Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
MTOR Suppresses Cigarette Smoke-Induced Airway Inflammation and MMP12 Expression in Macrophage in Chronic Obstructive Pulmonary Disease
Authors Dong L, Wang Y, Chen H, Li Z , Xu X, Zhou J, Shen H, Chen Z
Received 6 August 2023
Accepted for publication 7 January 2024
Published 23 January 2024 Volume 2024:19 Pages 269—279
DOI https://doi.org/10.2147/COPD.S426333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Lingling Dong,1,* Yong Wang,1,* Haipin Chen,1 Zhouyang Li,1 Xuchen Xu,1 Jiesen Zhou,1 Huahao Shen,1,2 Zhihua Chen1
1Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhihua Chen, Department of Respiratory and Critical Care Medicine, Key Laboratory of Respiratory Disease of Zhejiang Province, Second Affiliated Hospital, Zhejiang University School of Medicine, 88 Jiefang Road, Hangzhou, 310009, People’s Republic of China, Tel +86-571-8898-1913, Fax +86-571-8778-3729, Email [email protected]
Background: Macrophage-derived matrix metalloproteinase 12 (MMP12) can cause destruction of lung tissue structure and plays a significant role in the development and progression of chronic obstructive pulmonary disease (COPD). MTOR is a serine/threonine kinase that plays a crucial role in cell growth and metabolism. The activity of MTOR in the lung tissues of COPD patients also shows significant changes. However, it is unclear whether MTOR can regulate the development and progression of COPD by controlling MMP12. This study primarily investigates whether MTOR in macrophages can affect the expression of MMP12 and participate in the progression of COPD.
Methods: We tested the changes in MTOR activity in macrophages exposed to cigarette smoke (CS) both in vivo and in vitro. Additionally, we observed the effect of MTOR on the expression of MMP12 in macrophages and on lung tissue inflammation and structural damage in mice, both in vivo and in vitro, using MTOR inhibitors or gene knockout mice. Finally, we combined inhibitor treatment with gene knockout to demonstrate that MTOR primarily mediates the expression of MMP12 through the NF-κB signaling pathway.
Results: Exposure to CS can enhance MTOR activity in mouse alveolar macrophages. Inhibiting the activity of MTOR or suppressing its expression leads to increased expression of MMP12. Myeloid-specific knockout of MTOR expression can promote the occurrence of CS-induced pulmonary inflammation and emphysema in mice. Inhibiting the activity of NF-κB can eliminate the effect of MTOR on MMP12.
Conclusion: Macrophage MTOR can reduce the expression of MMP12 by inhibiting NF-κB, thereby inhibiting the occurrence of COPD inflammation and destruction of lung tissue structure. Activating the activity of macrophage MTOR may be beneficial for the treatment of COPD.
Keywords: matrix metalloproteinase 12, MMP12, pulmonary emphysema, cigarette smoke, CS, mechanistic target of rapamycin, MTOR
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with an inflammatory response to inhaled toxins, especially cigarette smoke (CS), and is a leading cause of mortality in the world.1,2 However, the underlying mechanisms of COPD pathogenesis are still unclear.
Mechanistic target of rapamycin (MTOR), a serine/threonine kinase, plays a central role in cellular growth and metabolism.3,4 MTOR exists in two distinct multiprotein complexes, MTORC1 and MTORC2.5 MTORC1 can activate multiple downstream molecules to regulate different cellular functions, including mRNA transcription, protein synthesis, lipid synthesis, nucleotide synthesis, and autophagy.4 It has been shown that MTOR plays a crucial role in a variety of physiological and pathological processes, containing neurodegenerative diseases, aging, cancer, and obesity.6–8 However, much remains to be explored about the in vivo function of MTOR in the pathogenesis of COPD. We have previously reported that reduced MTOR expression was observed in lung tissue of patients with COPD, and knockout of MTOR in bronchial epithelial or alveolar type 2 cells contributed to the formation of emphysema.9 In contrast, increased MTOR activity was found in peripheral blood monocytes from COPD patients.10 Besides, inhibition of MTOR activity can ameliorate corticosteroid resistance in COPD.10 These results suggest that MTOR may play different roles in distinct cell types.
