Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Monocyte-to-High-Density Lipoprotein-Cholesterol Ratio Predicts Prognosis of Hepatocellular Carcinoma in Patients with Metabolic-Associated Fatty Liver Disease
Authors Miao T, Lou X, Dong S, Zhang X, Guan W, Zhang Y, Li L , Yuan X, Ma D, Nan Y
Received 13 September 2023
Accepted for publication 13 January 2024
Published 18 January 2024 Volume 2024:11 Pages 145—157
DOI https://doi.org/10.2147/JHC.S439397
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Tongguo Miao,1 Xianzhe Lou,2 Shiming Dong,1 Xiaoxiao Zhang,1 Weiwei Guan,1 Ying Zhang,1 Lu Li,1 Xiwei Yuan,1 Dong Ma,2,* Yuemin Nan1,*
1Department of Traditional and Western Medical Hepatology, Hebei Medical University Third Hospital & Hebei International Joint Research Center for Liver Cancer Molecular Diagnosis, Hebei International Science and Technology Cooperation Base, Shijiazhuang, Hebei Province, 050051, People’s Republic of China; 2Department of Biochemistry and Molecular Biology, Key Laboratory of Neural and Vascular Biology, Ministry of Education, Hebei Medical University, Shijiazhuang, Hebei Province, 050017, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuemin Nan, Department of Traditional and Western Medical Hepatology, Hebei Medical University Third Hospital & Hebei International Joint Research Center for Liver Cancer Molecular Diagnosis, Hebei International Science and Technology Cooperation Base, No. 139, Ziqiang Road, Shijiazhuang, Hebei Province, 050051, People’s Republic of China, Tel +86 18533112266, Email [email protected] Dong Ma, Department of Biochemistry and Molecular Biology, Key Laboratory of Neural and Vascular Biology, Ministry of Education, Hebei Medical University, No. 361, Zhongshan East Road, Shijiazhuang, Hebei Province, 050017, People’s Republic of China, Tel +86 15081650349, Email [email protected]
Purpose: The incidence of non-B and non-C hepatocellular carcinoma (NBNC-HCC) is increasing globally. Metabolically associated fatty liver disease (MAFLD) has been a contributing factor to this rising trend in NBNC-HCC incidence. The monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) is a new prognostic marker that connects systemic inflammation with disorders of lipid metabolism. Therefore, MHR may be a potential prognostic predictor of patients with MAFLD-related HCC (MAFLD-HCC). This study aims to investigate the relationship between the MHR and prognosis of patients with MAFLD-HCC and construct a novel prognostic prediction tool for MAFLD-HCC.
Patients and Methods: This retrospective study of patients with MAFLD-HCC included training (n = 112) and internal validation (n = 37) cohorts. Univariate and multivariate Cox proportional hazard regression analysis was conducted to identify independent risk factors of survival. A visual nomogram was constructed to assess the performance of the two groups. Furthermore, receiver operating characteristic (ROC) curves and calibration curves were used to verify the prognostic discriminative ability of this nomogram, even in the MHR, ALBI grade, and MHR–ALBI model.
Results: Univariate and multivariate analyses revealed that extrahepatic metastases, Vascular invasion, Barcelona staging B, C, D, elevated ALBI Grade 3, C-reactive protein (CRP), and MHR were independent risk factors for the prognosis of MAFLD-HCC. Moreover, calibration plots showed good discrimination and consistency when the significant factors were entered into the nomogram. Meanwhile, the MHR strongly correlated with the prognosis of cancer under a background of MAFLD-HCC, with a sensitivity of 88.89% and a specificity of 79.61%. Importantly, the performance of the MHR alone (AUC = 86.2) was not only superior to the ALBI grade (AUC = 63.8) but was comparable to the combination of MHR and ALBI (AUC = 88.5).
Conclusion: The novel nomogram demonstrated good value in predicting the overall survival of patients with MAFLD-HCC. The MHR may be a potential predictor of prognosis.
