been developed to deliver a high-frequency nerve block (HFNB) current directly to targeted nerve fibers to block the propagation of pain signals in order to relieve PAP (Figure 1. The Altius® System, Neuros Medical, Inc., Willoughby Hills, Ohio).
The Altius System consists of a rechargeable implantable pulse generator (IPG) connected to one or two nerve cuff electrode(s) that are wrapped around the target nerve(s) (sciatic or its branches; tibial and peroneal). The IPG is programmed with therapeutically effective settings through in-clinic testing. Patients are then able to initiate therapy sessions based on their needs for pain control.
In an investigational device exemption (IDE) pilot study using HFNB, 70% (7/10) of subjects attained ≥50% pain reduction in ≥50% of their HFNB sessions during in-clinic testing and proceeded to in-home therapy.26 All subjects who received in-home therapy demonstrated significant pain relief through three months and this therapeutic effectiveness was sustained through 12 months. The pilot study provided preliminary evidence of the effectiveness and safety of the HFNB therapy and justified a larger sample size, randomized pivotal study.
Here, we report on the trial design for the High Frequency Nerve Block System in the Treatment of Patients with Post-Amputation Pain (QUEST) randomized controlled pivotal study being conducted as part of FDA’s premarket approval (PMA) process (ClinicalTrials.gov ID: NCT02221934). The study is designed to evaluate the effect size of the Treatment program group compared to a Control active-sham program group at a 3-month endpoint in randomized subjects suffering from lower limb post-amputation pain.
Methods/Results
The QUEST Study is a multicenter, double-blinded, randomized, active-sham-controlled clinical trial, and the purpose of the study is to evaluate the safety and effectiveness of the Altius System treatment for the management of PAP (Figure 1). The study enrollment of 180 subjects was completed in September 2021. Trial was registered on ClinicalTrials.gov (NCT02221934) prior to study enrollment. The protocol and informed consent were approved by the Western Investigational Review Board, and local site IRBs as appropriate and in accordance with the Declaration of Helsinki. In addition, written informed consent was provided by the patients. The 3-month effectiveness alternate hypothesis is used to demonstrate statistical superiority of the Treatment program group versus the Control active-sham program group during the randomized double-blinded testing phase (Figure 2).
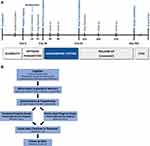 |
Figure 2 The QUEST subject schedule is presented in (A), and the QUEST subject flow chart is presented in (B). (A) QUEST schedule overview. (B) QUEST subject flow chart. |
The study population will consist of adult lower-limb amputees suffering from chronic (persistent >6 months), severe (≥4 episodes of pain per week on average with >5 NRS) post-amputation pain, which is resistant to pain medication. Subjects must demonstrate significant pain relief after ultrasound guided lidocaine sciatic nerve block injection proximal to the amputation site and must demonstrate little or no pain relief after a sham (saline) injection at the same location. The therapeutic response to lidocaine was demonstrated to be a strong predictor of the subject’s response to electrical nerve block in the pilot study.26
The implant procedure (Day 0) is conducted by neurosurgeon, vascular or general surgeon as an outpatient surgical visit under general anesthesia. The procedure for accessing the target nerve and for placing the cuff electrode on the previously severed nerve is akin to the conventional procedure for neuroma resection. Subjects are implanted with up to two cuff electrodes on either the sciatic nerve main trunk or on its branches, common peroneal and tibial nerves depending on the level of amputation. The lead(s) is/are tunneled subcutaneously to the rechargeable IPG. The IPG implantation location – typically, the lower abdomen resembles those selected for other commercially available neurostimulators and is within easy reach of the subject for communication with and recharging of the device. The IPG is placed in a subcutaneous pocket.
At 2 weeks post-surgery (Day 14), the system is activated, and initial treatment parameters are programmed and then adjusted on Day 21 (Figure 2). Subjects are randomized in a 1:1 ratio to receive either the Treatment program or the Control active-sham program. The programmable parameters include amplitude, frequency, lockout time, ramp-up time, and stimulation duration. Amplitude, ramp-up time, and stimulation duration can be programmed independently for each cuff electrode.
At Day 28, the subjects begin the 2-month randomized study period (Day 28 to Day 91). The Treatment group receives a high frequency (5000 or 10,000 Hz) stimulation program for 30 minutes. The Control active-sham group receives an ultra-low frequency (0.1 Hz) program for 8 minutes (Table 1). Exact program parameters are determined based on the subject’s response to the assigned treatment so that all subjects perceive a sensation when the device is activated. Subjects use the device via a patient controller (Figure 1) that communicates transcutaneously with the IPG as needed for pain relief.