Prior research has demonstrated that emphysema’s pathogenesis involves crucial stages such as protease-antiprotease imbalance, oxidative stress, chronic lung inflammation, alveolar cell apoptosis, and autophagy.11 Recently, alveolar macrophages have garnered increasing attention as significant endogenous suppliers of matrix-degrading proteinases in the lungs of emphysematous patients.12 Multiple proteases are released by macrophages and neutrophils, which can degrade loose connective tissue.13,14 These proteases are capable of causing the apoptosis of vascular endothelial cells and destruction of lung parenchyma. Among these proteases, matrix metalloproteinase 12 (MMP12) plays a crucial role in the progression of COPD.15,16 MMP12 is a 54-kDa enzymatic protease primarily expressed by macrophages, and an elevation in macrophage MMP12 has been noted both clinically and experimentally in conjunction with the development of emphysema and COPD.15–17 It has been reported that MMP12 protein level was increased in induced-sputum collected from COPD patients.18 Besides, mice lacking MMP12 have been reported to protect from emphysema.16 Follow-up study found out that elastin fragments degraded by MMP12 possessed monocyte chemotactic activity, which may account for the presence of chronic airway inflammation.19–21 However, further mechanisms concerning the regulation of MMP12 have yet to be clearly investigated.
Hence, we addressed whether MTOR might integrate environmental stresses due to CS with activation of inflammation and release of MMP12, leading to lung tissue damage and emphysematous destruction. We demonstrate that MTOR is important for CS-induced activation of nuclear factor-κB (NF-kB), and subsequent MMP12 production, airway inflammation and emphysema, adding fresh insight into the pathogenesis of emphysema.
Materials and Methods
Chemicals and Reagents
The antibodies against phosphorylated p-rpS6 (4858), rpS6 (2217), MTOR (2972), (p)-MTOR (5536), RELA (8242), p-RELA (4812) were sourced from Cell Signaling Technology. The antibody against MMP12 (ab52897) was acquired from Abcam, and the one against ACTB (sc-47778) was obtained from Santa Cruz Biotechnology. All these antibodies were diluted in accordance with the manufacturer’s instructions using either 5% nonfat dried milk (Sangon Biotech, A600669) or 5% BSA (Sangon Biotech). For flow cytometry, we used anti-mouse CD45 PE-Cyanine7 (25-0451-82), anti-mouse CD11c+ FITC (11-0114-82), anti-human/mouse phospho-MTOR (S2448) eFluor 450 (48-9718-42), and anti-human/mouse phosphor-rpS6 (S235/S236) APC (17-9007-42) antibodies from eBioscience. The anti-mouse Siglec-F PE (562068) antibody was purchased from BD Biosciences. BAY 11-7082 (HY-13453) was obtained from Medchem Express and was used at a final concentration of 2.5 μM in the culture medium. Rapamycin (S1039) and Torin 1 (S2827) were sourced from Selleck, and their final concentrations were 1.25 and 125 nM, respectively.
CSE Preparation and Vivo CS Exposures
CS extract (CSE) was prepared and incorporated into the culture media following previously described methods.9 In summary, mainstream smoke from 20 Marlboro cigarettes with filters was bubbled through 100mL RPMI 1640 (Gibco, C11875500BT) using a pump. The resulting smoke extract was then filter-sterilized, stored at −80°C, and used immediately after thawing. This CSE was considered to be 100% strength and was further diluted in complete RPMI 1640 medium for cell treatment. For the animal study, we randomly selected age- and gender-matched mice, starting at 6–8 weeks of age. These mice were exposed to total body CS in a CS chamber using a whole-body smoke exposure device (Teague Enterprises). The exposure regimen consisted of 2 hours per day, 5 days per week, for 3–6 months. The mice were exposed to both mainstream and sidestream smoke from 100 Marlboro cigarettes, which resulted in an average total particulate matter (TPM) of 150–200mg/m3.
