Back to Journals » International Journal of General Medicine » Volume 16
Morphological Study of Hashimoto Thyroiditis in Fine Needle Aspiration Cytology Specimens
Authors Ranabhat SK , Rijal NR , Dubey M, Dubey AK, Dwivedi N , Mohan AK, Ravikant R , Lolla R
Received 5 April 2023
Accepted for publication 6 July 2023
Published 24 July 2023 Volume 2023:16 Pages 3127—3137
DOI https://doi.org/10.2147/IJGM.S413230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sabin Kumar Ranabhat,1 Nishan Raj Rijal,2 Muskan Dubey,3 Arun Kumar Dubey,4 Neelam Dwivedi,5 Arun Kumar Mohan,6 Ravikant Ravikant,7 Ramesh Lolla4
1Department of Pathology, Xavier University School of Medicine, Oranjestad, Aruba; 2Department of Pathology, Chitwan Medical College Teaching Hospital, Bharatpur, Nepal; 3Xavier University School of Medicine, Oranjestad, Aruba; 4Department of Pharmacology, Xavier University School of Medicine, Oranjestad, Aruba; 5Department of Medicine, OSCE and SP Program, Xavier University School of Medicine, Oranjestad, Aruba; 6Department of Physiology, Xavier University School of Medicine, Oranjestad, Aruba; 7Department of Microbiology, Xavier University School of Medicine, Oranjestad, Aruba
Correspondence: Sabin Kumar Ranabhat, Email [email protected]
Background: Hashimoto thyroiditis is an autoimmune disease which is diagnosed based on well-defined clinical and cytological criteria.
Purpose: The objective of this research is to study cytomorphological features in patients of Hashimoto thyroiditis and compare the findings with other studies. Literature on morphology of multinucleated giant cells was found to be lacking, and this study has focused on the number and morphology of these cells in this study.
Material and Methods: FNAC was done in patients who met the clinical diagnostic criteria of Hashimoto thyroiditis formulated by “Japan Thyroid Association” and smears were analyzed by light microscopy. Data analysis was done by XLSTAT in Microsoft Excel 2010. The Wilcoxon Signed Rank Test was done to analyze the data on multinucleated giant cells. The null hypothesis was that the median of the population of differences between the paired data of small and large giant cells is zero.
Results: A total of 26 patients were included in a period of one year. Contrary to observations in other studies, multinucleated giant cells were found in most participants. The Wilcoxon Signed Rank Test proved that small multinucleated giant cells were significantly more common than large multinucleated giant cells in Hashimoto thyroiditis; P value (two-tailed) being < 0.0001 at significance alpha of 0.05. This study has also revealed that a few patients with Hashimoto thyroiditis can have large and very large multinucleated giant cells in a small number. Data on other cytomorphological features were no different than in other studies.
Conclusion: The presence of multinucleated giant cells in 92.3% of patients in this study is far higher than in other studies which can have important diagnostic implications. Few large multinucleated giant cells can be present in a small number in a few patients as in Hashimoto thyroiditis.