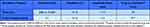 |
Table 1 Stimulation Programs for the Treatment Group and the Control Active-Sham Group during the Randomized Testing Period |
The randomized testing period occurs between Day 28 and Day 91 during which the subjects activate the system as needed for pain management. The subjects record their pain intensity using a 0–10 Numerical Rating Scale (NRS) score before therapy and after therapy in their electronic diary (eDiary) application on a study-provided, secure mobile phone (Axiom, Inc., Toronto, Canada and Samsung, Seoul, South Korea). After Day 91, subjects previously receiving the Control active-sham program crossover to the Treatment program. Follow-up continues through 12 months (Day 365). The complete study schedule is summarized in Figure 2.
The 3-month primary effectiveness data are the repeated measures of pain intensity before and after each therapy session from Day 28 to Day 91 by each subject using the 0–10 NRS. The 3-month primary effectiveness endpoint is the proportion of responders in the Treatment program group vs the Control active-sham program group. Specifically, a responder must attain ≥50% pain reduction in ≥50% of the treatment sessions during the randomized testing period of the study.
Secondary variables to be measured during this study include pain interference with activities of daily living (ADL) as assessed by the Brief Pain Inventory (BPI). Three additional questionnaires are being employed as well to assess a health-related quality of life: Medical Outcomes Study 12-item Short Form Health Survey (SF-12; OptumInsight Life Sciences, Inc.), European Quality of Life-Five Dimensional-Five Level (EQ-5D; EuroQol Group), and Patient Global Impression of Change.
Randomization is stratified by clinical sites with random permuted blocks to achieve an approximate balance of treatment allocation within each site. The block size is concealed from site personnel. Subjects and the investigators, or designees, performing study assessments are blinded to the subjects’ randomized group assignment from the time of randomization to study completion. Blinding aims to eliminate or minimize the confounding influence on outcomes caused by knowledge about treatment allocation and its related expectations. The implant procedure performed by the investigator is identical between treatment groups and device programming is conducted following a script to ensure that the subjects in each treatment group will have an identical experience. Parameter determination will be based on the subject’s response to the assigned treatment and selected so that all subjects, regardless of treatment group, experience a perceivable sensation when the device is activated. The parameters programmed by the Field Clinical Specialist (FCS) are stored separately from other study documents and are not accessible to the sponsor, investigator or study staff unless necessary for safety-related unblinding.
Data quality is maintained through study and device training, site monitoring, and data management. eDiary compliance and accuracy will be monitored by comparing the subject-reported device usage with the log that is automatically recorded by the Altius device. An Independent Physician Adjudicator (IPA) with expertise in relevant medical specialties is employed for adjudication of adverse events. At a minimum, the IPA will adjudicate all reported deaths, device- or procedure-related SAEs, and SAEs associated with the target limb or abdominal implant site.
Adverse event rates will be monitored for the entire duration of the study by an independent Data Monitoring Committee comprising medical and statistical expert reviewers. The primary safety endpoint is the assessment of all reported adverse events starting or worsening from the time of injection screening to 3 months post-implant. There is no formal statistical hypothesis for safety.
The study was powered according to the primary effectiveness endpoint. Total sample size calculation used a normal approximation multiplied by an inflation factor for group sequential design (PASS 15 Power Analysis and Sample Size Software (2017). NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass). With an estimated attrition rate of up to 10%, to account for subjects who received implantation but did not proceed to randomization due to various reasons, such as significant change in pain intensity or frequency, the sample size equaled 180 implanted subjects.
The primary analysis population for the effectiveness endpoint is a Full Analysis Set (FAS) group, which includes subjects who were randomized and had documented device treatment use. The primary analysis population for safety endpoints includes all subjects who underwent implant surgery.
Two additional analysis populations will be utilized to assess study effectiveness outcomes. First, the intent-to-treat (ITT) population consists of all randomized subjects. This analysis estimates the causal effect of being assigned to Treatment versus Control. Second, the per-protocol (PP) analysis set will be restricted to subjects who fulfil the protocol in terms of eligibility, treatment, and outcome assessment. This analysis attempts to estimate the treatment effect when complying with the protocol but is typically subject to selection bias.
Discussion
The Altius System is intended for the treatment of PAP using a novel neuromodulation therapy, which employs a high frequency alternating current to electrically induce nerve blocks. HFNB technology has the potential to provide an effective, mechanism-based treatment for managing intractable PAP.