Peritoneal Macrophages and Bone Marrow–Derived Macrophages Proliferation
The procedure for isolating and culturing bone marrow-derived macrophages (BMDM) followed a previously described method with some minor modifications.22 Briefly, mice aged 6–8 weeks were euthanized by cervical dislocation and immersed in 75% ethanol. The femurs and tibias were then extracted, and bone marrow (BM) cells were flushed out from all bones. After centrifugation at 400 × g for 5 minutes, erythrocytes were removed using RBC Lysing Buffer. The remaining cells were seeded in plates and incubated in DMEM with 10% (v/v) heat-inactivated FBS and 10ng/mL recombinant mouse M-CSF (Novoprotein, P07141) for 7 days to promote the growth of non-activated proliferative cells. Primary mouse peritoneal macrophages were acquired from the peritoneal exudates of mice aged 6–8 weeks. These mice were injected intraperitoneally (i.p.) with 1 mL of fluid thioglycolate medium three times. Afterward, the peritoneal exudate cells were washed twice with PBS and cultured in RPMI 1640 for 3–4 hours at 37°C and 5% CO2. The nonadherent cells were then removed by washing with warm PBS.
Flow Cytometry for AMs
Following euthanasia, mice underwent intracardial perfusion with approximately 20mL of PBS. The lungs were carefully extracted and cut into small pieces, followed by digestion in collagenase type I (Gibco, 17100017) within HBSS (with Ca2+/Mg2+) supplemented with FBS for 30 minutes at 37°C. Subsequently, the cells were filtered through a 40-mm filter. The cell pellets were resuspended in an antibody mix in PBS and subjected to staining at 4 °C for 15 minutes. CD45+CD11c+SlglecF+ cells were then selected for analyzing the intracellular expression of p-MTOR or p-rpS6 using Cytoflex (Beckman Coulter). The data were subsequently analyzed using FlowJo software (Tree Star).
Animals
Wild-type mice were acquired from the Animal Center of Slaccas (Shanghai, China). Mtorfl/fl mice, having a C57BL/6 background, were acquired from the Jackson Laboratory, while LysMCre mice with a C57BL/6 background were generously provided by Dr. G. Feng (University of California at San Diego, CA). Myeloid cell-specific MTOR conditional knockout mice (Mtorfl/fl-LysMCre) were generated through the crossing of Mtorfl/fl mice with those carrying Cre recombinase under the control of the lysozyme promoter (LysMCre). Control groups consisted of age- and gender-matched LysMCre-negative, Mtorfl/fl littermates. For in vitro BMDM experiments, mice aged 4–5 weeks were usually employed. Meanwhile, mice aged 6–8 weeks were used for in vitro peritoneal macrophage experiments and in vivo CS exposure models. All mice were housed in a specific pathogen-free facility. Ethical approval for all experimental procedures was granted by the Zhejiang University Medical Laboratory Animal Care and Use Committee and the Ethics Committee for Animal Studies at Zhejiang University. All operations adhere to the “Basic Requirements for Laboratory Animal Welfare and Ethics in Experiments” (GB/T 35892-2018). The following primers were utilized to identify genetically modified mice: MTOR forward: 5’- TTATGTTTGATAATTGCAGTTTTGGCTAGCAGT-3’, reverse: 5’- TTTAGGACTCCTTCTGTGACATACATTTCCT-3’; LysMcre common: 5’- CTTGGGCTGCCAGAATTTCTC-3’, WT: 5’- TTACAGTCGGCCAGGCTGAC-3’, mutant: 5’- CCCAGAAATGCCAGATTACG-3’.