Keywords: Hashimoto thyroiditis, cytomorphology, giant cells
Introduction
Hashimoto’s thyroiditis is the second most common cause of hypothyroidism worldwide, second to inadequate iodine intake. But in developed countries and in areas where dietary iodine intake is sufficient, it is the most common cause of hypothyroidism.1,2 It is 10 to 20 times more common in females.3 Hashimoto’s thyroiditis usually presents as diffuse, symmetric, and painless enlargement of thyroid, but enlargement may be nodular also. In early stages, patients are euthyroid, or few patients may have thyrotoxicosis (Hashitoxicosis) due to disruption of thyroid follicles leading to release of stored thyroid hormones. As the disease evolves, there is gradually progressive destruction of thyroid follicles, and patient presents with subclinical hypothyroidism and later with overt hypothyroidism.3–5 Anti-thyroid peroxidase (anti-TPO) antibodies, anti-thyroglobulin antibodies (Tg Ab) and TSH receptor blocking antibodies can be present in the disease. Among these, anti-TPO antibodies are the most common which are present in 90% of patients.7 However, in euthyroid patients, its sensitivity is only 50%. Because these antibodies are also present in Graves disease, positive test should be confirmed by fine needle aspiration cytology especially if the patient has hyperthyroidism. Tg Ab is markedly elevated in the early phase of Hashimoto thyroiditis, but in the late stages of the disease, it may disappear. On the other hand, TPO Ab is only mildly elevated in the early phase, but it persists for many years unlike Tg Ab. Patients with atrophic thyroiditis and mothers of athyreotic cretins have elevated levels of TSH-R blocking antibody. While their presence is helpful in diagnosis, these are not hundred percent specific for Hashimoto thyroiditis, because moderate titers are seen in Graves disease, multinodular goiter, and thyroid neoplasm; and low titers are found in the elderly.6,7 On the other hand, fine needle aspiration cytology (FNAC) has very good sensitivity and specificity for the diagnosis of Hashimoto thyroiditis in patients with all types of thyroid function and autoantibody status.7–9 In the cytomorphologic diagnosis of the disease, follicular epithelial cells, lymphoid cells, multinucleated giant cells, Hurthle cells, epithelioid cells, and amount of colloid are analyzed in detail and the diagnostic categorization is done according to “The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC)”.10 The aim of this study is to describe cytomorphologic characteristics in patients of Hashimoto thyroiditis and compare them with other studies. During literature review, it was found that the detailed study on multinucleated giant cells was lacking. Past studies have described only the presence or absence of the giant cells but not their size and number of nuclei in them. This information on size and number of nuclei in a giant cell is important from the diagnostic point of view because these cells are present in other thyroid diseases as well mainly subacute thyroiditis and papillary carcinoma. The current study aims to fill that lacuna in the cytomorphology of Hashimoto thyroiditis.
Materials and Methods
Study Design and Ethical Approval
A descriptive cross-sectional study was carried out in a cohort of 26 patients prospectively who visited a tertiary referral hospital in the central region of Nepal in different points in time in one year starting from August 1, 2020. Data collection instrument was formulated, and data were entered into it as the patients were included into the research as they fulfilled the inclusion criteria. Ethical approval to conduct this research was obtained from the Institutional Review Committee of Chitwan Medical College Teaching Hospital, Nepal (CMC-IRC/076/077-124) in compliance with the World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects (WMA 2008) and the Council for International Organizations of Medical Sciences’ International Guidelines for Ethical Review of Epidemiological Studies (CIOMS 1991). Informed written consent was taken from each patient.
Inclusion Criteria
A patient who had undergone fine needle aspiration cytology of thyroid and who was later diagnosed as having Hashimoto thyroiditis was included in the study. The thyroid function status of each patient was noted. The diagnosis of “Hashimoto thyroiditis” was based on the guidelines set out by “The Japan Thyroid Association”, with one modification11 (Table 1).
 |
Table 1 “Japan Thyroid Association” Diagnostic Criteria for the Diagnosis of Hashimoto Thyroiditis11 |
Patients with a single lobe enlargement of thyroid were also diagnosed to have Hashimoto thyroiditis if cytological features of the disease were present (Table 2).
 |
Table 2 Cytological Criteria for the Diagnosis of Hashimoto Thyroiditis |
The process of selection of patients is presented in the below-given flow diagram (Figure 1).