The QUEST Study protocol was designed to minimize intentional or unintentional bias. A randomized study design is employed to increase the probability of comparable treatment populations and remove investigator bias in the allocations of treatment assignments. The study design is based on previous sham-controlled IDE pivotal studies approved for neuromodulation devices for treating neurogenic pain as well as recommendations from The Initiative on Methods, Measurements, and Pain Assessment in Clinical Trials (IMMPACT).27,28 The 3-month primary endpoint randomized period represents a reasonable compromise between the scientific goals, which are best served by long-term parallel comparison and ethical considerations, which necessitate a shorter period of withholding a potentially beneficial treatment. The primary variable for the study, subject-reported pain intensity, will be measured by the NRS. The validity of the NRS has been well demonstrated by its positive and significant correlation with other measures of pain intensity such as the Visual Analog Scale (VAS).29,30 Its sensitivity to treatments that are expected to have an impact on pain intensity has also been demonstrated.31 The NRS is easy to administer and score, so it can be used in a variety of subjects.31–33 The secondary variables in this study include pain interference with ADLs and quality of life by using questionnaires recommended by IMMPACT and/or have been validated in previous chronic pain studies.29,30,33
QUEST unblinding risk was markedly reduced by using the same surgery to implant the same devices for both treatment and active-sham groups. Device activation occurred prior to randomization, and the same procedures were used in both groups to prevent the investigator or their staff from determining the treatment group. Programming by the company field clinical specialist (FCS) was done under the supervision of the investigator or designee; FCSs were not allowed contact with the subjects outside of the clinic. Optimization of treatment parameters ensured that all subjects experienced a sensation when using the device, reducing the potential that subjects discerned their treatment arm through device use. The pattern of stimulation used for the control group eliminated the possibility of non-intentional therapeutic effects in two ways:1 a minimal pulse width current was delivered at very low frequency as control program and2 this electrical stimulus was delivered at the initiation of therapy but was programmed to stop after 8 minutes. Finally, the 8 min control program duration was based on the observation by Soin et al that the “tingling-like” treatment-induced sensation increased then subsided within the first 10 minutes of a treatment session on average.26
QUEST is the first randomized longitudinal study with multiple, repeated measurements of a medical device therapy for treatment of chronic, intractable pain. As described by Detry and Ma:
It is possible to compare outcomes between treatments using only a final measurement to determine whether there was a difference at the end of the study; however, this approach would not include much of the information captured with repeated measurements and there would be no consideration of the pattern of outcomes each patient experienced in reaching his or her final outcome. When outcomes are measured repeatedly over time, a wide variety of clinically important questions may be addressed.34
The QUEST 3-month primary endpoint data set may contain thousands of before and after NRS pain score therapy sessions extrapolating from the repeated measurements of the Pilot Study subjects in their first 3-month period.26 The repeated measures data collection in QUEST is expected to accurately assess and precisely the alternate hypothesis difference between the Treatment and Control groups, but, when analyzed with secondary variables, the longitudinal measurement of health-related quality of life and use of pain medication may, for example, show effectiveness in the reduction of opioid use over time.
Conclusion
The QUEST Study is the first large multicenter, double-blinded, randomized, active-sham-controlled pivotal study in the field of neuromodulation to measure a response to HFNB therapy. This study was designed to evaluate the evidence required to support FDA premarket approval of the Altius System for PAP. By combining randomization and crossover time periods with repeated measures, the QUEST trial provides a unique opportunity to understand the safety and effectiveness of the Altius System – over time – as a therapy for chronic, intractable lower limb post-amputation pain.
Acknowledgments
The authors would like to thank John Doucet, PhD, and William R. Patterson, PhD, for their helpful manuscript discussions and preparation.
Funding
The QUEST study is supported by Neuros Medical, Inc., Willoughby Hills, OH.
Disclosure
Dr. Kapural receives research support as the principal investigator of the QUEST study. He also reports research grants provided by Neuros Medical to all clinical research sites. Dr. Mekhail receives research support as a site principal investigator. Dr. Fang and Mr. Syed Shah are employees of Neuros Medical, Inc. Mr. Syed Shah reports patents “High Frequency Electrical Nerve Block” and “Nerve cuff electrode for neuromodulation in large human nerve trunks” issued. The authors report no other conflicts of interest in this work.
References
1. Zieger-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States:2005 to 2050. Arch Phys Med Rehabili. 2008;89:422–429. doi:10.1016/j.apmr.2007.11.005
2. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat. 1998;13(139):1–119.
3. Neil MJE. Pain after amputation. BJA Educ. 2016;16(3):107–112. doi:10.1093/bjaed/mkv028
4. Luo Y, Anderson TA. Phantom limb pain: a review. Int Anesthesiol Clin. 2016;54(2):121–139. doi:10.1097/AIA.0000000000000095
5. Hanley MA, Jensen MP, Smith DG, Ehde DM, Edwards WT, Robinson LR. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain. 2007;8(2):102–109. doi:10.1016/j.jpain.2006.06.004
6. International Neuromodulation Society. Post-amputation pain and phantom limb pain. Available from: https://www.neuromodulation.com/amputation-pain.
