Back to Journals » Infection and Drug Resistance » Volume 13
Multi-Organ Abscesses and 5th Cervical Vertebra Bone Destruction Related with Klebsiella ozaenae Infection: A Case Report
Authors Wu Y, Yang D, Wang K, Liu C
Received 30 July 2020
Accepted for publication 11 November 2020
Published 27 November 2020 Volume 2020:13 Pages 4321—4325
DOI https://doi.org/10.2147/IDR.S274742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yonghong Wu,1,1,2 Dan Yang,1,1 Ke Wang,1,1 Chuntao Liu1,1
1Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Department of Critical Care Medicine, The People’s Hospital of Jianyang City, Jianyang, People’s Republic of China
Correspondence: Ke Wang; Chuntao Liu
Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, People’s Republic of China
Email [email protected]; [email protected]
Abstract: Klebsiella ozaenae (K. ozaenae) is a causative pathogen of some rare diseases such as primary atrophic rhinitis and ozena. Here, we describe one case of a potentially lethal kind of K. ozaenae infection in which multiple organs were implicated. A 40-year-old diabetic male patient presented to our hospital due to fever with right anterior chest mass and neck and shoulder pain for half a month. Based on all examination results, he was diagnosed with sepsis, bilateral pulmonary/right chest wall/liver abscesses and 5th cervical vertebra bone destruction with prevertebral abscesses, all related with K. ozaenae infection. During the first time of admission, he was treated with antimicrobials without operations. Twelve days after his first discharge, fever and pain occurred again, the patient was treated with antimicrobials, operations (anterior debridement, spinal canal decompression, iliac bone graft fusion and internal fixation) and rehabilitation at second admission. The patient recovered well and was discharged from hospital. This case report demonstrates that K. ozaenae can trigger a wide range invasive infections. Particularly, 5th cervical vertebra bone destruction was first reported as a clinical manifestation of K. ozaenae infection in our patient.
Keywords: Klebsiella ozaenae, infection, abscess, bone destruction
Introduction
Klebsiella ozaenae (K. ozaenae), a rare Gram-negative bacillus, is known as a causative pathogen of some rare diseases such as primary atrophic rhinitis and ozena.1 Generally, this organism is considered to be opportunistic and of low virulence; however, in some circumstances, it can trigger serious invasive infection such as meningitis,2 sepsis3 and endophthalmitis.4 We hereby present one case of a potentially lethal kind of K. ozaenae infection in which multiple organs were implicated.
Case Presentation
A 40-year-old male patient with a history of poorly controlled type 2 diabetes presented to our hospital due to fever with right anterior chest mass and neck and shoulder pain for half a month. Physical examination showed a 4 cm × 4 cm soft mass in the right anterior chest wall with tenderness, a small amount of fine moist rales in the lower lobe of both lungs, limited cervical mobility, with temperature of 38.2°C. Laboratory findings revealed an elevated white blood cell count of 12.77 ×109/L, hemoglobin of 107 g/L, C-reactive protein of 26.4 mg/L, procalcitonin of 0.21 ng/mL, blood albumin of 22 g/L, blood glucose of 16.23 mmol/L and glycated hemoglobin of 10.9%. Blood, sputum and bronchoalveolar lavage fluid cultures were then performed to find potential infection organisms. Other examinations such as bronchoscopy, electrocardiogram, hepatic and renal functions, as well as virus tests (syphilis, human immunodeficiency virus and hepatitis virus) were all normal.
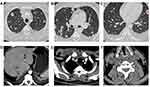
Enhanced computerized tomography (CT) of the chest, abdomen and cervical spine showed multiple pulmonary nodules and partial liquefaction and necrosis in bilateral lungs (Figure 1A–C). Suspected abscesses were noticed in the lower edge of the rightposterior lobe of the liver (Figure 1D) and the right anterior superior chest wall (Figure 1E). Bone destruction in the 5th cervical vertebra and suspected prevertebral abscesses were also noticed (Figure 1F).
Four days after admission, K. ozaenae was identified in blood, sputum and bronchoalveolar lavage isolates. Briefly, Gram-negative bacillus was confirmed in all isolates obtained from blood, sputum and bronchoalveolar lavage cultures by Gram staining; isolates were then rapidly identified using the VITEK compact 2 system according to the manufacturer’s instructions (bioMérieux, Marcy l’Etoile, France). Based on all examination results, the patient was diagnosed with 1) sepsis; 2) bilateral pulmonary abscesses; 3) right chest wall abscess; 4) liver abscess; and 5) 5th cervical vertebra bone destruction with prevertebral abscesses, not excluding tumor.
