Back to Journals » Nature and Science of Sleep » Volume 15
Mouth Puffing Phenomenon and Upper Airway Features May Be Used to Predict the Severity of Obstructive Sleep Apnea
Authors Jau JY, Kuo TB, Li LPH, Chen TY, Hsu YS , Lai CT, Yue WC, Huang PH, Yang CC
Received 6 August 2022
Accepted for publication 16 March 2023
Published 3 April 2023 Volume 2023:15 Pages 165—174
DOI https://doi.org/10.2147/NSS.S384387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Je-Yang Jau,1 Terry BJ Kuo,1– 3 Lieber PH Li,1,4– 6 Tien-Yu Chen,1,7 Ying-Shuo Hsu,1,8,9 Chun-Ting Lai,1,2 Weng-Cheu Yue,10 Pin-Hsuan Huang,11 Cheryl CH Yang1,2,4,12,13
1Institute of Brain Science, National Yang-Ming Chiao-Tung University, Taipei, Taiwan; 2Sleep Research Center, National Yang Ming Chiao Tung University, Taipei, Taiwan; 3Tsoutun Psychiatric Center, Ministry of Health and Welfare, Nantou, Taiwan; 4Department of Otolaryngology, Cheng Hsin General Hospital, Taipei, Taiwan; 5Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan; 6Department of Speech Language Pathology and Audiology, College of Health Technology, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan; 7Department of Psychiatry, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan; 8Department of Otolaryngology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; 9School of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan; 10DP Dental, Singapore; 11Department of Health Promotion and Health Education, National Taiwan Normal University, Taipei, Taiwan; 12Department of Education and Research, Taipei City Hospital, Taipei, Taiwan; 13Brain Research Center, National Yang Ming Chiao Tung University, Taipei, Taiwan
Correspondence: Cheryl CH Yang, Institute of Brain Science, National Yang-Ming Chiao-Tung University, No. 155, Sec. 2, Li-Nong St., Beitou, Taipei, 11221, Taiwan, Tel +886-2-28267058, Fax +886-2-28273123, Email [email protected] Lieber PH Li, Department of Otolaryngology, Cheng Hsin General Hospital, No. 45, Cheng Hsin St., Beitou, Taipei, 11221, Taiwan, Tel +886-2-28264400, Email [email protected]
Purpose: This study aimed to investigate (1) the role of mouth puffing phenomenon and upper airway features in obstructive sleep apnea (OSA) and (2) whether mouth-taping during sleep alleviated the severity of OSA.
Participants and Methods: Seventy-one participants underwent a two-night home sleep test (the first day sleeping normally; the second day sleeping with their mouths being taped); their oximetry desaturation index (ODI) and mouth puffing signals (non-mouth puffing, complete mouth puffing, intermittent mouth puffing (IMP), and side mouth puffing) were detected by a validated fingertip pulse oximeter and a mouth puffing detector. The participants were grouped into the ODI-improved group and the ODI-not-improved group according to their sleeping test results. The radiograph was taken by cone-beam computed tomography and cephalometries. Upper airway features including airways, soft tissues, and oral cavity variables were measured.
Results: Participants with severe OSA showed a higher IMP percentage compared with those with normal, mild, and moderate OSA (severe: 33.78%, moderate: 22.38%, mild: 14.55%, normal: 0.31%, p < 0.001). In all participants, the ODI and the percentage of SpO2 under 90 (T90) were positively related to body mass index (BMI) (r = 0.310 and 0.333, respectively), while ODI and T90 were negatively correlated with the minimum width of the airway (r = − 0.473 and − 0.474, respectively); all mentioned relationships were significant (p < 0.05).
Conclusion: IMP proportions were found to be higher in the half of participants whose ODI did not improve after mouth-taping and in those with severe OSA. Moreover, OSA patients with higher ODI, higher T90, and higher proportions of IMP were more likely to have a narrower upper airway.
