Back to Journals » Infection and Drug Resistance » Volume 15
Multi-Drug Resistance Profile, Prevalence of Extended-Spectrum Beta-Lactamase and Carbapenemase-Producing Gram Negative Bacilli Among Admitted Patients After Surgery with Suspected of Surgical Site Nosocomial Infection North East Ethiopia
Authors Tilahun M
Received 29 May 2022
Accepted for publication 21 July 2022
Published 26 July 2022 Volume 2022:15 Pages 3949—3965
DOI https://doi.org/10.2147/IDR.S376622
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mihret Tilahun
Department of Medical Laboratory Sciences, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
Correspondence: Mihret Tilahun, Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wollo University, Dessie and Borumeda, PO.BOX 1145, Ethiopia, Tel +251 920988307, Fax +251 333115250, Email [email protected]
Background: Antibiotic resistance is becoming a global issue, with estimated 2.8 million people in the United States developing antibiotic-resistant diseases each year. The carriage of ESBL and Carbapenemase-producing Enterobacteriaceae among hospitalized patients is a threat to the future of antibiotic treatment.
Objective: Multi-drug resistance profile, prevalence of extended-spectrum beta-lactamase and Carbapenemase-producing Gram-negative bacilli among admitted patients after surgery with suspected surgical site nosocomial infection north east Ethiopia.
Material and Methods: A hospital-based cross-sectional study was conducted from April 2021 to February 2022. Socio-demographic and clinical data were assessed using a structured questionnaire. A total of 384 relevant clinical samples (pus, pus aspirates, and wound swabs) were collected aseptically and processed within 30 min by placing the swabs into the sterile test tubes having 0.5 mL of sterile normal saline. The samples were cultured on MacConkey agar, chocolate agar and blood agar, and species identification was done using standard biochemical tests. Disk diffusion antimicrobial sensitivity test was done on Mueller–Hinton agar. All the cefoxitin resistant Enterobacteriaceae isolates were checked for the presence of AmpC beta-lactamase using four cartridges of disk diffusion tablets. ESBL output validation was conducted by the combination disk test. The production of Carbapenemase was checked using modified carbapenem inactivation method.
Results: The prevalence of significant bacterial nosocomial infection among surgical site infection 343 (89.32%). S. aureus 125 (36.4%) was predominant followed by E. coli 80 (23.3%) P. aeruginosa 31 (9.03%). The overall MDR rate of isolated bacteria was 251 (73.3%). About 150 (73.9%) bacteria were suspected for ESBL production and 67 (33%) AmpC beta-lactamase and 27 (13.3%) Carbapenemase-producing Gram-negative bacterial, respectively.
Conclusions and Recommendations: The severity of ESBL-PE was critical, and the CPE was alarming. Meropenem and imipenem were the most effective antibiotics against ESBL-producing Enterobacteriaceae. Therefore, strict infection prevention and control measures are needed.
Keywords: carbapenemase, ESBL, Enterobacteriaceae, surgical site infection
Corrigendum for this paper has been published.
Introduction
Antibiotic resistance is becoming a global issue every year, with estimated 2.8 million people in the United States developing antibiotic-resistant diseases. Higher rates of morbidity, mortality, and healthcare costs are linked to certain infections. Drug-resistant Gram-negative bacteria, on the other hand, are becoming more common in the United States and around the world. In the past, Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus and Clostridium difficile were the most concerning in terms of antibiotic resistance.1
Beta-lactam antimicrobial drugs are the most commonly prescribed antibiotics for bacterial infections, and they continue to be a major source of Gram-negative bacteria resistance globally. Bacterial strains exposed to a variety of Beta-lactam antibiotics have acquired an energetic and constant production and mutation of Beta-lactames, increasing their activity even against recently produced Beta-lactam antibiotics.2
In hospital settings around the world, ESBL-producing Enterobacteriaceae are a substantial threat. The problem is concerning since, with the exception of carbapenems and cephamycin, ESBL enzymes can hydrolyse beta-lactam antibiotics. Furthermore, genes located on plasmids that are extremely mobile, allowing for clonal and horizontal transfer, frequently encode these enzymes. Aminoglycosides, trimethoprim, sulphonamides, tetracyclines, and chloramphenicol resistance genes can all be conferred via these plasmids.3 Carbapenemase and ESBL are easily spread within and between Enterobacteriaceae by plasmid and transposon (mobile genetic element) mediated. Infection management becomes a problem with ESBL and Carbapenemase-producing Enterobacteriaceae, especially in hospitals.4
Multidrug resistance (MDR) among bacterial pathogens implicated in both nosocomial and community-acquired infections has emerged as a major threat to public health around the world.5 Antimicrobial resistance (AMR) is a serious hazard to public health that is rapidly growing all over the world. Multidrug resistance bacteria has been found in a variety of nations and continents, with large disparities in carriage prevalence rates.6 Multidrug resistant infections have a higher death rate than infections caused by non-MDR bacteria, and they have a significant economic impact, estimated at over $20 billion per year in the United States alone. According to the Centres for Disease Control and Prevention, an infection with an antibiotic-resistant bacterium kills at least 23,000 individuals in the United States per year.7
Antimicrobial resistance is increasing in both healthcare settings and in the community around the globe. The dissemination of MDR Enterobacteriaceae in particular extended spectrum beta-lactamase (ESBL) and Carbapenemase producing Enterobacteriaceae (CPE), is alarming. Severe infections due to MDR Enterobacteriaceae are associated with worse outcomes and increased mortality, especially when adequate antibiotic therapy is delayed. Low- and middle-income countries are mostly affected, but precise data from their hospitals are often deficient.8
Carbapenems are the last choice of antibiotics for the treatment of multi-drug-resistant Enterobacteriaceae (MDR-E) infections. However, carbapenem-resistant Enterobacteriaceae (CIRE) is an on-going public health problem globally.9,10 Resistant to carbapenems by production of Carbapenemase is becoming a challenge though limiting the treatment option leads to failure of beta lactam therapy which may result in high economic loss and mortality.11
Carriage of ESBL and Carbapenemase-producing Enterobacteriaceae among hospitalised patients are a threat to the future of antibiotic treatment. The high burden of ESBL and Carbapenemase-producing Enterobacteriaceae among asymptomatic individuals has a significant public health effect, for the treatments of both hospital and community-acquired infections.12
Although infections caused by ESBL, AmpC and Carbapenemase-producing Enterobacteriaceae are a global threat, the burden is high in low-income countries like sub-Saharan Africa (SSA), where widespread of self-treatment, overcrowding of hospitals, absence of antibiotic prescription guidelines, poor infection control practices and poor hygiene and antibiotic misuse are common.13
Although surgical site infection is a possible source of transmission and infection, little is known about it, and there is limited evidence in the literature on Enterobacteriaceae that produce extended spectrum beta-lactamase, AmpC beta-lactamase and Carbapenemase in settings. To prevent nosocomial infection caused by AMR Enterobacteriaceae, it is necessary to know the magnitude of fecal carriage of ESBL, AmpC beta-lactamase, and Carbapenemase-producing bacterial pathogens. This study will also aid in the careful selection of empirical therapy based on the local susceptibility pattern. Hence, the aim of this study was to determine the multi-drug resistance profile, Carbapenemase, and ESBL-producing health care bacterial pathogen among patients suspected of surgical associated nosocomial infection at Dessie and Borumeda comprehensive specialized hospital Dessie and Borumeda Specialized Hospital (DCSH), north east, Ethiopia.