 |
Figure 1 The process of selection of patients. |
Fine Needle Aspiration Cytology Procedure
Fine Needle Aspiration Cytology was done after clinical and biochemical examination of thyroid without ultrasonographic guidance. Local anesthetic or sedatives were not used before or during the procedure. At least two punctures were performed on each patient with a regular 10 mL syringe having bevel needle of size 25-gauge. Specimen in the first puncture was obtained utilizing the non-aspiration technique described by Zajdela et al in which suction is not applied; material flows into the needle by capillary action.12 A needle without barrel in a pencil grip was inserted into thyroid and multiple, back-and-forth repetitive passes were made in different directions till diagnostic material flowed into the needle and appeared in the hub. After withdrawal, the needle was attached to the syringe with air inside, and the material was expelled into the frosted glass slides. In the second attempt, aspiration technique was used to obtain diagnostic material as described in the Manual for Cytology.13 A regular 10 mL syringe having bevel needle of size 25-gauge was used. After reaching the desired depth into the lesion, suction was applied to create a vacuum inside the plunger. Keeping the plunger static, the syringe was moved back-and-forth multiple times in different directions. When material was seen in the hub of the needle, suction was released, while still inside the lesion and syringe with the needle was withdrawn. Material was expelled into the frosted slides, and smears were prepared. Six smears were prepared in the case of each patient. In three patients in whom smears were unsatisfactory, FNAC was repeated. The smears were air-dried at room temperature. Dried smears were fixed in 100% methanol for 20 minutes. The alcohol-fixed smears were stained by the Giemsa method.
Examination and Interpretation of FNAC Smears
Examination and interpretation of FNAC smears was done by the first author for all the cases. All the aspirations were also done by the first author with the objective of getting high-quality samples. Each smear was studied based on the cytological criteria (Table 2) outlined in the textbook of fine needle aspiration cytology by Orell and Sterrett.14 Apart from other cellular features, multinucleated giant cells were also studied objectively in this research. A total number of multinucleated giant cells and the nuclei within them were counted in each smear in all the patients. The number of nuclei within the largest giant cell was noted in each patient. Mean number of giant cells and mean number of nuclei within the largest giant cells were calculated. Detailed observations of cytological features are given in the results section.
Size Definition of Multinucleated Giant Cells
Multinucleated giant cells were categorized into three strata based on the number of nuclei inside them which are as mentioned below:
- Small: giant cells which contain <40 nuclei.
- Large: giant cells which contain 40 to 99 nuclei.
- Very large: giant cells which contain 100 or more nuclei.
Published guidelines for such categorization of multinucleated giant cells were not found in the literature for reference. Therefore, this definition has been developed for this study as an arbitrary classification of multinucleated giant cells.
Statistical Analysis
The data were entered into Microsoft Excel Spreadsheet 2010. XLSTAT was used to analyze data. The Shapiro–Wilk test was used to test the normality of data distribution. The Wilcoxon Signed Rank Test was done to analyze the data on multinucleated giant cells because the data did not follow normal distribution. The null hypothesis was that the median of the population of differences between the paired data of small and large giant cells is zero. The alternate hypothesis was that small giant cells are significantly more common in Hashimoto thyroiditis.
Results
A total of 26 patients participated in the study. Data regarding age, age-groups and gender ratio are presented in Table 3. Mean age was 35.2 (± 15.5) years.
 |
Table 3 Age-Group, Thyroid Function Profile and Type of Thyroid Enlargement |
Maximum numbers of patients were in the age-group of 20–35 years, with the total percentage of 53.8. The cumulative percentage of age-group 20 to 50 years, the reproductive age-group was 73. Gender distribution was skewed to female patients (92.3%): only 2 patients were male. A thyroid function test was done in all the 26 patients, the results of which are summarized in Table 4. Cases with hypothyroidism were the highest with 19 (73.1%). If subclinical patients are added to this figure, the total percentage of hypothyroidism and subclinical hypothyroidism comes out to be 80.7%. Only one patient had hyperthyroidism. About 84.6% of patients had thyroid dysfunction, while 15.4% of patients were euthyroid. Most of the patients (76.9%) came with diffuse enlargement of both lobes of thyroid gland. The rest of the patients had enlargement of only the right thyroid lobe (23.1%). No patient with enlargement of only the left thyroid lobe were seen during the time of study. Grading of thyroiditis is summarized in Table 4 which was done based on the criteria suggested by Bhatia et al.15
 |
Table 4 Cytological Grading of Thyroiditis Was Done According to the Criteria Mentioned15 |
Most of the patients in this study had grade II thyroiditis. A cytomorphological assessment of FNAC specimens stained by Giemsa was carried out which is summarized in Table 5.