Western Blot Assay
After treatment with CSE, BMDMs and peritoneal macrophages were lysed in radio immunoprecipitation assay lysis buffer (Biosharp, BL504A) containing protease (Roche Diagnostics, 04-693-116-001) and phosphatase inhibitors (Roche Diagnostics, 04-906-837-001). After obtaining the lysates, standard gel electrophoresis was performed, and the proteins were subsequently immunoblotted with relevant antibodies following standard procedures. ACTB was used as the protein loading control.
Isolation of RNA and Analysis Using Quantitative Real-Time PCR
RNA was extracted using RNAiso Plus reagent (Takara, 9109). Complementary DNA was synthesized with Oligo-dT primer using the Reverse Transcription Reagents (Takara, DRR037A) according to the manufacture’s protocols. Quantitative PCR was performed using SYBR Green Master Mix (Takara, DRR041A) on a StepOne real-time PCR system (Applied Biosystems). The samples were individually normalized to ACTB. The following primers were used: Actb forward: 5’- GGCTGTATTCCCCTCCATCG-3’, reverse: 5’-CCAGTTGGTAACAATGCCATGT-3’; Mmp12 forward: 5’-TGGTACACTAGCCCATGCTTT-3’, reverse: 5’-AGTCCACGTTTCTGCCTCATC-3’.
ELISA and MMP12 Activity Kit
The levels of secreted protein MMP12 (Rockland, KOA0622) and its activity (Anaspec, AS-71157) in cell culture supernatants or bronchoalveolar lavage fluid (BALF) supernatants were assessed using ELISA kits according to the manufacturer’s instructions.
BALF Collection and Analysis
At the 24-hour mark after the final CS exposure, bronchoalveolar lavage fluid (BALF) was obtained by instilling the lungs with three injections of 0.4 mL PBS, which was subsequently withdrawn to collect the cells. The total count of BALF cells was calculated, and the remaining BALF was centrifuged at 6000 × rpm for 10 minutes at 4°C. The resulting supernatants were stored at −80°C for cytokine analysis. The cell pellets were resuspended in 200 μL of PBS, and 50 μL of the suspension was then placed onto glass microscope slides. After staining the cells with Wright-Giemsa stain (Baso, BA-4017), differential counts were performed by examining 200 total cells.
Lung Morphometry
Upon euthanasia, the mouse lungs were fixed using 4% paraformaldehyde at a pressure of 30-cm H2O for 15 minutes and subsequently stored in 4% paraformaldehyde for 24 hours. The collected lungs were embedded in paraffin and subjected to standard H&E staining protocols.23,24 Airspace enlargement was quantified using the mean linear intercept (MLI) method, which has been previously described.25,26
Statistics
Statistical analysis was performed using GraphPad Prism software (GraphPad Software). The data are presented as the mean ± SEM. Differences in measured variables between experimental and control groups were evaluated using one-way ANOVA. A p-value of less than 0.05 was considered to indicate statistical significance.
Results
CS Activates MTOR in Macrophages in vivo and in vitro
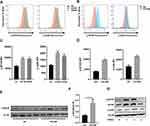
In order to investigate the potential biological modulation of MTOR in macrophages after exposure to CS, we first analyzed the expression of MTOR phosphorylation in vivo and in vitro. The mean fluorescence intensity of both p-MTOR and p-rpS6 in alveolar macrophages was elevated, irrespective of exposure duration to CS, as evidenced in both short-term (Figure 1A and C) and long-term (Figure 1B and D) scenarios. Similarly, the expression of p-MTOR was significantly increased in BALF cells after 12 weeks CS exposure (Figure 1E and F). Next, bone marrow derived macrophages (BMDM) were stimulated with CS extract (CSE), and the activities of MTOR and rpS6 were measured. The expression of MTOR and p-MTOR was quickly and transiently increased after CSE stimulation, and the levels of p-MTOR and p-rpS6 were gradually returned to basal levels at 4h post CSE stimulation (Figure 1G).