Materials and Methods
Study Design and Period
A hospital-based prospective cohort study was conducted from April 2021 to February 2022 at Dessie and Borumeda Comprehensive Specialized Hospital, which is found in Dessie town, north east, Ethiopia. Dessie and Borumeda is located 400 Km from the capital city of the country, Addis Ababa. According to the 2007 population and housing census of Ethiopia, the town has a total of 450,012 people. Dessie and Borumeda is situated at an altitude of 2840 m above sea level with a mean annual temperature that ranges from 10°C to 16°C. It has 500 beds and offers pediatrics, emergency, surgery, medical, gynecology, psychiatry, ophthalmology, antiretroviral therapy (ART), neonatal intensive care unit, microbiological laboratory, viral load, and other health care services.
Study Participants
Operating patients with any surgical site infection and emergency surgical procedures at general surgery, gynecology/obstetrics, and orthopaedics wards were included in the study. All operated patients with any surgical site infection visiting the health facilities suspected of having bacterial infection during the study period were included in this study, and all of these eligible patients were monitored for 30 days to see if they got SSI. A bacteriological sample was obtained from individuals who had any surgical site infection during the 30-day follow-up period. Patients who had any surgical site infection at the start of the experiment died within 48 hours of the start of the experiment, or refused to participate were excluded.
Sampling Size Determination and Sampling Technique
A total of 384 Surgical patients who met the criteria were calculated and collected by using a single population proportion sample size determination formula, by considering 95% confidence level, 5% margin of error, and 50% estimated proportion and observed for evidence of infection on a daily basis. The clinical criteria for surgical site infection development (superficial incisional any surgical site infection, deep incisional any surgical site infection, and organ/space any surgical site infection) from the CDC any surgical site infection categorization system were used.14
Data and Specimen Collection
Socio-demographic characteristics such as age, occupation, sex, educational level, and residence of patients were assessed using a short interview guided by a pre-tested structured questionnaire. The clinical evaluation of surgical sites (wounds) was done by physicians. The clinical features of the wounds such as pain, redness, swelling, warm skin around the wound, yellow or green discharge, unpleasant odor, fever, and chills were considered for the clinical diagnosis of surgical site infection. The clinical samples (pus, pus aspirates, and wound swabs) were collected aseptically and processed immediately in the microbiology laboratory within 30 minutes by placing the swabs into the sterile test tubes containing 0.5 mL of sterile normal saline. The swabs and pus aspirate were inoculated on the sterile nutrient broth medium and the Amies medium right away.15 Each sample bottle was labeled carefully and transported to the laboratory immediately for microbiological investigations.
Specimen Processing, Culture
The collected samples were inoculated onto MacConkey agar, Blood agar, Mannitol salt agar and chocolate agar plates.15,16 MacConkey agar, Blood agar and Mannitol salt agar were incubated at 37°C for 24–48 hours aerobic condition. Meanwhile, Chocolate agar plates were incubated in 5–10% CO2 atmosphere environment at 37°C for 24–48 hours. All the plates were incubated and examined for growth after 24 hours and the ones without growth were further incubated for up to 48 hrs.15,16
Isolation and Identification of Bacterial Isolates
Bacterial isolates were characterized using hemolysis pattern, colony morphology, Gram stain and using a panel of biochemical tests based on the Gram reaction (for Gram positives, mannitol fermentation, catalase, coagulase, bacitracin and optochin were disks used, and for Gram negatives, glucose and lactose fermentation, hydrogen sulfide production, indole production, motility tests, urease production, citrate utilization and lysine iron agar and Triple sugar iron agar tests) were implemented based on the standard microbiological methods.16
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility profiles of bacterial isolates were determined by Kirby-Bauer disc diffusion method, and the results were interpreted according to CLSI guidelines.17 The following antibacterial agents were selected based on local prescription habit and CLSI recommendations. The standard antibiotic discs (Liofilchem-Italy, HARDY Diagnosis-Santa Maria, USA) and its concentrations used as follows: penicillin (10μg), chloramphenicol (30μg), ciprofloxacin (5μg), clindamycin (30μg), cefoxitin (30μg), trimethoprim-sulfamethoxazole (1.25/23.75μg), cefotaxime (30μg), ceftriaxone (30μg), erythromycin (15μg) and oxacillin (30μg) are the antimicrobial agents that can be used by Gram-positive bacteria. Cefoxitin (FOX:30μg), gentamicin (GM: 10μg), amikacin (AM:30 μg) ciprofloxacin (CIP: 5μg), trimethoprim-sulfamethoxazole (SXT: 1.25/23.75 μg), imipenem (IMP: 10μg), meropenem (MEM: 10 μg), amoxicillin-clavulanic acid (AMC: 20/10 μg), cefotaxime (CTX: 30 μg), ceftazidime (CAZ: 30μg), ceftriaxone (CRO: 30μg), tetracycline (TE: 30 μg) and chloramphenicol (CL: 30 μg) can be used for Gram-negative bacteria Diameters of zones of inhibitions were measured using digital caliper. The interpretation of the results of antimicrobial susceptibility tests was based on a standardized table supplied by CLSI2020.17
MDR = resistant to at least one agent in three or more antimicrobial classes18
Screening of ESBLs, AmpC Beta-Lactamase and Carbapenemase
The gram-negative bacterial isolates which showed an inhibition zone size of ≤22 mm with ceftazidime (30 μg), and/or ≤27 mm with cefotaxime (30 μg) were considered as potential extended-spectrum beta-lactamase-producing bacteria and further examined for phenotypic confirmation of extended-spectrum beta-lactamase production. Gram-negative bacteria, which showed an inhibition zone size of ≤14 mm to cefoxitin (30 μg), were considered as potential AmpC beta-lactamase producers. On the other hand, all Gram-negative bacteria that showed resistance to imipenem or meropenem or zone of inhibition ≤19 mm for imipenem or meropenem were considered as carbapenemase producers.17
Phenotypic Confirmation of Extended-Spectrum Beta-Lactamase with Combination Disk Test
A disk of ceftazidime (30 μg) and cefotaxime (30 μg) alone and their combination with Clavulanic acid (30 µg/10 µg) were placed at a distance of 25 mm, centre to centre, on MHA plate that was seeded with a bacterial suspension of 0.5 McFarland turbidity standard and incubated overnight (18–24 hrs) at 37°C. Gram-negative bacteria that showed an increase in the inhibition zone diameter of ≥5 mm for a combination disk versus ceftazidime or cefotaxime disk alone were confirmed as ESBL producer.17
Phenotypic Confirmation of Carbapenemase Production