Keywords: lipid metabolism, MAFLD-HCC, prognostic prediction, nomogram, MHR
Introduction
Hepatocellular carcinoma (HCC) has a high morbidity and mortality. Worldwide, 906,000 new cases of HCC are recorded annually.1 Although chronic infections with hepatitis B (HBV) and C viruses (HCV), chronic alcohol consumption, and diabetes or obesity-related non-alcoholic steatohepatitis (NASH) mainly contribute to HCC, the incidence of virus-related HCC has decreased in recent years with HBV vaccination programs, control of HCV transmission, and changes in lifestyle. Notably, there is an increase in the number of patients with non-virus HCC (non-B, non-C HCC [NBNC-HCC]) associated with metabolic syndrome.2 Metabolically associated fatty liver disease (MAFLD) has been a contributing factor to this rising trend in NBNC-HCC incidence. MAFLD, which is the proposed new term instead of NAFLD as it better reflects the metabolic nature, is closely related to metabolic syndrome.3,4 Moreover, multiple complications, such as diabetes, hypertension, and hypertriglyceridemia, result in the limitation of treatment options that lead to poor prognosis of patients with HCC in the context of MAFLD. Additionally, levels of related indices, including C-reactive protein (CRP) or serum high-density lipoprotein (HDL)-cholesterol (HDL-C) are altered,5–7 which may also play an important role in MAFLD-HCC. However, the relationship between MAFLD and CRP or HDL-C remains unclear. Thus, understanding the risk factors associated with MAFLD-HCC and accurately assessing its prognosis are crucial for prognostic counseling, follow-up and surveillance, and treatment decisions.
Currently, there is no consensus regarding the optimal tool for prognosticating MAFLD-HCC, which may partially contribute to failure of clinical therapy. Prognostic prediction models, including the China liver cancer staging, Japan integrated staging score and Barcelona Clinic Liver Cancer (BCLC) system, utilize various clinical and pathological factors to stratify patients into different risk groups based on their likelihood of disease progression, recurrence, or survival, further enabling prognostic assessments. Meanwhile, the ALBI grading system, which relies on serum bilirubin and albumin levels as crucial indicators for assessing liver function, is frequently employed to assess the recurrence and prognosis of patients with HCC.8 Nevertheless, each prognostic prediction model has specific limitations, and may not be applicable to all clinical scenarios encountered. Additionally, the monocyte-to-HDL-C ratio (MHR), which is the ratio of the monocyte count to HDL-C level, has been proposed as a potential marker of systemic inflammation and oxidative stress.9–11 Several studies have suggested that an elevated MHR is associated with a higher risk for MAFLD and cardiometabolic diseases;12,13 the MHR may be a potential predictor of prognosis of MAFLD-HCC. However, using the MHR in the assessment and management of MAFLD-HCC has not been validated.
Therefore, in this study, we aimed to determine the following: 1) characterize MAFLD-HCC and reveal the related factors; 2) investigate the relationship between the MHR and prognosis of patients with MAFLD-HCC; and 3) construct a novel prognostic prediction tool for MAFLD-HCC.
Patients and Methods
Subjects
All patients with HCC (n=2311) were recruited from Hebei Medical University Third Hospital from January 2011 to December 2020. HCC was diagnosed according to the practice guidelines recommended by the American Association for the Study of Liver Diseases.14 The patients who were seronegative for hepatitis B surface antigen, HBV-DNA, hepatitis C antibody, and HCV-RNA test were considered patients with NBNC-HCC (n=321).15 The inclusion criteria were as follows: 1) pathological/clinical diagnosis of NBNC-HCC, 2) age≥18 years, 3) more than 48h of hospitalization, 4) MAFLD was defined by either MRI imaging findings or liver biopsy where the presence of >5% liver steatosis.5 After 172 patients were excluded including non-MAFLD, a history of other cancers, or incomplete clinical data, a total of 149 patients with MAFLD-HCC were included in this study. Additionally, we developed an internal validation dataset came from these patients through 30% selection randomly (shown in Figure 1). Written informed consent was obtained from all the participants of this study. This study was approved by the institutional ethics committee of the Third Hospital of Hebei Medical University (Approve Number: K2019-014-2), and it conformed to the ethical guidelines of the Declaration of Helsinki.
 |
Figure 1 Patients selection flow diagram. Abbreviations: HCC, hepatocellular carcinoma; NBNC-HCC, non-B and non-C hepatocellular carcinoma; MAFLD, metabolically associated fatty liver disease. |
Data Collection
All patients underwent routine examinations, which included hepatitis B and C immunology, HBV-DNA load, alpha-fetoprotein (AFP) levels, liver and kidney function examinations, ultrasonography, dynamic contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Clinical information and laboratory data of patients was collected from electronic medical records system. Patients’ information was involved in demographic characteristic (age, sex, smoking status, alcohol consumption), comorbidity (liver cirrhosis, hypertension, diabetes), and family history of cancers.