Pharmacological Inhibition or Genetic Knockdown of MTOR in Macrophages Deteriorates CSE-Induced MMP12 Production in Macrophages
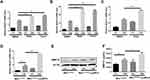
Previous studies have found that MMP12 was correlated with the progress of emphysema, and CS could induce MMP12.16,21 Therefore, we examined a possible role of MTOR in CSE-induced MMP12 expression. We utilized two MTOR inhibitor (rapamycin and torin 1) to avoid contingency. Interestingly, mRNA expression of Mmp12 induced by CSE were markedly upregulated in BMDMs (Figure 2A), peritoneal macrophages (Figure 2B) and alveolar macrophages (Figure 2C) treated with MTOR inhibitors.
To further examine the function of MTOR in regulation of MMP12 level in macrophages, we used Mtorfl/fl-LysMCre mice, which are known to have impaired MTOR expression in myeloid cells.27–29 As expected, Mtor-deficient BMDMs exhibited amplified MMP12 production upon CSE treatment, including mRNA transcripts (Figure 2D) and protein levels (Figure 2E and F).
Mtorfl/fl-LysMCre Mice Display Increased MMP12 Expression, Enhanced Airway Inflammation, and Airspace Enlargement in Response to CS Exposure
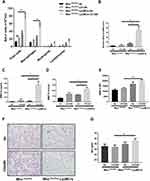
To further examine the role of MTOR in regulation of MMP12 production and airway inflammation in vivo, Mtorfl/fl-LysMCre mice were used for two experimental COPD models. After 12 weeks of chronic CS exposure, there was a notable rise in the overall count of inflammatory cells in the bronchoalveolar lavage fluid (BALF). Notably, these effects were even more pronounced in the Mtorfl/fl-LysMCre mice (Figure 3A). Moreover, Mmp12 mRNA level in BALF cells (Figure 3B) and MMP12 protein level (Figure 3C) and activity (Figure 3D) in BALF were notably increased in Mtorfl/fl-LysMCre mice in response to CS exposure relative to Mtorfl/fl-unimpaired controls. Similarly, mean fluorescence intensity of MMP12 in alveolar macrophages was higher in the Mtorfl/fl-LysMCre mice after 12 weeks CS exposure (Figure 3E). Moreover, the emphysema-like enlargement of airspaces, as indicated by the mean linear intercept (MLI), was significantly increased in CS-exposed Mtorfl/fl-LysMCre mice (Figure 3F and G).
MTOR Suppresses MMP12 Expression and Airway Inflammation in Mouse COPD Model Induced by CS and Elastin
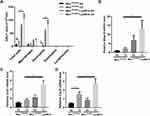
Next, we used an autoimmune-driven mouse COPD model,21 hereafter referred as CS-elastin model, in which mice were exposed to CS for 2 weeks, following a two-week period of rest, and were challenged intratracheally with elastin for 3 days. As expected, instillation of elastin for consecutive 3 days increased the number of total inflammatory cells and neutrophils in BALF, both of which were further exacerbated in the Mtorfl/fl-LysMCre mice (Figure 4A). Cxcl2 showed the same tendency, with a marked induction by CS-elastin treatment in wild-type mice, and a significant increase in Mtorfl/fl-LysMCre mice (Figure 4D). Similar to CS induced COPD mouse model, although the expression of mmp12 and cxcl1 was not significantly increased in the CS-elastin treatment in wild-type mice, their expression markedly elevated when MTOR expression is specifically knocked out in myeloid cells (Figure 4B and C). These findings further confirm the critical role of MTOR in macrophages in suppressing the inflammation associated with COPD.
MTOR Regulates CSE-Induced MMP12 Production Through NF-kB Signaling
NF-kB signaling has been shown to regulate MMP12 in macrophages.30 We next raised the question whether MTOR regulates CSE-induced MMP12 expression through NF-kB signaling. We observed that in macrophages, the levels of p-RELA were markedly induced by CSE in a time-dependent manner, which were exacerbated in MTOR-deficient macrophages (Figure 5A). Furthermore, the application of BAY-11-7082, a specific inhibitor of NF-kB activity, markedly reduced MMP12 mRNA expression induced by CSE (Figure 5B), and similarly decreased protein expression (Figure 5C). Crucially, the inhibition of NF-kB activity was also proved to eliminate the regulatory effect of MTOR on MMP12 expression (Figure 5D).