The gram-negative bacteria isolates that were not susceptible to imipenem or meropenem were checked for the presence of carbapenemase using a modified carbapenem inactivation method (mCIM) based on the CLSI guideline.17 Modified Hodge Test (MHT) were conducted that McFarland standard equivalent suspension of carbapenem-sensitive indicator organism (E. coli ATCC®25922) was evenly swabbed to MHA, and then the meropenem in the TSB was dispensed. After incubation for 24 hours at 37°C, the zone of inhibition for meropenem was measured. If the zone of inhibition was between 6 and 15 mm or 16 and18 mm with pin points at the inhibition zone, it was considered as a carbapenemase producer.17
Phenotypic Confirmation of AmpC Beta-Lactamase Production
All the cefoxitin non-susceptible Gram-negative bacterial isolates were checked for the presence of AmpC beta-lactamase using four cartridges of disk diffusion tablets. One cartridge of tablets with cefotaxime, one with ceftazidime, and two cartridges of the cephalosporins combined with cloxacillin (AmpC inhibitor). An increase in the inhibition zone diameter of ≥5 mm for a combination disk versus ceftazidime or cefotaxime disk alone was confirmed as AmpC beta-lactamase producing Gram-negative bacteria.17
Quality Assurance
Standard Operating Procedures (SOP) were strictly followed for each procedure. The specimens were processed and transported as soon after receipt as possible. Delayed specimens were placed in the refrigerator. Expiry date of the media, reagents and antibiotic disks were checked before use. Quality control of culture media was verified for sterility test by overnight incubation of 5% one uninoculated plate/tube of the prepared media from each batch. Positive and negative controls were used for biochemical media; and visual inspections of holes, uneven filling, and haemolysis, signs of freezing, bubbles and corrosion in media or plastic Petri dishes were conducted to check the quality of all prepared culture media. Moreover, standard reference strain of S. aureus (ATCC-25923), E. coli (ATCC-25922) and P. aeruginosa (ATCC-27853) were used as control strains. For ESBLs confirmatory test, ESBLs positive K. pneumoniae ATCC 700603 and ESBLs negative E. coli ATCC 25922 control strains were performed. E. cloacae (ATCC 1143) and E. coli (ATCC 25922) were used as positive and negative QC strains for AmpC beta-lactamase producing Gram-negative, respectively. E. coli ATCC 25922 control strain was used as negative control for Carbapenem resistance detection.
Statistical Analysis
The data generated were entered every day into the epi-data version 4.6.0.4. The data were then exported and analysed using the Social Sciences Statistical Package (SPSS) version 25. The frequency and percentage descriptive statistics were calculated and presented using graphs and tables.
Ethical Approval
Ethical approval was obtained from the ethical review committee of the College of Medicine and Health Sciences, Wollo University and the official letter of cooperation was written to respective health facilities prior to data collection. Moreover, prior to commencing the study, a written informed consent was obtained from each study participant. Study participants, aged less than 18 years, were asked for assent and written consent was taken from their parents or legal guardians. Throughout the study, confidentiality and any unique data security needs were maintained and ensured. Moreover, this study was conducted in accordance with the Declaration of Helsinki.
Result
Socio-Demographic Characteristics
A total of 384 surgery patients were enrolled in this study with a 100% response rate. Among the study participants, 204 (53.1%) were males. The mean age of the participants was 42 (SD ± 6.92) years, with an age range of 5 to 87 years. Majority 290 (75.5%) of the study participants were from urban dwellers, 126 (32.8%) were Government employed, 105 (27.3%) were illiterate, 288 (75%) were married and 101 (26.3%) had >2000 Birr monthly income (Table 1).
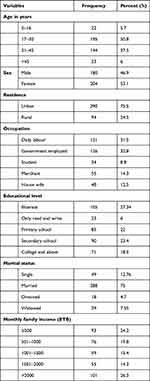 |
Table 1 Socio-Demographic Characteristics of the Study Participants Debre Birhan Comprehensive Specialized Hospital, Ethiopia, from April 2021 to February 2022 |
Behavioural and Clinical Related Factors
From the perspective of behavioural-related factors, most of the study participants 75 (19.5%) were smokers, 96 (25%) were chat chewers; and 86 (22.4%) were alcohol drinkers, respectively (Table 2). Whereas it related to clinical factors, most of 257 (66.9%) of the study subjects were admitted in the surgical ward. The majority of 191 (49.7%) wounds were located on the abdomen and leg 91 (23.7%). There were 165 (23.7%) elective and 105 (23.5%) censorial section operations. High proportions of participants 129 (33.6%) were found in the contaminate wound, 120 (31.3%) in clean-contaminated, 87 (22.6%) in dirty, and low proportions of participants 48 (12.5%) were found in clean wound class. About 114 (29.7%) study participants had developed discharge. Most of the study participants were 339 (88.3%) HIV negative and 306 (79.7%) non-diabetic patients, respectively. Furthermore, 176 participants (45.8%) were hospitalized for more than two weeks (Table 2).
 |
Table 2 Distribution of Bacterial with Clinical and Procedural Condition of Study the Study Participants Debre Birhan Comprehensive Specialized Hospital, Ethiopia, from April 2021 to February 2022 |
The Magnitude of Surgical Site Infection
The magnitude of bacterial growth was high among surgical patients 245 (95.3%). Among 165 elective surgery patients 150 (90.9%) developed bacterial infection. Bacterial surgical site infection occurred in 115 (95.8%) of the 120 clean contaminate wounds, 83 (95.4%) of the 87 dirty wounds, and 120 (93%) of the 129 contaminate wounds. Regarding comorbidity conditions, 83.3% (65/78) diabetic patients developed SSI. The majority of the HIV positive patients 88.9% (40/49) developed surgical site infections (Table 2).
Profile of Bacterial Isolates from Surgical Site Infections
In this study, out of 384 cultured specimens, 343 had significant bacterial growth of surgical site nosocomial infection. The prevalence of significant bacterial infection among surgical site infection 343 (89.32%) with (95% CI 84.2–9.2%). Majority 203 (59.2%) of the bacterial isolates were Gram negative bacterial. S. aureus 125 (36.4%) was predominant bacterial isolates followed by E. coli 80 (23.3%) P. aeruginosa 31 (9.03%), whereas P. vulgaris 9(2.6%) and Acinetobacter species 3(0.88%) were the least isolated bacteria (Figure 1).
 |
Figure 1 Frequency of bacteria isolated from surgical site infection in Dessie Comprehensive Specialized and Borumeda General Hospital, from April 2021- February 2022. |
Antimicrobial Susceptibility Profile of Isolates
Gram Positive Bacteria
In general, Gram-positive bacteria showed a high level of resistance to tetracycline 118 (84.29%) and medium resistance to chloramphenicol 105 (75%) and trimethoprim-sulfamethoxazole 96 (65.87%). On the other hand, 64% and 52% of Gram-positive isolates were susceptible to ciprofloxacin and cefotaxime, respectively. More than 60% of S. aureus isolates were resistant to tetracycline, chloramphenicol, trimethoprim-sulfamethoxazole, and penicillin. Similarly, CONS showed 86.7%, 80% and 73.3% resistance to tetracycline, penicillin, and trimethoprim-sulfamethoxazole, respectively. About 81.6% of S. aureus isolates also showed resistance to methicillin. However, 66% of S. aureus isolates were susceptible to erythromycin (Table 3).