Keywords: mouth breathing, mouth puffing, sleep phenomena, oximeter, sleep apnea, accelerometer
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder wherein there is a recurrent resistance to the breathing of patients during sleep. Loud snoring, gasping during sleep, obesity, and enlarged neck circumference are risk factors for OSA.1 The exact prevalence is unknown but estimated to be 2–14%.2 This disorder is characterized by repetitive episodes of airway obstruction, resulting in decreased airflow despite ongoing respiratory efforts during sleep, which suggests that recurrent collapse of the upper airway during sleep might be the most important factor for OSA.3
OSA patients had a significantly smaller average airway area and average airway volume compared with non-OSA subjects.2,4 The craniofacial abnormalities that are most associated with OSA are mandibular deficiency, maxillary hypoplasia, the inferior position of the hyoid bone, a narrowed posterior air space, and a greater flexion of the cranial base.5–7 Overall, different oromandibular structures may affect the size of the soft tissues of the oropharynx, and when the soft tissues of the oropharynx are so large that they compress the space of the upper airway, it is likely that sleep apnea occurs. Breathing with the mouth open during sleep is a common phenomenon for patients with OSA.8 A previous study found that keeping the mouth open resulted in a prolonged airway and shrank the oropharyngeal lumen irrespective of the severity of OSA. Simultaneously, sleeping with the mouth wide open combined with larger tonsils contributes to a narrower oropharyngeal airway.9 A recent study showed that people breathing through their mouths might have a more elongated and narrower upper airway, increasing the pharyngeal resistance and the collapse of the pharyngeal airway, thus increasing the OSA severity.3–5 Moreover, mouth breathing has been related to hypoxia, and patients with OSA who have mouth breathing symptoms can have a relatively high chance of being hypoxemic.6,7
Mouth breathing has been identified as a risk factor for OSA in recent years. For this reason, oral devices or mouth-taping have been used to avoid mouth breathing. The porous oral patch is a useful device to reduce snoring severity and can improve the apnea/hypopnea index (AHI) in patients with mild OSA and those who habitually mouth-breath during sleep, regardless of the body mass index (BMI) of the patients.10 A previous study showed that using a porous oral patch, a mandibular advancement device (MAD), a continuous positive airway pressure (CPAP, the gold standard for treating OSA), or other oral devices to prevent mouth breathing had improved the AHI of OSA patients and their other sleep-disordered symptoms.1,10,11 However, the AHI of one-third of the participants became worse in the study by Huang,10 indicating that the device to prevent mouth breathing may not be suitable for every OSA patient and can even make OSA symptoms worse for reasons that need further investigations. Clinically, the condition of a patient with OSA was found to worsen after being mouth taped during sleep. After an otorhinolaryngological check-up, a mouth puffing phenomenon was observed when the patient was asleep, indicating that the patient was trying to breathe through the mouth despite that the oral device was preventing them to do so. In our previous study, the mouth puffing phenomenon had been observed and detected by a mouth puffing detector (MPD) in participants during polysomnography.12
Therefore, it is important to clarify the correlation between the mouth puffing phenomenon and OSA. This study aimed to investigate (1) the role of mouth puffing phenomenon and upper airway features in obstructive sleep apnea (OSA), and (2) whether mouth-taping during sleep alleviated the severity of OSA.