 |
Table 5 Cytomorphological Features and Grade of Hashimoto's Thyroiditis in This Study (N = 26) |
Follicular epithelial cells, lymphocytes and plasma cells were present in all the cases. Epithelioid histiocytes, multinucleated giant cells and Hurthle cells were also present in a very high number of patients (73%, 92.3% and 80.8%, respectively). The range of number of giant cells in patients was 0 to 32. Two patients did not have giant cells, three patients had only one giant cell in their smear, and one patient had 32 giant cells in all the smears. The number of nuclei was counted in the largest giant cell in each patient. About 42.3% patients had less than 40 nuclei in their largest giant cell, 42.3% of patients had 40 to 99 nuclei and one patient (3.8%) had very large giant cell with 125 nuclei (Figures 2 and 3).
 |
Figure 2 Large multinucleated giant cell with 60 nuclei (Giemsa, x400). |
 |
Figure 3 Very large multinucleated giant cell with 125 nuclei (Giemsa, x400). |
According to the size definition outlined in materials and methods, the percentage of patients having large and very large multinucleated giant cells comes out to be 50.0%. In total, 195 giant cells were counted in all cases. Total number of small giant cells in all the 26 patients was 172, and the total number of large and very large giant cells was 25. The mean number of nuclei per giant cell was 39 when the largest giant cell in each patient was taken into calculation. The mean number of nuclei per giant cell was 15 when giant cells of all sizes were considered in each patient. Small giant cell Figure 4.
 |
Figure 4 Small multinucleated giant cell with 5 nuclei (Giemsa, x400). |
Most of the patients (50%) did not have colloid in their specimens, and 42.3% had scant colloid. The cumulative percentage of absent and scant colloid comes out to be 92.3%. Follicular epithelial cells were present in all the patients; only in very few (7.7%) were plenty in number. Follicular cell infiltration by lymphocytes was seen in 69% of patients. Endocrine atypia was present in 51.4% of patients. Nuclei of some follicular epithelial cells and Hurthle cells were hyperchromatic (Figure 5).
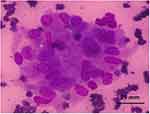 |
Figure 5 Hurthle cells (Giemsa, x400). |
There was Anisokaryosis. However, nucleoli were not obviously enlarged. All the cases had infiltration by lymphocytes and plasma cells. Follicular epithelial cells were impinged by lymphocytes (Figure 6).
 |
Figure 6 Plenty of lymphocytes with focal impingement of follicular epithelial cells (Giemsa, x400). |
Discussion
Hashimoto thyroiditis is an autoimmune disease. Most of the autoimmune diseases are more common in females.16 Female to male ratio of Hashimoto thyroiditis in this study was 11.3:1. There is a wide variation in the gender ratio within the same country and between different countries. In three studies from India, the researchers have found the ratio as 18.3:1, 9:1 and 6.1:1.17–19 A study from Sri Lanka found the ratio to be 10.3:1.20 But research from Italy concluded that the ratio was 3.2:1.21 In women, Hashimoto’s thyroiditis mainly occurs in the reproductive age-group because of high level of estrogen during that period.22 In our study, 73% of all the patients were seen in the reproductive age-group of 20 to 50 years. When the age-group was made smaller, the highest number of patients (53.8%) were found in the age-group of 20 to 35 years. Hashimoto thyroiditis was less common below 20 years and above 50 years. Other studies from South Asia corroborate our findings.17,23–25 However, Hashimoto thyroiditis is most prevalent in the age-group of 45 and 65 years in developed countries which is 10 to 20 years later in comparison to developing countries.3 Yaqiong et al found the mean age of patients as 57.7 years in their study in Kobe, Japan which is 22.5 years higher than in our research, a significant difference.26 Inflammation that occurs due to autoimmunity causes destruction of thyroid follicles. In late stages, patients present with hypothyroidism. Progression toward overt hypothyroidism is a gradual process taking several years. But in early stages, patients can be euthyroid or they may manifest subclinical hypothyroidism or even may be hyperthyroid. Therefore, hyperthyroid state, euthyroid state and subclinical state do not rule out Hashimoto’s thyroiditis. Hashitoxicosis is a transient hyperthyroid phase of Hashimoto’s thyroiditis. Its incidence in Hashimoto’s thyroiditis varies widely from 6% to 25%.24,27 In our study, only one patient had hyperthyroidism. In the same studies mentioned above, euthyroid state was found in 