Gram Negative Bacteria
In this study, the majority of isolated Gram-negative bacteria showed a higher resistance rate of 93.3% for tetracycline, 84.6% for ampicillin and 81.9% for amoxicillin-clavulanic acid. Gram-negative bacteria against ceftazidime, tetracycline, trimethoprim-sulfamethoxazole, chloramphenicol, amoxacillin-clavulinic acid, cefotaxime, amikacin, ceftriaxone, and gentamicin showed resistance rates ranging from 78 (43.8%) to 126 (93.3%). However, Gram-negative bacterial isolates showed relatively high sensitivity against gentamicin 113 (63.5%) and ciprofloxacin 100 (56.2%). The resistance rate of E. coli to tetracycline, amoxicillin-clavulanate, co-trimoxazole, chloramphenicol, and ceftazidime was 76 (95%), 69 (86.3%), 65 (81.3%), 48 (60%), and 42 (52.5%), respectively. However, the resistance rate was less than 50% for ciprofloxacin 39 (48.5%), amikacin (45%), meropenem (42.5%), and gentamicin 30 (37.5%). Isolates of P. aeruginosa showed more resistance to ceftazidime 16 (511.6%), amikacin 18 (58.1%) and piperacillin tazobactam 16 (511.6%). Similarly, K. pneumoniae isolates were more resistant to tetracycline (90%), amoxicillin-clavulanate (80%) and chloramphenicol (60%). C. freundii isolates were also resistant to tetracycline, amoxicillin-clavulanate, co-trimoxazole, and chloramphenicol, but not gentamicin or ciprofloxacin (Table 4).
Multiple Drug Resistance Patterns of the Isolates
Overall, 331 (96.5%) bacterial isolates were shown to be resistant to at least one antimicrobial class and 294 (85.7%) isolates were resistant to ≥2 antimicrobial classes. About 72 (21%) isolates had developed resistance to five or more antimicrobials. The overall, MDR rate of isolated bacteria was 251 (73.3%). The MDR rate of Gram-positive and Gram-Negative isolates were 65% and 78.8%, respectively. About 83.4% of E. coli, 80% of K. pneumoniae, 77.4% of P. aeruginosa, 76% of C. freundii and 68% of S. aureus isolates developed MDR (Table 5).
ESBL, AmpC Beta-Lactamase and Carbapenem-Producing Gram-Negative Bacteria
Of a total of 203 Gram-negative bacterial isolates, 150 (73.9%) were suspected for ESBL production with cefotaxime zone of inhibition ≤27 mm and ceftazidime zone of inhibition ≤22 mm. Out of the 150 suspected Gram-negative bacterial isolates 108 (72%) were confirmed ESBL production by using a combination disc test. The overall magnitude of ESBL producing Gram negative bacteria was 108 (53.3%). The overall magnitude of AmpC beta-lactamase and Carbapenemase-producing Gram-negative bacterial isolates was 67 (33%) and 27 (13.3%), respectively (Table 6).
Discussion
Surgical site infection is one of the most communal health complications that is caused and provoked by the invasion of pathogenic organisms as well as the main cause of disease and death in the world.19 The present study was conducted to determine the prevalence of Multi drug resistance, Carbapenemase and extended-spectrum beta-lactamase producing bacterial isolates among surgery patients with suspected surgical site nosocomial infection. In the current study, the significant bacterial growth among surgical site infections was 343 (89.32%) % (83.3–94.3%). This finding was consistent with the previous reports in Jimma Ethiopia 87.3%,20 Addis Ababa (84.1%),21 and Gondar 83.9%,22 and similar findings were also reported in Bangladesh 92.3%.23 However, it was higher than research conducted in Debre-Markos, Ethiopia 72.6%,24 Nepal 65%,25 India 68%,26 Nigeria 64.8%27 and Nigeria 82%.28 These variations among different studies might be due to the fact that the wound bed preparation, sample selection, transportation, and culturing technique, wound care and safety, treatment for rapid healing, sterilization method, and local surgical site treatment can have an impact on the significant bacterial growth rate. The other factor might be different circulations of pathogens among healthcare workers’ dress, inpatients’ hospital equipment, or interventional procedures. However, the prevalence of Gram-negative bacteria among surgical site infections was 52.9% (203/384). It is in line with a study conducted in Addis Ababa, 54%29 and Nigeria (50.9%).30 However, the present study presented that higher than study done in Bahir Dar Ethiopia 35.2%,31 in Ethiopia (25.13%),32 Uganda (32%)33 and India (37%),34 Mexico (19%),35 Nipa (19.5%)36 and Nepal (12.6%).37 However, it is significantly lower in comparison to other study results from Bosnia (65.2%)38 and India (62%)39.
The current study revealed that Gram-negative bacteria had shown higher antibiotic resistance rates of 93.3% for tetracycline, 84.6% for ampicillin, and 81.9% for amoxicillin-clavulanic acid. Relatively, in the present study, Gram-negative bacteria showed lower resistance to ceftazidime, tetracycline, trimethoprim-sulfamethoxazole, chloramphenicol, amoxicillin-clavulinic acid, cefotaxime, amikacin, ceftriaxone, and gentamicin in ranges from 43.8% to 93.3%. However, it is relatively responsive to gentamicin (63.5% sensitivity) and ciprofloxacin (56.2% sensitivity). This finding is consistent with studies done in Ethiopia where 72 (94.7%), 68 (89.5%), 60 (78.9%), 57 (75%), and 56 (73.7%) were found to be resistant to ampicillin, cephazolin, cefuroxime sodium, amoxicillin/clavulanic acid, cefotaxime21 and Iran, where 36% of Gram negative bacteria were resistant to ciprofloxacin.40 Similarly, a Mexican study found that the Gram-negative bacterial isolated was resistant to ampicillin (95.85%), cefuroxime (84.17%), piperacillin (82.93%), cefotaxime (78.07%), ceftriaxone (77.41%), aztreonam (75.23%), cefazolin (75.00%), and ceftazidime (73.19%).35 According to Nepal research, E. coli is most sensitive to cephalosporins and tetracycline, and resistant to quinolones, fluoroquinolones, and sulphonamides.41 This is despite the fact that a wide range of antibiotics may be used to treat Gram-negative surgical site infections. However, some resistant strains have emerged, resulting in life-threatening superbugs. In the fight against diseases, the rising rates of antimicrobial resistance in our environment expose partners to the need for improved antibiotic prescription tracking and management.