To find suitable antibiotics for this patient, antimicrobial susceptibility tests were performed using the VITEK compact 2 system (bioMérieux, Marcy l’Etoile, France) according to the manufacturer’s instructions. Results showed that isolates from blood, sputum and bronchoalveolar lavage fluid cultures of this patient were simultaneously susceptible to imipenem, meropenem, moxifloxacin, levofloxacin, amikacin and piperacillin/tazobactam. All susceptible antimicrobials and part-resistant antimicrobials for blood, sputum and bronchoalveolar lavage fluid isolates are summarized in Table 1. Based on antimicrobial susceptibility tests results and literature review, imipenem (500 mg iv q8h; Merck) was then used as an anti-infection treatment for 5 days, accompanied by 3 days of gamma globulin (20g ivgtt qd; Chengdu Rongsheng Pharmaceuticals). The patient’s symptoms were relieved, and body temperature dropped to 36.5°C; imipenem was subsequently changed to levofloxacin (500 mg iv qd; Daiichi Sankyo). After 21 days of treatment, chest CT showed a decrease in both the lung and liver abscesses; however, the patient was discharged due to his refusal of operation.
 |
Table 1 Antimicrobial Susceptibility Tests and Interpretation Based on Blood, Sputum and Bronchoalveolar Lavage Fluid Isolates |
After discharge, oral levofloxacin (500 mg qd; Daiichi Sankyo) was continued; 12 days later, fever occurred again, accompanied by aggravation of pain in the neck and shoulders. Ceftriaxone (2.0 g ivgtt qd; Shanghai Roche Pharmaceuticals) combined with moxifloxacin (400 mg ivgtt qd; Bayer) were used for anti-infection; unfortunately, the treatment effect was unsatisfactory. Imipenem (1.0 g iv q8h; Merck) was restarted. The pain was relieved, and the body temperature dropped to normal. The patient was subsequently admitted to the orthopaedic department of our hospital for further surgical treatment.
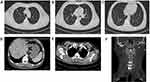
Before surgical operation, magnetic resonance imaging (MRI) was performed on the cervical vertebra of the patient and found bone destruction in the 5th cervical vertebra and prevertebral abscess in neck 1 to neck 7 (Figure 2). With the patient under general anesthesia, anterior debridement, biopsy, spinal canal decompression, iliac bone graft fusion and internal fixation were performed. Postoperative pathological result from the 5th cervical vertebra confirmed chronic suppurative inflammation (Figure 3). After the operation, the patient was treated with moxifloxacin (500 mg iv qd; Bayer) and piperacillin/tazobactam (4.5 g iv drip q8h; Wyeth), together with rehabilitation treatment. Half a month later, re-examination of thoracic and abdominal CT (Figure 4A–E) and cervical vertebra MRI (Figure 4F) showed that all focuses were significantly improved. After wearing a cervical brace, the patient was discharged from the hospital.
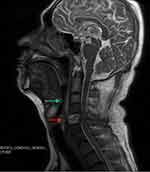 |
Figure 2 Magnetic resonance imaging (MRI) of the cervical vertebra before operation. Green arrow indicates prevertebral abscess, and red arrow indicates bone destruction in the 5th cervical vertebra. |
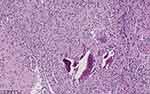 |
Figure 3 Postoperative H&E staining of the 5th cervical vertebra showing typical characteristics of chronic inflammation. |
Discussion
K. ozaenae, a member of the Enterobacteriaceae family, has long been considered a respiratory tract and oral colonizer. The organisms not only cause non-lethal infections, such as rhinitis,5 rhinoscleroma6 and cholecystitis,7 but also lead to life-threatening infections. Tang et al reported two cases of K. ozaenae meningitis.2 Murray et al reported two cases of K. ozaenae septicemia, one fatal and one nonfatal.8 Strampfer et al reported one case of cerebral abscess related with K. ozaenae.9 Kim et al reported one case of K. ozaenae endophthalmitis leading to terminal blindness.4 K. ozaenae infections rarely involved multiple organs; however, in our patient, it caused sepsis and multiple organ abscesses involving lung, liver, right chest wall and cervical vertebra. Besides, bone destruction of 5th cervical vertebra was first reported as a clinical manifestation of K. ozaenae infection in our patient. Considering K. ozaenae was found in the sputum, blood and bronchoalveolar lavage cultures in our patient, we suspected that K. ozaenae might have reached the lung, liver, right chest wall and cervical vertebra from the oral cavity by hematogenous dissemination, the most common route for spread of infection.
Diabetes mellitus may be one of the risk factors for K. ozaenae infection. In a case report, Baig et al presented one case of K. ozaenae cholecystitis in a 65-year-old morbidly obese woman with history of diabetes mellitus. The authors also summarized the clinical characteristics of 10 K. ozaenae bacteremia patients reported in the literature and found 2 of them had diabetes. In their paper, the authors considered diabetes as one of the risk factors for their patient’s infection.7 After checking the medical records, we found the clinical characteristics of our patient were pretty unobtrusive except for a history of poorly controlled diabetes, which may be an important predisposing factor for his K. ozaenae infection. As for bone destruction of 5th cervical vertebra, we suspected that K. ozaenae in the blood may reach the 5th cervical vertebra by hematogenous dissemination, slowly causing chronic suppurative inflammation and bone destruction.