10% to 48.5% percent of patients and incidence of hypothyroidism ranged from 39.7% to 84%. According to the data, hypothyroidism occurs in the majority of patients of Hashimoto thyroiditis. In our study, 73% of patients had overt hypothyroidism at the time of presentation. The cumulative percentage of overt hypothyroidism and subclinical hypothyroidism was 80.8%. Thyroid enlargement in Hashimoto thyroiditis can be diffuse or nodular. All the studies are in total agreement that most cases present as diffuse enlargement although the proportion varies from 68% to 82%.17,18 We found diffuse enlargement of thyroid in 76.9% of cases. In the rest, only the right lobe was enlarged. Enlargement of only left lobe was not seen in any patient. Fine needle aspiration cytology has the central role in the diagnosis of etiology of enlarged thyroid. In Hashimoto thyroiditis, FNAC is considered as the gold standard diagnostic test because it is highly sensitive and specific for the disease. We analyzed cytomorphological features of our study participants in detail and we have reached interesting conclusions. While our findings are in agreement with other studies regarding lymphoid cells, Hurthle cell change and amount of colloid, we have found quite different picture regarding the presence or absence and size of multinucleated giant cells. Cytological literature states that multinucleated foreign-body type giant cells are infrequent in Hashimoto thyroiditis. They further claim if multinucleated giant cells are in large number and if they have many nuclei, granulomatous thyroiditis should be suspected. According to the literature, multinucleated giant cells are small and contain fewer nuclei in Hashimoto thyroiditis.28,29 These giant cells are present in up to 40% of cases.14 The studies carried out in India and Malaysia reported giant cells to be present in 6% to 40% of patients which is far lower than what we found in our study.18,19,24,25,27 Contrary to these observations, we found multinucleated giant cells in the vast majority (92.3%) of study participants with number of nuclei per giant cell ranging from two to 125. A total of 195 giant cells were counted in all the patients. The reason for this might be due to the fact that it is a prospective study and FNAC slides were examined after research design in a total of six smears from each patient. In one patient, two very large giant cells were present containing 110 and 125 nuclei. At least one large giant cell, defined by the presence of 40 to 99 nuclei, was present in 12 (46.2%) patients. However, their total number was only 21. Small giant cells, defined by the presence of less than 40 nuclei, were present in 23 (88.5%) of patients, and their total number was 172. One of these patients also had large and very large giant cells. Three patients also had large giant cells in addition. Because very large multinucleated giant cells are seen in subacute thyroiditis, we were careful to rule it out by clinical and cytological features. A two-tailed Wilcoxon Signed Rank Test was performed on the paired data of each patient to find out the exact statistical significance level of difference between the sum of small giant cells and sum total of large plus very large giant cells. The test revealed that small multinucleated giant cells are significantly more common than large and very large multinucleated giant cells in Hashimoto thyroiditis patients; P value (two-tailed) of <0.0001 at significance alpha of 0.05. Nevertheless, this study has revealed that a few patients with Hashimoto thyroiditis can have large and very large multinucleated giant cells in a small number. Because of the lack of research literature on the morphology of giant cells, the data of this study could not be compared with the data of other studies. We reiterate that researchers should spend some time assessing the size of the giant cells and number of nuclei in them while studying the cytomorphological aspect of Hashimoto thyroiditis. Data of our study on the other cytomorphological features viz., Hurthle cell change, amount of colloid, follicular epithelial cells and lymphoid cells were in agreement with the data of other studies.17–20,23–25
Limitations
The study was conducted in a small number of patients at a tertiary hospital. New findings of this study in relation to multinucleated giant cells should be validated by studies with larger sample sizes. Future researchers should include patients with all thyroid diseases in which giant cells are found. By doing so, the significance of multinucleated giant cells can be studied in all types of thyroid pathologies.