The overall prevalence of MDR in this study was 73.2% (95% CI: 68.2%, 78.2%), which was comparable to studies done in Addis Abeba, where the MDR was 75.2%.21 On the other hand, the overall MDR rate of Gram-negative bacteria was 78.8%. Even though the result was comparable with the study reports in Bahir Dar, Ethiopia, the MDR of Gram-negative was 80.5%,31 in Nepal MDR Gram-negative which accounted 82.5%.42 Previous antibiotic treatment, unnecessary medication therapy, chronic respiratory infections, liver problems, and intellectual disease, previous multidrug resistance, recent hospital readmissions, longer hospital stays, endobronchial tracheostomy, and mechanical ventilation could all contribute to this. Antibiotic resistance has mostly resulted in the emergence of antibiotic misuse and overuse in humans and livestock. In our hospital and community, overcrowding, cleaning errors, and inadequate infection control procedures are the possible risk factors for the development of resistant bacteria.
In the current study, Gram-negative bacteria which showed the highest MDR rates were observed in E. coli 83.8%, K. pneumoniae 80%, Citrobacter species and P. mirabilis 76% each, P. aeruginosa 67.7%, P. vulgaris 66.7% and Acinetobacter species 33.3%. A similar report was found at Bahir Dar, Ethiopia, were Klebsiella pneumoniae which accounted MDR rate of 87.6% followed by E. aerogenes 83.3%, E. coli 82.6%, E. cloacae 77.8%.43 In contrast to Mexico, it also showed that the highest percentage of MDR profiles seen in E. coli accounted 91.57% and Acinetobacter baumannii 86.79%.35 In Iran, lower MDR Gram-negative bacteria were reported in 25.8% of Acinetobacter species, 20% of Klebsiella species, and 16.6% of Pseudomonas species. The antimicrobials that were the most effective were vancomycin 93.5% followed by amikacin 71.5% and gentamicin 46%.40 Excessively broad coverage raises the risk of antibiotic resistance in the majority of instances. Underlying diseases or conditions such as diabetes, chronic renal disease, or skin lesions are examples of underlying illnesses for which drugs have been administered in the past and might be the contributing factors to multi-drug resistance. Ciprofloxacin, meropenem, and amikacin were found to be relatively active antibiotics in this study. Moreover, unlike other common antimicrobial drugs with an oral route, amikacin was not as accessible to the general public in the study setting.
In the present study, the prevalence of ESBL-producing Gram-negative bacteria was 53.2%, which is concurrent in Bahir Dar Ethiopia 57.8%,44 Nepal, 55.6%,42 Cameroon, 55.3%;45 and India 54.3%.46 However, the present study was higher than study done in Bahir Dar Ethiopia 24.8%,31 India 35.2%,47 France (25%),48 Nepal (28.2%),37 Qatar (26%,49 India (21.4%),34 Saudi (38.8%)50 and in Algeria 47.6%,51 whereas the current result of ESBL was lower than 72% reported at a tertiary care hospital in Riyadh capital,52 Pakistan 60%53 and Nigeria 65%.54 This suggested that beta-lactamase-producing bacteria were widely disseminated around the world, particularly in developing countries like Ethiopia. This could be due to a lack of antibiotic treatments and lack of sanitation in undeveloped countries. Additionally, growing world trade and international travel have been identified as significant risk factors for the emergence of resistant microorganisms. For ESBL-producing Enterobacteriaceae, Colistin is an alternative treatment only in the study area where carbapenems cannot be utilized.55 A genotypic analysis of distinct families of beta-lactamase resistant genes may be required to build a complete picture of beta-lactamase resistance mechanisms in Gram-negative bacteria. As a result, local and national surveillance, as well as an international report on the spread of beta-lactamase-producing microbes, are necessary.
Citrobacter species, E. coli, K. pneumoniae and P. aeruginosa were the most common Gram-negative ESBL producer’s bacteria in this investigation. This is supported by various research in Africa,56,57 Indonesia58 Algeria59 and India47 Uganda,60 Nepal,37 Brazil,61 Saudi Arabia62 and Mexico.35 This could be because the bacteria are frequently exposed to a range of beta-lactams, prompting them to produce beta-lactamases. Furthermore, beta-lactamase enzymes, which are mediated by plasmid and chromosomal genes, produce antibiotic resistance.
The current study exhibited a higher prevalence of AmpC β-lactamase producers 33%. This corresponds to many research conducted in Saudi Arabia 32.5%,62 India 36.5%.63 However, it was higher than research conducted in Saudi Arabia (5.5%).64 The predominant bacteria that were AmpC β-lactamase producers P. aeruginosa (38.5%) followed by K. pneumoniae (33.3%) and P. mirabilis (3.3%). These findings suggest that regularly implementing infection control methods may contribute to limiting the spread of AmpC-lactamase-producing bacteria. Antibiotic-resistant bacteria could have an overwhelming number of efflux pumps on their surface. The combination of a beta-lactam antibiotic and a beta-lactamase inhibitor is a novel approach to treating bacterial beta-lactamases. There seem to be several combinations of the antagonist clavulanic acid, sulbactam, and tazobactam currently available.
In our investigation, the prevalence of Carbapenemase-resistance Gram-negative bacteria was 13.7%, which is comparable with the research conducted in Taiwan 15.4%,65 Indonesia 13.7%,58 Nepal 11.2%37 and Romania66 although our findings were lower than study done at Addis Ababa 36%67 in Nigeria 34.5%,30 India 34.5%47 and 44.1%.68 It was higher than the study conducted in Bahir Dar, Ethiopia (5.2%),31 Addis Ababa Ethiopia (2%)32 and CDC reports from 1.2% (2001) to 4.2%.2011.69 The most common Carbapenemase-producing bacteria in our study were P. aeruginosa (19.4%), E. coli 11.2% and K. pneumoniae (13.3%). A similar finding in Addis Ababa67 and Tanzania70 supports this, which accounts for the prevalence of carbapenemase-producing E. coli (14%), followed by K. pneumoniae (10.57%) had carbapenem resistance. For patients suspected of having carbapenem resistance, polymyxins are feasible initial treatment71 and alternatively combination treatment is also another option to treat carbapenem resistance.72,73 Differences in local antibiotic prescribing trends and infection control practices in different health facilities could also be to blame. Antimicrobial susceptibility testing must be done on a regular basis to detect multidrug-resistant bacteria. Precise identification of carbapenem-resistant bacterial pathogens is critical for patient treatment,74 as well as for implementing suitable contamination control measures to prevent the pathogens from spreading quickly.