Clinical Variables
For hematological investigations, we utilized the initial results from the hospitalization upon admission. The formulas for calculating the indices (MHR, NLR, and NγLR) have been previously described in studies.11,12,16–18 The imaging data, including macrovascular invasion, extrahepatic metastases, was obtained from contrast-enhanced computed tomography or contrast-enhanced magnetic resonance imaging. Tumor diameter was the diameter of the largest tumor. ALBI scores were calculated using the following formula: −0.085 (albumin g/l) + 0.66 log (bilirubin μmol/l). ALBI grades 1, 2, and 3 are defined as ALBI scores of ≤-2.60, >-2.60 to ≤-1.39, and > −1.39, respectively.8 Macrovascular invasion was defined as vascular invasion of large vessels detectable radiologically.
Survival Outcome
Patient follow-up data were carried out through outpatient visits, hospitalization records, and regular telephone follow-ups. The primary survival outcomes observed in this study was the overall survival (OS) of patients with HCC, defined as the time from the initial cancer diagnosis to either death or the last censored data point.
Statistical Methods
The 149 patients were randomly divided into either a training cohort for creating a new prognostic model or a validation cohort for evaluating the resulting prognostic index, roughly in a 3:1 ratio. Categorical variables were presented as percentages and continuous variables were expressed as the mean ± standard deviation [SD] or median with interquartile range [IQR], the latter for variables with highly skewed. Categorical data were compared by the Pearson χ2 test or the Fisher exact test, while continuous variables were assessed by Student’s t test. OS was calculated using the Kaplan-Meier method and compared with the Log rank test. Univariate analysis was performed to identify significant prognostic factors by using the Cox regression model. The multivariate Cox proportional hazards regression model was performed to identify independent predictors by including all the variables demonstrated to be significant in the univariate analyses. All statistical analyses were performed with SPSS18.0 (SPSS Inc., Chicago, IL). Statistical significance was defined as P<0.05.
Results
Clinical Characteristics
Overall, 149 patients with MAFLD-HCC were randomly divided into training (n = 112) and validation (n = 37) cohorts in a 3:1 ratio. There was no statistical difference in clinical characteristics between the two cohorts (P > 0.05). The average ages in the training and validation cohorts were 61.88 ± 10.3 and 62.59 ± 9.89 years, respectively. Most of the patients with MAFLD-HCC were men (60.7–62.2%) and had cirrhosis (69.6%–81.1%). In the training cohort, 20 patients (17.9%) were classified as BCLC-A, 27 (24.1%) were BCLC-B, 51 (45.5%) were BCLC-C, and 14 (12.5%) were BCLC-D. Meanwhile, 21 patients (18.8%) were classified as ALBI-1, 73 (65.2%) were ALBI-2, and 18 (16.1%) were ALBI-3. In the validation cohort, 5 patients (13.5%) were classified as BCLC-A, 12 (32.4%) were BCLC-B, 17 (45.9%) were BCLC-C, and 3 (8.1%) were BCLC-D. Additionally, 9 patients (24.3%) were classified as ALBI-1, 14 (37.8%) were ALBI-2, and 14 (37.8%) were ALBI-3. In the training and validation cohorts, there were 89 (79.5%) and 29 (78.4%) patients with diabetes, as well as 24 (21.4%) and 10 (27%) patients with hypertension, respectively. In both cohorts, cirrhosis was present in 69.6% and 81.1% of MAFLD-HCC patients. (Table 1).