Conclusion
The presence of multinucleated giant cells in 92.3% of patients in this study is far higher than in other international studies in which the same data stand at up to 40% and this finding can be of diagnostic importance for the cytologists. The reason for the high percentage of giant cells might be due to the fact that it is a prospective study and FNAC slides were meticulously examined after research design. Our study also shows that a few large multinucleated giant cells can be present in a small number in a few patients as in subacute thyroiditis. Cytologists should bear this in mind during diagnostic work-up. On the other hand, this study reaffirms the fact that small multinucleated giant cells are significantly more common in patients of Hashimoto thyroiditis.
Abbreviations
FNAC, Fine Needle Aspiration Cytology.
Data Sharing Statement
All the data underlying the results are available which can be obtained through the corresponding author.
Ethics Approval and Consent to Participate
Ethical approval to conduct this research was obtained from the Institutional Review Committee of Chitwan Medical College Teaching Hospital, Nepal (CMC-IRC/076/077-124) on March 20, 2020, in compliance with the World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects (WMA 2008) and the Council for International Organizations of Medical Sciences’ International Guidelines for Ethical Review of Epidemiological Studies (CIOMS 1991). Informed written consent was taken from each patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. 2022;8(1):30. doi:10.1038/s41572-022-00357-7
2. Mincer DL, Jialal I. Hashimoto Thyroiditis. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459262/.
3. Kumar V, Abbas AK, Ester JC. Robbins and Cotran Pathologic Basis of Disease.
4. Parvathaneni A, Fischman D, Cheriyath P. Hashimoto’s thyroiditis. Semantic scholar. Springer; 1967. Available from: https://www.semanticscholar.org/paper/%5BHashimoto’s-thyroiditis%5D.-Parvathaneni-Fischman/12dd95c4e73322cbe9685e5518a552d18b9c6fb8.
5. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8(May). doi:10.3389/fimmu.2017.00521
6. Sanyal D. Spectrum of Hashimoto’s thyroiditis: clinical, biochemical & cytomorphologic profile. India J Med Res. 2014;140(December):710–712.
7. John JA, Basheer A, Govindarajan D, Phansalkar M, Iqbal N. Diagnostic accuracy of anti-thyroid antibodies in Hashimoto’s thyroiditis. J Evid Based Med Healthc. 2018;5(12):1045–1047. doi:10.18410/jebmh/2018/216
8. Staii A, Mirocha S, Todorova-Koteva K, Glinberg S, Jaume JC. Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state. Thyroid Res. 2010;3(1):11. PMID: 21172028; PMCID: PMC3016247. doi:10.1186/1756-6614-3-11
9. Esfandiari NH, McPhee SJ. Thyroid Disease. In: Hammer GD, Stephen J, editors. Access Medicine. New York, NY: McGraw-Hill Education; 2019. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=2468§ionid=198224090.
10. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346. doi:10.1089/thy.2017.0500
11. Hishinuma A. Guidelines| Japan Thyroid Association; 2022. Available from: https://www.japanthyroid.jp/en/guidelines.html#Chr.