K. ozaenae is sensitive or intermediately sensitive to antimicrobials including cotrimoxazole, cephalosporins, quinolones, tetracyclines and aminoglycosides; however, which kind of antimicrobial to use in the treatment of K. ozaenae infections remains uncertain due to drug-resistance.10 Therefore, an antibiotic susceptibility test should be performed before treatment to guide the antimicrobials use. Huang et al reported one highly multi-drug-resistant strain of K. ozaenae which was isolated from an ICU patient and resistant to most of the tested antimicrobials, including imipenem, ertapenem, ampicillin/sulbactam, piperacillin–tazobactam, aztreonam, ceftazidime, cefepime, levofloxacin and ciprofloxacin, but it was sensitive to gentamicin.10 Abbas et al reported similar results: in their study, the K. ozaenae isolate was multi-resistant to most tested antibiotics, including amoxicillin, cephalothin, ceftazidime, cefotaxime, amikacin, tetracycline, naldixic acid, erythromycin and trimethoprim, but it was sensitive to imipenem and gentamicin.11 In another cross-sectional retrospective study, performed by Radji et al, the authors collected specimens from 385 patients, of which 249 (64.68%) were cultured positive, including 21 that were positive for K. ozaenae. The top five most effective antibiotics against K. ozaenae were levofloxacin (93.8%), imipenem (90.5%), meropenem (90.5%), amikacin (90.5%) and fosfomycin (76.2%).12 In this study, imipenem, meropenem, moxifloxacin, levofloxacin, amikacin and piperacillin/tazobactam were simultaneously regarded as “susceptible” to our isolates from blood, sputum and bronchoalveolar lavage fluid cultures by the VITEK compact 2 system. Among these antimicrobials, imipenem has the lowest MIC value, at ≤0.25 μg/mL. Based on the results of the antimicrobial susceptibility test and literature review, imipenem was used as the main drug for anti-infection treatment and achieved good results.
Conclusion
In conclusion, K. ozaenae is not just an opportunistic bacterium; in certain circumstances, it can trigger serious invasive infections and even death in humans. Therefore, it should be treated as seriously as other pathogenic Gram-negative bacteria.
Patient Consent and Ethics Statement
The patient provided informed consent for publication of the case. No ethical committee approval was required for this study as the data had been analyzed in a retrospective manner.
Author Contributions
All authors contributed to data collection, drafting and revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Funds of China (grant nos.82070019 and 81870034).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Jain T, Sanju HK, Guerrieri M, Ralli M, Di Mauro R. Primary atrophic rhinitis: ozaena and other infective forms. In: Atrophic Rhinitis. Springer; 2020:3–9. doi:10.1007/978-3-030-51705-2_1
2. Tang L-M, Chen S-T. Klebsiella ozaenae meningitis: report of two cases and review of the literature. Infect. 1994;22(1):58–61. doi:10.1007/BF01780771
3. Endailalu YW, Sealy P, Michael M, Kauter Al Khalloufi M, Nabhani H. Klebsiella ozaenae sepsis in a young healthy male. Malays J Pathol. 2012;34(2):153.
4. Kim INH, Moon SM, Chi MJ. Endogenous endophthalmitis due to klebsiella ozaenae. J Korean Ophthalmol Soc. 2016;57(7):1139–1143. doi:10.3341/jkos.2016.57.7.1139
5. Botelho-Nevers E, Gouriet F, Lepidi H, et al. Chronic nasal infection caused by Klebsiella rhinoscleromatis or Klebsiella ozaenae: two forgotten infectious diseases. Int J Infect Dis. 2007;11(5):423–429. doi:10.1016/j.ijid.2006.10.005
6. Gonzales Zamora J, Murali AR. Rhinoscleroma with pharyngolaryngeal involvement caused by Klebsiella ozaenae. Case Rep Infect Dis. 2016;2016:6536275. doi:10.1155/2016/6536275
7. Baig A, Gujral M, Hameed R, Borra S. Klebsiella ozaenae cholecystitis. Am J Med Sci. 2011;342(3):259–261. doi:10.1097/MAJ.0b013e318220ac6f
8. Murray KA, Clements BH, Keas SE. Klebsiella ozaenae septicemia associated with Hansen’s disease. J Clin Microbiol. 1981;14(6):703–705. doi:10.1128/JCM.14.6.703-705.1981
9. Strampfer MJ, Schoch PE, Cunha BA. Cerebral abscess caused by Klebsiella ozaenae. J Clin Microbiol. 1987;25(8):1553–1554. doi:10.1128/JCM.25.8.1553-1554.1987
10. Huang J, Li X, Zhu N, Li G. Genetic characteristics of one highly multi-drug-resistant strain of Klebsiella ozaenae. J Med Microbiol. 2012;61(9):1303–1305. doi:10.1099/jmm.0.044115-0
11. Abbas AF, Al-Saadi A-S, Hussein AK, Al-Thaheb AO Role of outer membrane proteins in virulence of Klebsiella ozaenae and antibiotic sensitivity.
12. Radji M, Fauziah S, Aribinuko N. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac J Trop Bio. 2011;1(1):39–42. doi:10.1016/s2221-1691(11)60065-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.