7. Flahaut M, Laurent NL, Michetti M, Hirt-Burri N, Jensen W, Lontis R. Patient care for postamputation pain and the complexity of therapies: living experiences. Pain Manag. 2018;8(6):441–453. doi:10.2217/pmt-2018-0033
8. Sekonyela-Rakolanyana R, Ferguson M, Hunziker S. Managing phantom limb pain with medication: pharmacologic options for the treatment of this multifactorial, neuropathic condition, including gabapentin, TCAs, opioids, ketamine, memantine, lidocaine, and bupivacaine. Pract Pain Manag. 2019;19:54–57.
9. Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016;10(10):CD006380. doi:10.1002/14651858.CD006380.pub3
10. Yousef AA, Aborahma AM. The preventive value of epidural calcitonin in patients with lower limb amputation. Pain Med. 2017;18:1745–1751. doi:10.1093/pm/pnw249
11. Ayling OG, Montbriand J, Jiang J, et al. Continuous regional anaesthesia provides effective pain management and reduces opioid requirement following major lower limb amputation. Eur J Vasc Endovasc Surg. 2014;48:559–564. doi:10.1016/j.ejvs.2014.07.002
12. Economides JM, DeFazio MV, Attinger CE, Barbour JR. Prevention of painful neuroma and phantom limb pain after transfemoral amputations through concomitant nerve coaptation and collagen nerve wrapping. Neurosurgery. 2016;79:508–513. doi:10.1227/NEU.0000000000001313
13. Page DM, George JA, Kluger DT, et al. Motor control and sensory feedback enhance prosthesis embodiment and reduce phantom pain after long-term hand amputation. Front Hum Neurosci. 2018;12:352. doi:10.3389/fnhum.2018.00352
14. Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. doi:10.2147/JPR.S32299
15. Aiyer R, Barkin RL, Bhatia A, Gungor S. A systematic review on the treatment of phantom limb pain with spinal cord stimulation. Pain Manag. 2017;7(1):59–69. doi:10.2217/pmt-2016-0041
16. Gilmore C, Ilfeld B, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2019;44(6):637–645. doi:10.1136/rapm-2018-100109
17. Petrini FM, Bumbasirevic M, Valle G, et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat Med. 2019;25:1356–1363. doi:10.1038/s41591-019-0567-3
18. Herta F. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 2002;1:182–189. doi:10.1016/S1474-4422(02)00074-1
19. Sturm V, Kroger M, Penzholz H. Problems of peripheral nerve surgery in amputation stump pain and phantom limbs. Chirurg. 1975;46:389–391.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78:714–719. doi:10.3171/jns.1993.78.5.0714
21. Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77:427–438. doi:10.1097/00006534-198603000-00016
22. Miles JD, Kilgore KL, Bhadra N, et al. Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. J Neural Eng. 2007;4(4):390–398. doi:10.1088/1741-2560/4/4/005
23. Bhadra N, Kilgore KL. High-frequency nerve conduction block. Conf Proc IEEE Eng Med Biol Soc. 2005;2004:4729–4732.
24. Tai C, Guo D, Wang J, Roppolo JR, de Groat WC. Mechanism of conduction block in amphibian myelinated axon induced by biphasic electrical current at ultra-high frequency. J Comput Neurosci. 2011;31(3):615–623. doi:10.1007/s10827-011-0329-9
25. Waataja JJ, Tweden KS, Honda CN. Honda CN Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng. 2011;8(5):056013. doi:10.1088/1741-2560/8/5/056013
26. Soin A, Shah NS, Fang ZP. High-frequency electrical nerve block for postamputation pain: a pilot study. Neuromodulation. 2015;18(3):197–205. doi:10.1111/ner.12266
27. Dworkin RH, Turk DC, Farrar JT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
28. Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149(2):177–193. doi:10.1016/j.pain.2010.02.018
29. Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi:10.1016/0304-3959(86)90228-9
30. Wilkie DJ, Holzemer WL, Tesler MD, et al. Measuring pain quality: validity and reliability of children’s and adolescents’ pain language. Pain. 1990;41(2):151–159. doi:10.1016/0304-3959(90)90019-A
31. Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20(2):88–93. doi:10.1097/00002820-199704000-00002
32. Jensen IB, Bergstrom GBergström G, Ljungquist T, Bodin L, Nygren AL. A randomized controlled component analysis of a behavioral medicine rehabilitation program for chronic spinal pain: are the effects dependent on gender? Pain. 2001;91(1–2):65–78. doi:10.1016/S0304-3959(00)00420-6
33. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Cleeland CS Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi:10.1097/00002508-200409000-00005
34. Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315(4):407–408. doi:10.1001/jama.2015.19394
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.