Materials and Methods
Participants, Instrumentation, and Study Protocol
This study recruited 71 participants, aged from 35 to 60 years. Figure 1 shows the participant recruitment flowchart. All participants took the home sleep test for two consecutive nights. The wireless fingertip pulse oximeter (AT101C-XB, Taiwan) was attached to a finger to detect the SpO2-related variables, the oxygen saturation signal, and physical activity data, which were gathered every second.13 It has an 81.25% accuracy in OSA diagnosis.13 To detect the phenomenon of mouth puffing when mouth-taped, the participants were asked to sleep with their mouths sealed by the breathable tape throughout the night (Figure 2). We have also developed a mouth puffing detector (MPD).12–14 It is the combination of two originally designed accelerometers (BLEACT, 7 g, 4.2×1.8 × 0.75 cm3, Taiwan) to record the ranges of mouth puffing. In the present study, the accelerometers were placed on both cheeks of the participants and fixed with tape (shown in Figure 2). According to the number of mouth puffing by minute, the mouth puffing signals (MPSs) were distinguished into four types, including non-mouth puffing (NMP), complete mouth puffing (CMP), intermittent mouth puffing (IMP), and side mouth breathing (SMP). Moreover, we obtained the basic information about the participants at their first intake, including their sex, age, height, weight, and neck circumference. We also collected their answers to the sleep-related scales, including Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS). Participants with reported psychiatric diseases, neurological disorders, diabetes, chronic renal diseases, cancer, cardiovascular diseases (hypertension), cigarette or alcohol addiction, or sleep disorders were excluded. All participants had provided written informed consent. The procedures used in this study were approved by the Human Research Committee of the National Yang-Ming University, Taipei, Taiwan (YM107083E).
 |
Figure 1 Flowchart summarizing the study procedure. Abbreviations: PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; w/o MT, when not mouth-taped; w/ MT, when mouth-taped. |
 |
Figure 2 Images to demonstrate how the participants slept with their mouths sealed by the breathable tape throughout the night. |
Upper Airway, Soft Tissue, and Oral Cavity Variables
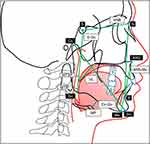
All participants took the cone beam computed tomography and cephalometries, from which we measured their upper airways, soft tissues, and oral cavity variables. The followings are the descriptions of the measured variables (shown in Figures 3 and 4). Upper airway variables included the narrowest dimension between the posterior part of the uvula and the anterior wall of the nasopharynx (MTSP), the narrowest dimension between the posterior part of the tongue and the anterior wall of the oropharynx (MTIP), and the minimum width of the airway (MWA). Soft tissue variables included the distance between the hyoid bone to the mandibular plane (MP), and the length of the uvula (UL). The oral cavity consisted of variables coming from three dimensions. Sagittal variables included the mandible length (Co-Gn), and the angle between the sella-nasion and nasion-B point (SNB). Vertical variables included the anterior lower facial height (ANS-Me), and posterior facial height (S-Go). Transverse variables included the distance between the upper canine tips (U3W) and upper inter-molar width (U6W).
Statistical Analysis
Statistical analysis was performed using the SPSS software (24.0 version for Windows). For the statistical analysis of the present study, we first examined whether the variables were normally distributed. To compare the differences in the SpO2-related variables and MPSs between the ODI-improved and ODI-not-improved groups, we used the independent sample t-test or Mann–Whitney U-test (if a variable was not normally distributed). The correlations of upper airway variables with the SpO2-related variables and MPSs were analyzed with Pearson’s correlation coefficient or Spearman correlation coefficient (if a variable was not normally distributed). The comparisons across the normal, mild, moderate, and severe OSA groups in terms of the SpO2-related variables and MPSs were analyzed by the analysis of variance (ANOVA) with Scheffe’s post-hoc test, if a variable was not normally distributed, then Kruskal–Wallis test with Dunn post-hoc test. Different biotypes, such as BMI and Angle’s Classification, were also compared with ANOVA, if a variable was not normally distributed, then Kruskal–Wallis test with Dunn post-hoc test. For all analyses, p < 0.05 was considered statistically significant.