 |
Table 1 Baseline Characteristics of Patients with MAFLD-HCC |
Development of Prognostic Nomogram for Prediction of Survival
Univariate Cox proportional hazard regression analyses showed that there was a significant relationship between the MHR, ALBI, and survival, and that the monocyte count, neutrophil count, ALT, AST, TP, ALB, TB, CRP, LDH, γ-GT, WBC, PLT, AFP, UREA, and CRE levels, BCLC stage, extrahepatic metastases, and macrovascular invasion were optimal indicators (Table 2). Moreover, multivariate analyses revealed that extrahepatic metastases (hazard ratio with 95% confidence interval [CI] = 2.014 [1.077–3.766], P = 0.028), macrovascular invasion (2.075 [1.068–4.03], P = 0.031), BCLC-B (2.369 [1.091–5.144], P = 0.029), BCLC-C (4.449 [1.957–10.114], P < 0.001), BCLC-D (26.473 [7.978–87.843], P < 0.001), ALBI grade 3 (11.687 [1.625–84.029], P = 0.015), CRP level (1.018 [1.006–1.03], P = 0.003), LDH level (0.998 [0.997–1.004], P = 0.003), and MHR (1.491 [1.24–1.791], P < 0.001) were independent risk factors (Table 3). A prognostic nomogram model of patients with MAFLD-HCC was also constructed by integrating statistically significant variables (Figure 2).
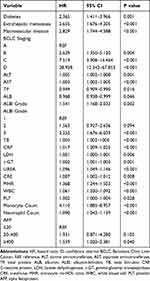 |
Table 2 Univariate Cox Regression Analysis of Risk Factors of Survival in Training Cohort |
 |
Table 3 Multivariate Cox Regression Analyses of Risk Factors of Survival in Training Cohort |
 |
Figure 2 Nomogram predicting OS in patients with MAFLD-HCC. Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CRP, C-reactive protein; LDH, lactate dehydrogenase; MHR, monocyte -to-HDL ratio. |
Performance of Nomogram in Prognostic Prediction
Calibration plots revealed 1-, 2-, and 3-year survival in patients with MAFLD-HCC (Figure 3). Additionally, receiver operating characteristic (ROC) curves also showed the survival of patients with MAFLD-HCC with high accuracy in the training (1-year, area under the curve [AUC] = 90.6 [95% CI: 0.854–0.959]; 2-year, AUC = 92.4 [95% CI: 0.91–0.997]; 3-year, AUC = 91.6 [95% CI: 0.899–0.988]), and validation (1-year, AUC = 92.4 [95% CI: 0.832–1.00]; 2-year, AUC = 92.4 [95% CI: 0.836–1.00], 3-year, AUC = 89.2 [95% CI: 0.786–0.999]) cohorts (Figure 4).
 |
Figure 3 The calibration curves for predicting the 1-, 2-, and 3-year nomogram in the training cohort(A) and validation cohort(B). |
 |
Figure 4 ROC curve analysis for 1‑, 2‑, and 3‑year in the training cohort(A) and validation cohort(B). |
Analyses of the Prognostic Value of MHR, ALBI Grade, and MHR–ALBI
Next, we used the MHR to predict the survival of patients with MAFLD-HCC. As shown in Figure 5, the AUCs of MHR at different time points (1-, 2-, and 3-year) were 0.734 (95% CI: 0.636–0.832), 0.749 (95% CI: 0.657–0.842), and 0.862 (95% CI: 0.773–0.955), respectively. Meanwhile, the sensitivity and specificity for 3-year survival was 88.89% and 79.61%, respectively. Similarity, according to the quartile of MHR (Q1–Q4), Kaplan–Meier survival curves at 12-, 24-, 36-month were the following: Q1, <0.32; Q2, ≥0.32, <0.56; Q3: ≥0.56, <1.35; Q4: ≥1.35. Kaplan–Meier analysis also showed a significant relationship between MHR and overall survival (OS). Additionally, the MHR was significantly associated with poor OS in patients with MAFLD-HCC (Log rank test; HR, 1.491, 95% CI: 1.24–1.791, P < 0.05) (Figure 6). Based on the ALBI grade, we plotted the ROC curves at 1-year (0.644 [95% CI: 0.540–0.749]), 2-year (0.647 [95% CI: 0.530–0.763], and 3-year (0.638 [95% CI: 0.464–0.811]) and the KM curve, which showed that patients with ALBI grade 3 had the lowest survival rate (Figures 7–8).