Conclusion and Recommendation
The prevalence of surgical sites associated with infection in the current study was 343 (89.32%). Among them, 203(59.2%) were Gram-negative bacteria isolates. S. aureus, was predominantly followed by E. coli and P. aeruginosa. In general, Gram-positive bacteria showed high level of resistance for tetracycline and medium resistance for chloramphenicol and trimethoprim-sulfamethoxazole, while Gram-positive isolates were susceptible to ciprofloxacin and cefotaxime, respectively, whereas the majority of isolated Gram-negative bacteria showed resistance rates for tetracycline,ampicillin and amoxicillin-clavulic acid. Rates of resistance of Gram-negatives against ceftazidime, tetracycline, trimethoprim-sulfamethoxazole, chloramphenicol, amoxicillin-clavulinic acid, cefotaxime, amikacin, ceftriaxone and gentamicin. Among Gram-negative bacteria, a relatively higher rate of MDR was seen in Klebsiella spp, Pseudomonas, Acinetobacter, Enterobacter and Citrobacter. The predominant bacterial isolate was K. pneumoniae. P. aeruginosa, E. coli and K. pneumoniae were the most frequent ESBL and Carbapenemase-producing Enterobacteriaceae. In the future, anaerobic bacteria, fungi, and other microorganisms that can be sources of infections should be included in the prevalence and drug susceptibility pattern of surgical site infections, Empirical surgical site infection treatment might lead to antibiotic resistance and Selection of antibiotics should be based on the results of culture and sensitivity tests. There is a need for hospitals to encourage periodic reviews to do culture and sensitivity tests. Physicians and healthcare professionals provide education and surveillance of antimicrobial resistance in order to control the ESBL and Carbapenemase-producing Gram-negative bacilli transmission in hospitals. Despite comprehensive specialized hospital practices in relation to preventing surgical site infections, the hospital-based data obtained from the regular surveillance should provide feedback to each ward on a regular basis. Treatment of surgical site infections has to be made based on the culture and susceptibility test results.
Limitation of the Study
Isolated Enterobacteriaceae were not subjected to a molecular assay for ESBL and Carbapenemase gene characterization. Even though it was beyond the scope of the study, healthy community members were not used as a control group.
Abbreviations
AMR, Antimicrobial Resistance; ATCC, American Type Cell Culture; Clinical and Laboratory Standard Institute; BAP, Blood Agar Plate; CAP, Chocolate Agar Plate; MDR, Multidrug resistance; MHA, Muller Hilton Agar; SOPs, Standard Operating Procedures; SPSS, Statistical Package for Social Science.
Data Sharing Statement
Data supporting the conclusions of this article are within the manuscript.
Acknowledgment
The authors would like to acknowledge the College of Medicine and Health Sciences, Wollo University for providing the laboratory set up and facilities to conduct the experiments. Data collectors and all study participants and data collectors are gratefully acknowledged for their kind cooperation during data collection.
Disclosure
The authors state that they have no conflicts of interest in this work.
References
1. Morris S, Cerceo E. Trends, epidemiology, and management of multi-drug resistant gram-negative bacterial infections in the hospitalized setting. Antibiotics. 2020;9(4):196. doi:10.3390/antibiotics9040196
2. Rawat D, Nair D. Extended-spectrum β-lactamases in gram negative bacteria. J Glob Infect Dis. 2010;2(3):263. doi:10.4103/0974-777X.68531
3. Cantón R. Co qu e TM. The CTX-M Be Ta-Lac Tama Se Pan de Mic. Curr Opin Mic Ro Bi Ol. 2006;9(5):466–475.
4. Wilmore SS, Kranzer K, Williams A, et al. Carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in HIV-infected children in Zimbabwe. J Med Microbiol. 2017;66(5):609–615. doi:10.1099/jmm.0.000474
5. Nature E. The antibiotic alarm. Nature. 2013;495(7440):141.
6. Brooks PT, Mansfield LS. Effects of antibiotic resistance (AR) and microbiota shifts on Campylobacter jejuni-mediated diseases. Anim Health Res Rev. 2017;18(2):99–111. doi:10.1017/S1466252318000014
7. Control CfD, Prevention. Antibiotic Resistance Threats in the United States, 2013. Atlanta, GA: CDC; 2013.
8. Sangare SA, Rondinaud E, Maataoui N, et al. Very high prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in bacteriemic patients hospitalized in teaching hospitals in Bamako, Mali. PLoS One. 2017;12(2):e0172652. doi:10.1371/journal.pone.0172652
9. Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791. doi:10.3201/eid1710.110655
10. Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci. 2018;6(1):1. doi:10.3390/medsci6010001
11. Thomson KS. Extended-spectrum-β-lactamase, AmpC, and carbapenemase issues. J Clin Microbiol. 2010;48(4):1019–1025. doi:10.1128/JCM.00219-10
12. Babu R, Kumar A, Karim S, et al. Faecal carriage rate of extended-spectrum β-lactamase-producing Enterobacteriaceae in hospitalised patients and healthy asymptomatic individuals coming for health check-up. J Glob Antimicrob Resist. 2016;6:150–153. doi:10.1016/j.jgar.2016.05.007
13. Vialle‐Valentin C, Lecates R, Zhang F, Desta A, Ross‐Degnan D. Predictors of antibiotic use in African communities: evidence from medicines household surveys in five countries. Trop Med Int Health. 2012;17(2):211–222. doi:10.1111/j.1365-3156.2011.02895.x
14. Khan M, Khalil J, Zarin M, et al. Rate and risk factors for surgical site infection at a tertiary care facility in Peshawar, Pakistan. J Ayub Med Coll Abbottabad. 2011;23(1):15–18.
15. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge university press; 2005.
16. Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology E-book. Elsevier Health Sciences; 2020.
17. Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. 2020;58(3):e01864–e01919. doi:10.1128/JCM.01864-19
18. Magiorakos A-P, Srinivasan A, Carey R, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
19. Lashoher A, Schneider EB, Juillard C, et al. Implementation of the World Health Organization Trauma Care Checklist Program in 11 centers across multiple economic strata: effect on care process measures. World J Surg. 2017;41(4):954–962. doi:10.1007/s00268-016-3759-8
20. Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob. 2014;13(1):1–10. doi:10.1186/1476-0711-13-14
21. Dessie W, Mulugeta G, Fentaw S, Mihret A, Hassen M, Abebe E. Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals, Addis Ababa, Ethiopia. Int J Microbiol. 2016;2016:1–8. doi:10.1155/2016/2418902
22. Mohammed A, Seid ME, Gebrecherkos T, Tiruneh M, Moges F. Bacterial isolates and their antimicrobial susceptibility patterns of wound infections among inpatients and outpatients attending the University of Gondar Referral Hospital, Northwest Ethiopia. Int J Microbiol. 2017;2017:1–10. doi:10.1155/2017/8953829
23. Roy S, Ahmed MU, Uddin BMM, et al. Evaluation of antibiotic susceptibility in wound infections: a pilot study from Bangladesh. F1000Research. 2017;6:2103. doi:10.12688/f1000research.12887.1
24. Shimekaw M, Tigabu A, Tessema B. Bacterial profile, antimicrobial susceptibility pattern, and associated risk factors among patients with wound infections at Debre Markos Referral Hospital, Northwest, Ethiopia. Int J Low Extrem Wounds. 2020;21:1534734620933731.