Results
Basic Information of Participants and Relationships of OSA Severity with SpO2-Related Variables and MPSs
A total of 71 participants’ (45 males and 13 females; mean age: 45.01 years old) MPSs, SpO2 data, upper airway variables, and general information were collected. The mean BMI was 26.80, the mean neck circumference was 39.25 cm, the mean PSQI score was 8.63, and the mean ESS score was 10.75 (Table 1 and Table 2). All participants were classified into four different OSA severity groups based on their ODI, there were 13 participants without OSA (Normal/control group), 17 participants with mild OSA, 28 participants with moderate OSA, and 13 participants with severe OSA. In Table 1, Kruskal–Wallis test of variance and Dunn post-hoc test. BMI, Neck circumference, PSQI, ODI, T90, mean SpO2, the minimum SpO2, NMP, IMP, CMP, and SMP of the four groups were found to be significantly different.
 |
Table 1 Compare the ODI Improved Group and the ODI Not-Improved Group After Mouth-Taped |
 |
Table 2 Correlation Between the SpO2-Related Variables and MPS Variables of Participants When Not Mouth-Taped |
Comparing the ODI-Improved Group and the ODI-Not-Improved Group When Mouth-Taped
Table 2 shows that the ODI of 30 participants improved (51.72%, ODI-improved group/Group 1) when mouth-taped compared with when not, while those of the other 28 participants did not (48.28%, ODI-not-improved group/Group 2). Comparing the two groups when mouth-taped, only the ODI was statistically different (p = 0.001), with the mean ODI of Group 1 (16.37) significantly lower than that of Group 2 (30.50); further, a significant difference (p = 0.037) was found between the two groups in terms of their IMP ratios (Group 1: 19.06; Group 2: 26.47). On the other hand, comparing the two groups when not mouth-taped, none of the four SpO2-related variables was found to be statistically different between Group 1 and Group 2.
Correlation Between the SpO2-Related Variables and MPS Variables of Participants When Not Mouth-Taped
Table 2 shows the correlation between SpO2-related variables and MPS variables. IMP ratio was moderately positively correlated with ODI (r = 0.56, p < 0.05) and T90 (r = 0.45, p < 0.001), IMP ratio was negatively correlated with mean SpO2 (r = −0.55, p < 0.001) and the minimum SpO2 (r = −0.33, p < 0.05), NMP ratio was negatively correlated with ODI (r = −0.45, p < 0.001) and T90 (r = −0.39, p < 0.001), and NMP ratio was positively correlated with mean SpO2 (r = 0.50, p < 0.001) and the minimum SpO2 (r = 0.27, p <0.05). In short, ODI and T90 of the participants were positively correlated with the proportion of IMP and were negatively related to the proportion of NMP.
Correlations of the SpO2-Related Variables with Upper Airway, Soft Tissue, and Oral Cavity Variables
Table 3 presents the correlations of the SpO2-related variables with the upper airway, soft tissue, and three-dimensional oral cavity variables, among which was the soft tissue most related to SpO2. The proportion of IMP was significantly positively related to soft tissue, implying that OSA patients with a higher proportion of IMP had larger uvula (r = 0.301, p < 0.05). ODI was negatively related to the minimum width of the airway (r = −0.473, p < 0.001) and nasal width (r = −0.381, p < 0.001). T90 was significantly negatively related to the minimum width of the airway (r = −0.474, p < 0.001) and nasal width (r = −0.316, p < 0.001). In terms of the oral cavity variables, SpO2-related variables were not statistically related to the oral cavity variables. In addition, ODI, T90, and the proportion of MPS were significantly related to BMI and neck circumference, such that OSA patients with higher ODI, higher T90, and higher proportions of MPS were more likely to have a narrower upper airway, greater soft tissue, a thicker neck, and an overweight body.
 |
Table 3 The Data from All the Participants are Categorized into Four MPSs to Assess the Difference in SpO2-Related Variables by Minutes |
Discussion
This study evaluated the relationships between different mouth puffing phenomena and the severity of OSA. Several novel findings were noticed in the study. ODI in half of the participants deteriorated after they were mouth-taped during sleep. The percentage of IMP was found to be positively associated with OSA severity.