 |
Figure 5 ROC curve analysis of MHR for 1‑year (A), 2‑year (B), and 3‑year (C). Abbreviations: MHR, monocyte -to-HDL ratio; ROC, receiver operating characteristic; AUC, area under ROC curve. |
 |
Figure 6 Kaplan-Meier curve analysis of MHR for 12-month (A), 24-month (B), and 36-month (C). Abbreviation: MHR, monocyte -to-HDL ratio. |
 |
Figure 7 ROC curve analysis of ALBI Grade for 1‑year (A), 2‑year (B), and 3‑year (C). Abbreviations: ALBI, albumin-bilirubin; ROC, receiver operating characteristic; AUC, area under ROC curve. |
 |
Figure 8 Kaplan-Meier curve analysis of ALBI Grade for 12-month (A), 24-month (B), and 36-month (C). Abbreviation: ALBI, albumin-bilirubin. |
We also found that the predictive ability of the MHR in patients with MAFLD-HCC may be enhanced by integrating the ALBI grade. We compared the predictive values of the MHR, ALBI grade, and MHR–ALBI for survival in 3-year periods, revealing that the AUCs were 0.862, 0.638, and 0.885, respectively (Figure 9). In terms of predictive value, although the MHR–ALBI was not superior over the MHR, the MHR–ALBI model had a higher specificity (88.9%) than the MHR-only model (79.61%).
Kaplan–Meier curves for MHR and MHR–ALBI effectively stratified the survival outcomes of patients with MAFLD-HCC. According to the cutoff value of the 36-month ROC curve for MHR, patients were divided into high and low expression groups. Survival curves were subsequently plotted for 12-, 24-,36-month in conjunction with the ALBI grade. In the MHR–ALBI model, the median OS was longest in patients with an ALBI grade of 1 and low MHR (MHR < 0.325), whereas it was shortest in patients with an ALBI grade of 2 and 3 and high MHR (MHR > 0.325); the differences were statistically significant (p < 0.05) (Figure 9).
Discussion
It is necessary to accurately predict the prognosis of patients with NBNC-HCC as the disease has an insidious onset with a poor prognosis when compared with HCC associated with viral hepatitis.18 Among patients in this study, 82.1% had intermediate to advanced HCC, but only 17.9% received curative treatment. Metabolic syndrome is regarded as a risk factor for HCC, particularly for NBNC-HCC. NBNC-HCC caused by metabolic syndrome and MAFLD, which is characterized by abnormal lipid accumulation, may be associated with each other.19–21 The incidence rate of MAFLD in China has significantly increased. In this study, MAFLD-HCC comprised 46.4% of the NBNC-HCC cases. Several studies have shown that the prevalence of MAFLD is greater in individuals with type 2 diabetes (T2D), ranging from 60% to 75%.22,23 MAFLD-HCC and T2D are becoming increasingly clinically significant, with 79.5% of MAFLD-HCC patients also having T2D in this study. Moreover, 69.6% of patients with MAFLD-HCC had liver cirrhosis, similar to that in other studies on HCC in the background of metabolic syndrome or MAFLD.24–26
Due to the complicated nature of this disease, the prognosis and treatment decisions for patients with MAFLD-HCC can be challenging. Presently, the most used staging system for HCC is the BCLC;27 however, it may not fully capture the unique characteristics and prognosis of patients with MAFLD-HCC. Developing staging systems specifically customized for MAFLD-HCC could significantly improve the precision of prognosis prediction and treatment decisions. Additionally, metabolic dysregulation stands as a defining feature of MAFLD-HCC. Factors such as obesity, diabetes, and dyslipidemia can impact the progression of the disease and response to treatment. Hence, to better predict the prognosis and guide therapeutic choices for MAFLD-HCC, it is essential to construct a suitable model combining representative factors of metabolic syndrome and inflammation. Nomogram, a valuable and convenient tool for individualized cancer prognoses, represents an application direction from transforming complex regression equations into visual graphics.28 By generating an intuitive graph for a statistical predictive model, nomograms are utilized not only for predicting the risk of tumor occurrence but are also widely employed in the assessment of various cancer prognoses, such as lung cancer,29 liver cancer,30–32 and other malignancies, serving as valuable references for clinicians in devising tailored preventive and treatment strategies for patients. Hence, we developed and validated a novel nomogram that can provide individual prediction of OS for patients with MAFLD-HCC. This practical prognostic model incorporates BCLC staging and lipid metabolism parameters as well as extrahepatic metastases, macrovascular invasion, and CRP and LDH levels.