25. Rijal BP, Satyal D, Parajuli NP. High burden of antimicrobial resistance among Bacteria causing pyogenic wound infections at a tertiary Care Hospital in Kathmandu, Nepal. J Pathog. 2017;2017:1–7. doi:10.1155/2017/9458218
26. Shabnum M. Microbial profile and antibiotic susceptibility pattern of orthopedic infections in a tertiary care hospital: a study from South India. Int J Med Sci Public Health. 2017;6(5):838–842.
27. Omoregie AYEEC, Ohiorenuan R II, Onemu S, Onemu S. Microbiology of wound infections and its associated risk factors among patients of a Tertiary hospital in Benin City, Nigeria. J Res Health Sci. 2011;11:109–113.
28. Zuarez-Easton S, Zafran N, Garmi G, Salim R. Postcesarean wound infection: prevalence, impact, prevention, and management challenges. Int J Womens Health. 2017;9:81. doi:10.2147/IJWH.S98876
29. Mitiku M, Ayenew Z, Desta K. Multi-drug resistant, extended spectrum beta-lactamase and carbapenemase producing bacterial isolates among septicemia suspected under five children in Tikur Anbessa specialized Hospital, Addis Ababa Ethiopia: cross-sectional study; 2019.
30. Kano C. Carbapenem-Resistant Enterobacteriaceae (CRE) in Intensive Care Units and surgical wards of hospitals with no history of carbapenem usage in Kano, North West Nigeria. Niger J Microbiol. 2015;27(1):2612–2618.
31. Alebel M, Mekonnen F, Mulu W. Extended-spectrum β-lactamase and carbapenemase producing gram-negative bacilli infections among patients in intensive care units of felegehiwot referral hospital: a prospective cross-sectional study. Infect Drug Resist. 2021;14:391. doi:10.2147/IDR.S292246
32. Beyene D, Bitew A, Fantew S, Mihret A, Evans M. Multidrug-resistant profile and prevalence of extended spectrum β-lactamase and carbapenemase production in fermentative Gram-negative bacilli recovered from patients and specimens referred to National Reference Laboratory, Addis Ababa, Ethiopia. PLoS One. 2019;14(9):e0222911. doi:10.1371/journal.pone.0222911
33. Kołpa M, Wałaszek M, Gniadek A, Wolak Z, Dobroś W. Incidence, microbiological profile and risk factors of healthcare-associated infections in intensive care units: a 10 year observation in a provincial hospital in Southern Poland. Int J Environ Res Public Health. 2018;15(1):112. doi:10.3390/ijerph15010112
34. Gupta R, Malik A, Rizvi M, Ahmed M. Presence of metallo-beta-lactamases (MBL), extended-spectrum beta-lactamase (ESBL) & AmpC positive non-fermenting Gram-negative bacilli among Intensive Care Unit patients with special reference to molecular detection of blaCTX-M & blaAmpC genes. Indian J Med Res. 2016;144(2):271. doi:10.4103/0971-5916.195043
35. Uc-Cachón AH, Gracida-Osorno C, Luna-Chi IG, Jiménez-Guillermo JG, Molina-Salinas GM. High prevalence of antimicrobial resistance among gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Medicina. 2019;55(9):588. doi:10.3390/medicina55090588
36. Singh N, Pattnaik D, Neogi DK, Jena J, Mallick B. Prevalence of ESBL in Escherichia coli isolates among ICU patients in a tertiary care hospital. JCDR. 2016;10(9):DC19. doi:10.7860/JCDR/2016/21260.8544
37. Kayastha K, Dhungel B, Karki S, et al. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children’s Hospital, Kathmandu, Nepal. Infect Dis. 2020;13:1178633720909798. doi:10.1177/1178633720909798
38. Kovacevic P, Zlojutro B, Kovacevic T, Baric G, Dragic S, Momcicevic D. Microorganisms profile and antibiotics sensitivity patterns in the only medical intensive care unit in Bosnia and Herzegovina. Microbial Drug Resist. 2019;25(8):1176–1181. doi:10.1089/mdr.2018.0458
39. Siddique SG, Bhalchandra MH, Wyawahare AS, Bansal VP, Mishra JK, Naik S. Prevalence of MRSA, ESBL and Carbapenemase producing isolates obtained from endotracheal and tracheal tubes secretions of ICU patient at tertiary care centre. Int J Curr Microbiol App Sci. 2017;6(4):288–299. doi:10.20546/ijcmas.2017.604.032
40. Hamishehkar H, Shadmehr P, Mahmoodpoor A, Mashayekhi SO, Entezari-Maleki T. Antimicrobial susceptibility patterns among bacteria isolated from intensive care units of the largest teaching hospital at the northwest of Iran. Braz J Pharm Sci. 2016;52(3):403–412. doi:10.1590/s1984-82502016000300006
41. Lamichhane B, Thakur C, Jain S. Antibiotic resistance patterns of Gram-negative isolates in a tertiary care hospital of Nepal. Asian J Pharm Clin Res. 2014;7(3):30–33.
42. Ghimire A, Acharya B, Tuladhar R. Extended Spectrum β-Lactamase (ESBL) producing multidrug resistant gram-negative bacteria from various clinical specimens of patients visiting a Tertiary Care Hospital. TUJM. 2017;4:1–8. doi:10.3126/tujm.v4i0.21667
43. Moges F, Eshetie S, Abebe W, et al. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS One. 2019;14(4):e0215177. doi:10.1371/journal.pone.0215177
44. Abera B, Kibret M, Mulu W. Extended-Spectrum beta (β)-lactamases and Antibiogram in Enterobacteriaceae from clinical and drinking water Sources from Bahir Dar City, Ethiopia. PLoS One. 2016;11(11):e0166519. doi:10.1371/journal.pone.0166519
45. Lonchel CM, Melin P, Gangoué-Piéboji J, Assoumou M-C, Boreux R, De Mol P. Extended-spectrum β-lactamase-producing Enterobacteriaceae in Cameroonian hospitals. Eur J Clin Microbiol Infect Dis. 2013;32(1):79–87. doi:10.1007/s10096-012-1717-4
46. Vinodhini R, Moorthy K, Palanivel P, et al. Detection and antimicrobial susceptibility pattern of ESBL producing Gram negative bacteria. Asian J Pharm Clin Res. 2014;7(1):243–247.