Discussion
The main discoveries of this study can be outlined as follows: 1) CSE increases the expression and activity of MTOR in macrophages; 2) MTOR activation acts as an adaptive signal, leading to the suppression of CSE-induced MMP12 expression; 3) Mice with targeted MTOR knockdown in myeloid cells display noticeably exacerbated airway inflammation and MMP12 production; 4) The likely mechanism of MTOR in reducing CSE-induced MMP12 levels involves the inhibition of the NF-kB pathway.
Our previous research discovered a decrease in MTOR expression in lung tissue of COPD patients, and knocking out MTOR in bronchial epithelial cells or alveolar type 2 cells contributed to the development of emphysema.9 However, other studies have indicated the activation of MTOR in lung tissue.31 We hypothesized that there may be differences in MTOR expression among different cell types. A study measuring the corticosteroid sensitivity of peripheral blood mononuclear cells from COPD patients, smokers, and non-smoking control subjects found increased MTOR activity in peripheral blood mononuclear cells of COPD patients.10 These findings align with our results.
Previous reports have indicated that MMP12, secreted by macrophages, plays a crucial role in the pathogenesis of COPD by degrading elastin.32 TGF-β has been shown to inhibit the production of MMP12.17 Our data provides the evidence that MTOR can also suppress the secretion of MMP12 from macrophages, both in vivo and in vitro. Another study revealed that a complete knock-out of the a disintegrin and metalloproteinase domain-15 (ADAM15) gene could counteract macrophage apoptosis and MMP12 secretion triggered by CS in mice, meanwhile, the activation of MTOR.33 In this study, the authors suggest that enhanced MTOR activity may help macrophages resist apoptosis, thereby increasing the secretion of MMP-12. However, direct evidence of a correlation between MTOR and MMP-12 is lacking. Our research serves as a complementary addition to this article, demonstrating that the increase in MMP12 secretion due to the lack of ADAM15 is not a result of enhanced MTOR activity, but rather through other mechanisms. The MTOR complex is divided into MTORC1 and MTORC2. Studies have confirmed that inhibiting the activity of MTORC2 can alleviate lung remodeling in rats with COPD.34 Therefore, it may be MTORC1, but not MTORC2, that exhibits benefits in COPD by inhibiting the production of MMP12. However, specific evidence requires further experimental validation. Besides, some studies showed that inhibition of MTOR could ameliorate steroid resistance in COPD,10 whereas others suggested that activation of MTOR could induce pulmonary cell senescence and mimic change in the development of COPD, with rapid progress of emphysema.31 These studies suggest that MTOR may play a different role in distinct cells. Consequently, it is advised to use MTOR related drugs according to disease traits.
Our study revealed that MTOR deficiency mice showed a significant increase in inflammation and total BALF cells compared with wild-type mice after CS exposure. This is in line with our previous study that MTOR suppresses CS -induced airway inflammation in bronchial epithelial and alveolar type 2 cells.9 This suggests that pulmonary epithelium cells and macrophages exist similar mechanism in regulation of inflammation. MMP12 could facilitate the infiltration of inflammatory cells, release of inflammatory cytokines and activation of gelatinase.35 So it can be surmised that the MTOR may participate in the development of inflammation of COPD by regulating the expression of MMP12. Whether MMP12 could contribute to lung inflammation needs the verification of double knockout of MMP12 and MTOR in macrophages.