The present study revealed the relationship between MPSs and OSA severity. Higher percentage of IMP, lower percentage of NMP, higher ODI and T90, and lower mean SpO2 were significantly correlated. This signified that mouth breathing, especially IMP, was related to ODI severity. Recent studies showed that patients with a higher percentage of oral and oronasal breathing periods had severer OSA and lower SpO2 than common snorers and healthy subjects did.4,6,8,15 Previous studies showed that participants who completely breathed with the nose (NMP) tended to have more stable SpO2 during sleep,4,7,8 which is consistent with the findings of the present study. However, in we further classified mouth puffing into IMP, CMP, and SMP. IMP was the worst breathing pattern and was correlated with OSA severity the most (Table S1). While CMP and NMP are both types of regular breathing, the former was found to be more positively correlated with lower SpO2 than the latter, implying that CMP was a worse breathing pattern than NMP. SMP came in different values because of external influences (eg, sleep posture), which should be further explored in the future. Blood oxygen was found to be higher during SMP than during IMP, probably due to the patient’s attempt to move their body as a reaction to the respiratory obstruction. In short, IMP could be used as a mouth breathing severity index (MPSI) to assist in the diagnosis of OSA.
We also investigated the relationships between different biotypes and OSA, including BMI (Table 4) and Angle’s Classification (Table S2). We divided BMI into three groups—normal, overweight, and obese; we found that the higher the BMI value, the severer OSA and the higher the proportion of IMP the participants had. Concerning the Angle’s Classification, significant between-group differences were found only for the ANB angle.
 |
Table 4 Correlation Between the SpO2-Related Variables and Basic Information/Upper Airway/Stomatognathic Structure Variables |
There have been several previous studies on the relationship between palate structure and OSA, but there have been rare studies that investigated the relationship between stomatognathic structure and mouth breathing. In the present study, we found that OSA patients tended to have a narrower upper airway. This finding is consistent with the findings of previous studies, stating that the soft tissues of OSA patients are characterized by narrow posterior palate and posterior tongue airway space, thicker and longer soft palate, smaller angle between the tip of the uvula and the anterior nasal spine, and larger tongue. Oral breathing is a common phenomenon of OSA patients during sleep and it occurs more frequently right before and after events of apnea and hypopnea.16–18 Past studies have shown that mouth breathing tends to cause airway collapse and is associated with more serious and prevalent lateral pharyngeal wall collapse and tongue base collapse.4 For example, when viewed from the front posterior, the face and anterior cranial base of patients with OSA tended to shrink posteriorly, reducing the cranial base angle, and resulting in less available airway space. The inconsistency in the findings between the present study and previous studies might be due to differences in sample size: smaller in ours and larger in previous studies.
Although MPD is not a defined detection tool as Out-of-Center Sleep Testing (OCST), it complements the latter in the aspect of mouth puffing. Traditionally, OCST detects OSA by monitoring airflow, respiratory effort, and blood oxygenation. However, according to our explorations in the dental clinic, we discovered the association between mouth puffing and OSA severity, which then became the topic of our published article.12 This previous study took place in the sleep center, through which we confirmed the significant and positive correlation between the frequency of mouth puffing and OSA severity and the accuracy of MPD in detecting mouth puffing based on the comparison with the polysomnography results. Now in the present study, we aimed to investigate whether these two findings were still consistent based on the home sleep apnea test. In short, MPD is as convenient as OCST, since it is also portable, and it detects OSA from a different angle, which OCST does not look at.
In recent years, more studies have investigated therapies to alleviate OSA symptoms, such as continuous positive airway pressure, MAD, porous oral patch, and others.1,10,11,19 A study from Japan demonstrated a device for continuous tongue suction as a potential therapy,19 which shed light on the necessity to design an effective device for keeping the tongue flat against the palate to alleviate OSA, further implying that myofunctional therapy (oropharyngeal exercises) could be an alternative OSA therapy.20,21 In other words, more studies on healthy individuals without OSA are needed to investigate whether MPSs, BMI, neck circumference, tongue pressure, sleep habits, and other variables are still highly correlated with ODI, and whether the tongue function training, an MAD, or other oral appliances can truly alleviate MPSs and ODI.