47. Oberoi L, Singh N, Sharma P, Aggarwal A. ESBL, MBL and Ampc β lactamases producing superbugs–havoc in the intensive care units of Punjab India. JCDR. 2013;7(1):70. doi:10.7860/JCDR/2012/5016.2673
48. Baron EJ, Miller JM, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM) a. Clin Infect Dis. 2013;57(4):e22–e121. doi:10.1093/cid/cit278
49. Ahmed MAS, Bansal D, Acharya A, et al. Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae from intensive care units at Hamad Medical Corporation, Qatar. Antimicrob Resist Infect Control. 2016;5(1):1–6. doi:10.1186/s13756-015-0100-5
50. Saeed NK, Kambal AM, El-Khizzi NA. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med J. 2010;31(12):1341–1349.
51. Nedjai S, Barguigua A, Djahmi N, et al. Prevalence and characterization of extended spectrum beta-lactamase-producing Enterobacter cloacae strains in Algeria. J Infect Dev Ctries. 2013;7(11):804–811. doi:10.3855/jidc.3127
52. Marie MA, John J, Krishnappa LG, Gopalkrishnan S. Molecular characterization of the β‐lactamases in Escherichia coli and Klebsiella pneumoniae from a tertiary care hospital in Riyadh, Saudi Arabia. Microbiol Immunol. 2013;57(12):805–810. doi:10.1111/1348-0421.12104
53. Habeeb MA, Sarwar Y, Ali A, Salman M, Haque A. Rapid emergence of ESBL producers in E. coli causing urinary and wound infections in Pakistan. Pak J Med Sci. 2013;29(2):540. doi:10.12669/pjms.292.3144
54. Olowo-Okere A, Ibrahim YKE, Olayinka BO. Molecular characterisation of extended-spectrum β-lactamase-producing Gram-negative bacterial isolates from surgical wounds of patients at a hospital in North Central Nigeria. J Glob Antimicrob Resist. 2018;14:85–89. doi:10.1016/j.jgar.2018.02.002
55. Katip W, Yoodee J, Uitrakul S, Oberdorfer P. Efficacy of loading dose colistin versus carbapenems for treatment of extended spectrum beta lactamase producing Enterobacteriaceae. Sci Rep. 2021;11(1):1–8. doi:10.1038/s41598-020-78098-4
56. Andrew B, Kagirita A, Bazira J. Prevalence of extended-spectrum beta-lactamases-producing microorganisms in patients admitted at KRRH, Southwestern Uganda. Int J Microbiol. 2017;2017:1–5. doi:10.1155/2017/3183076
57. Ibrahim ME, Bilal NE, Magzoub MA, Hamid ME. Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from Hospitals in Khartoum State, Sudan. Oman Med J. 2013;28(2):116. doi:10.5001/omj.2013.30
58. Saharman YR, Lestari DC. Phenotype characterization of Beta-lactamase producing Enterobacteriaceae in the intensive care unit (ICU) of Cipto Mangunkusumo hospital in 2011. Acta Medica Indonesiana. 2016;45:1.
59. Hecini-Hannachi A, Bentchouala C, Lezzar A, Laouar H, Benlabed K, Smati F. Multidrug-resistant bacteria isolated from patients hospitalized in Intensive Care Unit in University Hospital of Constantine, Algeria (2011–2015). Afr J Microbiol Res. 2016;10(33):1328–1336. doi:10.5897/AJMR2016.8257
60. Qaddumi JA, Nazzal Z, Yacoup AR, Mansour M. Quality of death notification forms in North West Bank/Palestine: a descriptive study. BMC Res Notes. 2017;10(1):1–6. doi:10.1186/s13104-017-2469-0
61. Leite CAK, Oizumi KY, Caleffi-Ferracioli KR, et al. β-lactamase-producing Gram-negative bacteria in an intensive care unit in southern Brazil. Braz J Pharm Sci. 2017;22:53.
62. Ibrahim ME, Abbas M, Al-Shahrai AM, Elamin BK. Phenotypic characterization and antibiotic resistance patterns of extended-spectrum β -lactamase- and AmpC β -lactamase-producing gram-negative bacteria in a referral hospital, Saudi Arabia. Can J Infect Dis Med Microbiol. 2019;2019:1–9. doi:10.1155/2019/6054694
63. Manoharan A, Sugumar M, Kumar A, Jose H, Mathai D. Group I-Es. Phenotypic & molecular characterization of AmpC β-lactamases among Escherichia coli, Klebsiella spp. & Enterobacter spp. from five Indian Medical Centers. Indian J Med Res. 2012;135(3):359.
64. Abdalhamid B, Albunayan S, Shaikh A, Elhadi N, Aljindan R. Prevalence study of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking inducible ampC from Saudi hospitals. J Med Microbiol. 2017;66(9):1286–1290. doi:10.1099/jmm.0.000504
65. Chang -Y-Y, Chuang Y-C, Siu LK, et al. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect. 2015;48(2):219–225. doi:10.1016/j.jmii.2014.05.010
66. Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452–464. doi:10.1016/S1474-4422(17)30118-7
67. Makharita RR, El-Kholy I, Hetta HF, et al. Antibiogram and genetic characterization of carbapenem-resistant gram-negative pathogens incriminated in healthcare-associated infections. Infect Drug Resist. 2020;13:3991. doi:10.2147/IDR.S276975
68. Ramana K, Rao R, Sharada CV, Kareem M, Reddy LR, Mani MR. Modified Hodge test: a useful and the low-cost phenotypic method for detection of carbapenemase producers in Enterobacteriaceae members. J Nat Sci Biol Med. 2013;4(2):346. doi:10.4103/0976-9668.117009
69. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis. 2014;59(suppl_3):S97–S100. doi:10.1093/cid/ciu542
70. Mushi MF, Mshana SE, Imirzalioglu C, Bwanga F. Carbapenemase genes among multidrug resistant gram negative clinical isolates from a tertiary hospital in Mwanza, Tanzania. Biomed Res Int. 2014;2014:1–6. doi:10.1155/2014/303104
71. Katip W, Uitrakul S, Oberdorfer P. Clinical efficacy and nephrotoxicity of the loading dose colistin for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients. Pharmaceutics. 2021;14(1):31. doi:10.3390/pharmaceutics14010031
72. Katip W, Oberdorfer P. Clinical efficacy and nephrotoxicity of colistin alone versus colistin plus vancomycin in critically ill patients infected with carbapenem-resistant Acinetobacter baumannii: a propensity score-matched analysis. Pharmaceutics. 2021;13(2):162. doi:10.3390/pharmaceutics13020162
73. Katip W, Oberdorfer P, Kasatpibal N. Effectiveness and nephrotoxicity of loading dose colistin–meropenem versus loading dose colistin–imipenem in the treatment of carbapenem-resistant Acinetobacter baumannii infection. Pharmaceutics. 2022;14(6):1266. doi:10.3390/pharmaceutics14061266
74. Wanla W, Katip W, Supakul S, Apiwatnakorn P, Khamsarn S. Effects of an antimicrobial restriction system on appropriate carbapenem use in a hospital without infectious diseases consultation. Int J Gen Med. 2017;10:443. doi:10.2147/IJGM.S145133
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