12. Zajdela A, Zillhardt P, Voillemot N. Cytological diagnosis by fine needle sampling without aspiration. Cancer. 1987;59(6):1201–1205. doi:10.1002/1097-0142(19870315)59:6<1201::AID-CNCR2820590628>3.0.CO;2-P
13. Varghese C, Venkataraman K, Bhagwat S. Manual for Cytology. National Cancer Control Programme, Government of India; 2005.
14. Orell SR, Sterrett GF. Orell, Orell and Sterrett’s Fine Needle Aspiration Cytology E-Book. First. Elsevier Health Sciences; 2011.
15. Bhatia A, Rajwanshi A, Dash RJ, Mittal BR, Saxena AK. Lymphocytic thyroiditis--is cytological grading significant? A correlation of grades with clinical, biochemical, ultrasonographic and radionuclide parameters. Cytojournal. 2007;4:10. PMID: 17470291; PMCID: PMC1877811. doi:10.1186/1742-6413-4-10
16. Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10(11):2005–2011. PMID: 15550215; PMCID: PMC3328995. doi:10.3201/eid1011.040367
17. Chowdappa V, Shetty A. Cytomorphological spectrum of Hashimoto’s thyroiditis and its correlation with hormonal profile and hematological parameters. J Cytol. 2019;36(3):137. doi:10.4103/joc.joc_50_18
18. Thomas T, Sreedharan S, Khadilkar UN, et al. Clinical, biochemical & cytomorphologic study on Hashimoto’s thyroiditis. Indian J Med Res. 2014;140(6):729–735. PMID: 25758571; PMCID: PMC4365346.
19. Rathi M, Ahmad F, Budania SK, Awasthi S, Kumar A, Dutta S. Cytomorphological aspects of Hashimoto’s Thyroiditis: our experience at a tertiary center. Clin Med Insights Pathol. 2014;7:1–5. PMID: 24526840; PMCID: PMC3921154. doi:10.4137/CPath.S13580
20. Siriweera EH, Ratnatunga NVI. Profile of Hashimoto’s Thyroiditis in Sri Lankans: is there an increased risk of ancillary pathologies in hashimoto’s thyroiditis? J Thyroid Res. 2010;2010:1–5. doi:10.4061/2010/124264
21. Lanzo N, Patera B, Fazzino GFM, et al. The old and the new in subacute thyroiditis: an integrative review. Endocrines. 2022;3:391–410. doi:10.3390/endocrines3030031
22. University of Turku. New findings link estrogen and T cell immune response to autoimmune inflammation. ScienceDaily; 2018. Available from: https://www.sciencedaily.com/releases/2018/05/180531131116.htm.
23. Anila KR, Nayak N, Jayasree K. Cytomorphologic spectrum of lymphocytic thyroiditis and correlation between cytological grading and biochemical parameters. J Cytol. 2016;33(3):145. doi:10.4103/0970-9371.188055
24. Chandanwale SS, Nair R, Gambhir A, et al. Cytomorphological spectrum of thyroiditis: a review of 110 cases. J Thyroid Res. 2018;2018:1–7. doi:10.1155/2018/5246516
25. Kartha S, Shruthi B, Roni AD, Narain CD. Cytomorphological features of Hashimoto thyroiditis-with sonological and serological findings-analysis of 80 cases. J Evol Med Dent Sci. 2014;3(42):10551–10556. doi:10.14260/jemds/2014/3375
26. Yaqiong L, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of Hashimoto’s Thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010;95(3):1309–1317. doi:10.1210/jc.2009-1794
27. Jayaram G, Iyengar KR, Sthaneshwar P, Hayati JN. Hashimoto’s Thyroiditis - a Malaysian Perspective. J Cytol. 2007;24(3):119. doi:10.4103/0970-9371.41898
28. Kini SR. Thyroid Cytopathology: An Atlas and Text. Second. Lippincott Williams & Wilkins (LWW); 2015.
29. Orell SR, Philips J. The Thyroid: Fine-Needle Biopsy and Cytological Diagnosis of Thyroid Lesions. Vol. 14. Basel; Paris; London: Karger; 1997.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