Mice lacking MMP12 have been previously reported to protect from the development of COPD.16 In our study, dysregulation of MTOR in macrophages results in considerably increased levels of MMP12 and an exacerbated emphysematous phenotype in mouse models after CS exposure. The nuclear factor-κB family is a key player in controlling both innate and adaptive immunity.36 NF-kB has been showed to be involved in the production of MMP12.30 In our study, we found that MTOR decreased the expression of MMP12 by inhibiting NF-kB signaling.
In our CS-induced mouse model of COPD, the neutrophil inflammation in mice is not very apparent, and emphysema is also not obvious. Yet, in mice engineered with myeloid-specific MTOR knockout, CS exposure markedly increases neutrophil accumulation in the airways and precipitates the onset of emphysema. This contrast leads us to a compelling hypothesis: the pronounced activation of MTOR in the alveolar macrophages of these mice might play a pivotal role in mitigating airway inflammation and in the delayed development of emphysema, even under continuous exposure to CS. Hence, inhibiting MTOR activity in the alveolar macrophages of mice may enhance the efficacy of developing a successful COPD mouse model. Drawing inspiration from the established atherosclerosis mouse modeling techniques, specifically the complete knockout of apolipoprotein (ApoE) paired with a high-fat diet, a potentially effective method to refine the development of a COPD mouse model might be the inhibition of MTOR activity in alveolar macrophages, followed by concurrent exposure to CS.
Although we have drawn some conclusions, our study still has significant limitations. First, since the CS-induced COPD mouse model we constructed did not show a pronounced phenotype related to COPD, the specific role of MTOR in the development and progression of COPD remains to be further experimentally validated. Second, the MTOR complex mainly consists of two types, MTORC1 and MTORC2, which play different roles. However, our study did not clarify which of these complexes is responsible for the effects observed. Third, although we have demonstrated that MTOR can regulate the expression and secretion of MMP12, whether MTOR influences the progression of COPD through MMP12 needs to be further proven, for instance, through experiments using double knockout mice.
In conclusion, we show here that MMP12 can be regulated by MTOR in macrophages. We further demonstrated that MTOR can downregulate the expression of MMP12 by inhibiting the expression of NF-κB, thereby suppressing lung inflammation and the destruction of lung tissue structure. Activating MTOR in macrophages could be an effective therapeutic approach for airway inflammation and emphysema induced by CS exposure.
Funding
This work was supported by the General Project of National Natural Science Foundation of China (31970826 to Zhihua Chen), the Major project of Zhejiang Natural Science Foundation (LD21H010001 to Zhihua Chen), the Key Project of National Natural Science Foundation of China (81930003 to Huahao Shen), the Youth Project of National Natural Science Foundation of China (81800033 to Jiesen Zhou and 82100042 to Zhouyang Li), the General Project of National Natural Science Foundation of China (82070047 to Yong Wang).
Disclosure
The authors declare that they have no competing interests.
References
1. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi:10.1016/S0140-6736(11)60968-9
2. Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. doi:10.1016/S2213-2600(12)70060-7
3. Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203.
4. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi:10.1016/j.cell.2017.02.004
5. Kim J, Guan K-L. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21(1):63–71. doi:10.1038/s41556-018-0205-1
6. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi:10.1038/nrm2672
7. Barnes PJ. Senescence in COPD and its comorbidities. Annu Rev Physiol. 2017;79(1):517–539. doi:10.1146/annurev-physiol-022516-034314
8. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi:10.1038/nrm3025
9. Wang Y, Liu J, Zhou JS, et al. MTOR suppresses cigarette smoke-induced epithelial cell death and airway inflammation in chronic obstructive pulmonary disease. J Immunol. 2018;200(8):2571–2580. doi:10.4049/jimmunol.1701681
10. Mitani A, Ito K, Vuppusetty C, Barnes PJ, Mercado N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am J Respir Crit Care Med. 2016;193(2):143–153. doi:10.1164/rccm.201503-0593OC
11. Agustí A, Hogg JC, Drazen JM. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248–1256. doi:10.1056/NEJMra1900475
12. Ishii T, Abboud RT, Wallace AM, et al. Alveolar macrophage proteinase/antiproteinase expression in lung function and emphysema. Eur Respir J. 2014;43(1):82–91. doi:10.1183/09031936.00174612
13. Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51. doi:10.1016/j.matbio.2018.01.018
14. Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44–46:167–174. doi:10.1016/j.matbio.2015.02.002
15. Shibata S, Miyake K, Tateishi T, et al. Basophils trigger emphysema development in a murine model of COPD through IL-4-mediated generation of MMP-12-producing macrophages. Proc Natl Acad Sci U S A. 2018;115(51):13057–13062. doi:10.1073/pnas.1813927115
16. Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. doi:10.1126/science.277.5334.2002
17. Morris DG, Huang X, Kaminski N, et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422(6928):169–173. doi:10.1038/nature01413
18. Demedts IK, Morel-Montero A, Lebecque S, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61(3):196–201. doi:10.1136/thx.2005.042432
19. Houghton AM, Quintero PA, Perkins DL, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116(3):753–759. doi:10.1172/JCI25617
20. Lee SH, Goswami S, Grudo A, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi:10.1038/nm1583
21. Zhou JS, Li ZY, Xu XC, et al. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice. Eur Respir J. 2020;56(3):2000404. doi:10.1183/13993003.00404-2020
22. Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83(1). doi:10.1002/0471142735.im1401s83
23. Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006;20(3):455–465. doi:10.1096/fj.05-5045com
24. McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med. 2002;195(1):51–57. doi:10.1084/jem.20011732
25. Chen ZH, Kim HP, Sciurba FC, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3(10):e3316. doi:10.1371/journal.pone.0003316
26. Chen ZH, Lam HC, Jin Y, et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA. 2010;107(44):18880–18885. doi:10.1073/pnas.1005574107
27. Baker AK, Wang R, Mackman N, Luyendyk JP. Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-alpha expression in macrophages by reducing IL-10 expression. Mol Immunol. 2009;46(11–12):2249–2255. doi:10.1016/j.molimm.2009.04.011
28. Xia L, Hua W, Jin Y, et al. Eosinophil differentiation in the bone marrow is promoted by protein tyrosine phosphatase SHP2. Cell Death Dis. 2016;7(4):e2175. doi:10.1038/cddis.2016.74
29. Yang C-S, Kim -J-J, Lee H-M, et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy. 2014;10(5):785–802. doi:10.4161/auto.28072
30. Kim MJ, Nepal S, Lee ES, Jeong TC, Kim SH, Park PH. Ethanol increases matrix metalloproteinase-12 expression via NADPH oxidase-dependent ROS production in macrophages. Toxicol Appl Pharmacol. 2013;273(1):77–89. doi:10.1016/j.taap.2013.08.005
31. Houssaini A, Breau M, Kebe K, et al. mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight. 2018;3(3). doi:10.1172/jci.insight.93203
32. Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA. 1996;93(9):3942–3946. doi:10.1073/pnas.93.9.3942
33. Wang X, Rojas-Quintero J, Zhang D, et al. A disintegrin and metalloproteinase domain-15 deficiency leads to exaggerated cigarette smoke-induced chronic obstructive pulmonary disease (COPD)-like disease in mice. Mucosal Immunol. 2021;14(2):342–356. doi:10.1038/s41385-020-0325-3
34. Liu L, Qin Y, Cai Z, et al. Effective-components combination improves airway remodeling in COPD rats by suppressing M2 macrophage polarization via the inhibition of mTORC2 activity. Phytomedicine. 2021;92:153759. doi:10.1016/j.phymed.2021.153759
35. Nénan S, Planquois J-M, Berna P, et al. Analysis of the inflammatory response induced by rhMMP-12 catalytic domain instilled in mouse airways. Int Immunopharmacol. 2005;5(3):511–524. doi:10.1016/j.intimp.2004.10.011
36. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi:10.1038/nri910
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.