There are several limitations to our study. First, the sample size of the present study was rather small, making it inappropriate to generalize the findings to all populations. However, it was also due to the small sample size that we could obtain more detailed information about each participant, such as their SpO2-related variables, cone-beam computed tomography, and mouth puffing signals, and observe the relationships among them. Another limitation resulting from the small sample size was that it was easy for the immature MPS-detecting program to generate an overfitting model. If there had been more data collected, the model could have been trained to analyze MPSs without manually adjusting the parameters and come up with more accurate study results. For the current MPS-detecting program, we still needed to manually adjust the parameters, yet by observing the collected signals, we could further understand the facial movements of the participants during different sleep stages, such as yawning, coughing, etc., which can be included in future analysis. Finally, this was a cross-sectional study that could only suggest a correlation between MPSs and OSA and could not explain the cause and effect between the two.
Conclusion
MPD could effectively detect the mouth puffing phenomenon during sleep when mouth-taped and verify the effects of four types of MPSs, especially those of IMP, which was found to have a positive correlation with ODI and narrower upper airway. In the present study, we found that the mouth puffing phenomenon and OSA had a close correlation.
Abbreviations
OSA, obstructive sleep apnea; ODI, oxygen desaturation index; MPD, mouth puffing detector; IMP, intermittent mouth puffing; NMP, non-mouth puffing; AHI, apnea-hypopnea index; MPS, mouth puffing signal; CMP, complete mouth puffing; SMP, side mouth puffing; SpO2, oxygen saturation; T90, percentage of SpO2 under 90; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; MTSP, the narrowest dimension between the posterior part of the uvula and the anterior wall of the nasopharynx; MTIP, the narrowest dimension between the posterior part of the tongue and the anterior wall of the oropharynx; MWA, minimum width of the airway; NW, nasal width; U6sW, upper inter-molar width; U3W, the distance between the upper canine tips; UL, length of uvula; MP, the distance between hyoid bone to mandibular plane; SNB, Angle between sella-nasion and nasion-B point; Co-Gn, Mandible length; ANS-Me, Anterior lower facial height; S-Go, Posterior facial height.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Human Research Committee of the National Yang-Ming University, Taipei, Taiwan (project number YM107083E).
Acknowledgments
All data accessed complied with relevant data protection and privacy regulations. Je-Yang Jau contributed as first authors. Cheryl C. H. Yang and Lieber P. H. Li contributed equally as corresponding authors.
Funding
This study is financially supported by a grant (CY10615, & CY10820) from the Cheng Hsin General Hospital (Taiwan), and a grant (109BRC-B504) from the project “Aim for the Top University” from the Ministry of Education, Taiwan. The authors do not receive any other financial support from any other organization or person.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22(2):6.
2. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355–360.
3. Kim EJ, Choi JH, Kim KW, et al. The impacts of open-mouth breathing on upper airway space in obstructive sleep apnea: 3-D MDCT analysis. Eur Arch Otorhinolaryngol. 2011;268(4):533–539. doi:10.1007/s00405-010-1397-6
4. Fitzpatrick MF, McLean H, Urton AM, Tan A, O’Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003;22(5):827–832. doi:10.1183/09031936.03.00047903
5. Hsu YB, Lan MY, Huang YC, Kao MC, Lan MC. Association between breathing route, oxygen desaturation, and upper airway morphology. Laryngoscope. 2021;131(2):E659–E664. doi:10.1002/lary.28774
6. Niaki EA, Chalipa J, Taghipoor E. Evaluation of oxygen saturation by pulse-oximetry in mouth breathing patients. Acta Med Iran. 2010;48(1):9–11.
7. Gleeson K, Zwillich CW, Braier K, White DP. Breathing route during sleep. Am Rev Respir Dis. 1986;134(1):115–120. doi:10.1164/arrd.1986.134.1.115
8. Tsuda H, Lowe AA, Chen H, Fleetham JA, Ayas NT, Almeida FR. The relationship between mouth opening and sleep stage-related sleep disordered breathing. J Clin Sleep Med. 2011;7(2):181–186. doi:10.5664/jcsm.28107
9. Hu B, Ye J, Yin G, Zhang Y. The influential factors on the morphological changes of upper airway associated with mouth opening. Laryngoscope. 2018;128(12):2902–2909. doi:10.1002/lary.27212
10. Huang TW, Young TH. Novel porous oral patches for patients with mild obstructive sleep apnea and mouth breathing: a pilot study. Otolaryngol Head Neck Surg. 2015;152(2):369–373. doi:10.1177/0194599814559383
11. Chen H, Eckert DJ, van der Stelt PF, et al. Phenotypes of responders to mandibular advancement device therapy in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101229.
12. Jau JY, Kuo TBJ, Li LPH, et al. Mouth puffing phenomena of patients with obstructive sleep apnea when mouth-taped: device’s efficacy confirmed with physical video observation. Sleep Breath. 2022;27:153–164. doi:10.1007/s11325-022-02588-0
13. Wu CH, Lee JH, Kuo TBJ, Lai CT, Li LPH, Yang CCH. Improving the diagnostic ability of the sleep apnea screening system based on oximetry by using physical activity data. J Med Biol Eng. 2020;40(6):858–867. doi:10.1007/s40846-020-00566-z
14. Hsieh IT, Chen CY, Lin YC, Li JY, Lai CT, Kuo TBJ. Application of cloud computing in physical activity research. In: Sensors. Taipei, Taiwan: IEEE; 2012:1–4.
15. Koutsourelakis I, Vagiakis E, Roussos C, Zakynthinos S. Obstructive sleep apnoea and oral breathing in patients free of nasal obstruction. Eur Respir J. 2006;28(6):1222–1228. doi:10.1183/09031936.00058406
16. Finkelstein Y, Wolf L, Nachmani A, et al. Velopharyngeal anatomy in patients with obstructive sleep apnea versus normal subjects. J Oral Maxillofac Surg. 2014;72(7):1350–1372. doi:10.1016/j.joms.2013.12.006
17. Huang L, Gao X. The interaction of obesity and craniofacial deformity in obstructive sleep apnea. Dentomaxillofac Radiol. 2021;50(4):20200425. doi:10.1259/dmfr.20200425
18. Thapa A, Jayan B, Nehra K, Agarwal SS, Patrikar S, Bhattacharya D. Pharyngeal airway analysis in obese and non-obese patients with obstructive sleep apnea syndrome. Med J Armed Forces India. 2015;71(Suppl 2):S369–S375. doi:10.1016/j.mjafi.2014.07.001
19. Fukuda T, Takei Y, Nakayama H, Inoue Y, Tsuiki S. Continuous tongue suction as a potential therapy for obstructive sleep apnea: a feasibility study. J Dent Sleep Med. 2020;7:3. doi:10.15331/jdsm.7134
20. Suzuki M, Okamoto T, Akagi Y, et al. Efficacy of oral myofunctional therapy in middle-aged to elderly patients with obstructive sleep apnoea treated with continuous positive airway pressure. J Oral Rehabil. 2021;48(2):176–182. doi:10.1111/joor.13119
21. Rueda JR, Mugueta-Aguinaga I, Vilaró J, Rueda-Etxebarria M. Myofunctional therapy (oropharyngeal exercises) for obstructive sleep apnoea. Cochrane Database Syst Rev. 2020;11:CD013449. doi:10.1002/14651858.CD013449.pub2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.