Extrahepatic metastases and macrovascular invasion are known independent prognostic factors correlated with advanced tumor stage and poor clinical outcomes. The incidence of HCC accompanied by portal vein tumor thrombus ranged from 44% to 62.2%. Moreover, the median survival period in the absence of any interventions was 2.7 months.33 In this study, in both training and validation cohorts, patients with macrovascular invasion comprised 20.5% and 24.3%, respectively. CRP is not only an inflammatory marker but is also a prognostic indicator in MAFLD-HCC. Studies have shown that elevated CRP levels are associated with poorer outcomes and an increased risk for disease progression in patients with MAFLD-HCC,34–37 suggesting that monitoring CRP levels in patients with MAFLD-HCC may provide valuable insights into the presence and severity of inflammation as well as more severe liver injury and disease state and a high risk for complications or unfavorable prognosis.38 Monocytes play a significant role in the initial immune response and inflammation in HCC, recruiting cells to the tumor microenvironment and differentiating into tumor-associated macrophages, which contribute to increased tumor progression, metastasis, and reduced patient survival.39–41 HDL-C removes cholesterol from peripheral tissues, including the liver, through reverse cholesterol transport, thereby attenuating inflammation and oxidative stress properties, positively impacting the prognosis of patients with NAFLD-HCC.42,43 The role of HDL should be further researched in patients with MAFLD-HCC.
The MHR has been proposed as being representative of both systemic inflammation and abnormal lipid metabolism.44–46 While the specific influence of MHR on the prognosis of MAFLD-HCC remains unknown, it has been studied as a potential prognostic marker in various diseases, especially cardiovascular diseases.47,48 Moreover, an elevated MHR represents high levels of systemic inflammation linked to a worse prognosis in various cancers.49 Furthermore, dysregulated lipid metabolism is a hallmark of MAFLD and has the potential to contribute to tumor growth, angiogenesis, and metastasis.50–52 Therefore, the MHR may provide insights into the interaction between inflammation and lipid metabolism in MAFLD-HCC. Our study revealed that the constructed nomogram was a valuable prognostic tool for patients with MAFLD-HCC that performed well, and the time-dependent ROC curves also demonstrated that the nomogram possessed superior discrimination, prognostic prediction ability, and clinical benefit. Moreover, we integrated the MHR and ALBI grade into a new model to enhance survival prediction for patients with MAFLD-HCC. Interestingly, combining the MHR and ALBI grade did not result in a better predictive power than the MHR alone. The MHR model needs to be validated in prospective cohorts of diverse populations to confirm its applicability.
In summary, we developed an effective nomogram with a novel lipid/inflammation index through the MHR to predict the prognosis of patients with MAFLD-HCC. Adopting the MHR would be beneficial for facilitating the timely management of patients with MAFLD-HCC in clinical practice by using simple demographic and clinical variables.
Conclusion
The novel nomogram demonstrated good value in predicting the overall survival of patients with MAFLD-HCC. The MHR may be a potential predictor of prognosis.
Funding
This work was supported by Key Research and Development Program of Hebei Province (Grant No. 19277779D).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249.
2. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Estes et al Hepatol. 2018;67:123–133.
3. Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Ceramides. N Engl J Med. 2019;381:1866–1869.
4. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: a Meta-analysis. Diabetes Care. 2018;41:372–382.
5. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209.
6. Koh YX, Tan HJ, Liew YX, et al. Liver resection for nonalcoholic fatty liver disease-associated hepatocellular carcinoma. J Am Coll Surg. 2019;229:467–478.e1.
7. Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100:113–121.
8. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of Liver Function in Patients with Hepatocellular Carcinoma: a New Evidence-Based Approach—The ALBI Grade. J Clin Oncol. 2015;33:550–558.
9. Yılmaz M, Kayançiçek H. A New Inflammatory Marker: elevated Monocyte to HDL Cholesterol Ratio Associated with Smoking. J Clin Med. 2018;7:76.
10. Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46:1619–1625.
11. Wei X, Chen F, Huang J, et al. Novel Risk Biomarker for Infective Endocarditis Patients with Normal Left Ventricular Ejection Fraction ― Monocyte to High-Density Lipoprotein Cholesterol Ratio. Circ J. 2018;82:283–288.
12. Stefan N, Häring H-U, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–324.
13. Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus — mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612.
14. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380.
15. Nishikawa H, Osaki Y. Non-B, non-C hepatocellular carcinoma. Int J Oncol. 2013;43:1333–1342.
16. Li J, Liao Y, Suo L, et al. A novel prognostic index—neutrophil times γ-glutamyl transpeptidase to lymphocyte ratio (NγLR) predicts outcome for patients with hepatocellular carcinoma. Sci Rep. 2017;7:9229.
17. Zheng J, Seier K, Gonen M, et al. Utility of Serum Inflammatory Markers for Predicting Microvascular Invasion and Survival for Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2017;24:3706–3714.
18. Zhang W, Tan Y, Jiang L, et al. Prognostic nomogram for patients with non-B non-C hepatocellular carcinoma after curative liver resection. Int J Surg. 2017;44:160–165.
19. Le MH, Le DM, Baez TC, et al. Global incidence of non-alcoholic fatty liver disease: a systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol. 2023;S0168827823002192.
20. Zhou J, Zhou F, Wang W, et al. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851–1864.
21. Dewidar B, Kahl S, Pafili K, Roden M. Metabolic liver disease in diabetes – from mechanisms to clinical trials. Metabolism. 2020;111:154299.
22. Ciardullo S, Monti T, Perseghin G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults with Type 2 Diabetes. Diabetes Care. 2021;44(2):519–525.
23. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced Liver Fibrosis Is Common in Patients with Type 2 Diabetes Followed in the Outpatient Setting: the Need for Systematic Screening. Diabetes Care. 2021;44(2):399–406.
24. Myers S, Neyroud-Caspar I, Spahr L, et al. NAFLD and MAFLD as emerging causes of HCC: a populational study. JHEP Rep. 2021;3:100231.
25. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–37. e2.
26. Kodama K, Kawaguchi T, Hyogo H, et al. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J Gastroenterol Hepatol. 2019;34:1626–1632.
27. Lin YP, Wang PM, Chuang CH, et al. Metabolic Risks Are Increasing in Non-B Non-C Early-Stage Hepatocellular Carcinoma: a 10-Year Follow-Up Study. Front Oncol. 2022;12:816472.
28. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80.
29. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869.
30. Lei Z, Li J, Wu D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151(4):356–363.
31. Serenari M, Han KH, Ravaioli F, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73(4):855–862.
32. Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638.
33. Zhang ZM, Lai EC, Zhang C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8–16.
34. Park SH, Kim BI, Yun JW, et al. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol. 2004;19(6):694–698.
35. Oruc N, Ozutemiz O, Yuce G, et al. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: a case control study. BMC Gastroenterol. 2009;9:16.
36. Foroughi M, Maghsoudi Z, Khayyatzadeh S, Ghiasvand R, Askari G, Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;5:28.
37. Lee J, Yoon K, Ryu S, Chang Y, Kim H-R. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS One. 2017;12:e0172666.
38. Wang Y, Li Z, Huang Z, et al. C-Reactive Protein Is an Indicator of the Immunosuppressive Microenvironment Fostered by Myeloid Cells in Hepatocellular Carcinoma. Front Oncol. 2022;11:774823.
39. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322.
40. Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18(1):45–56.
41. Sung PS. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin Mol Hepatol. 2022;28(3):333–350.
42. Crudele L, De Matteis C, Piccinin E, et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. 2022;5(1):100627.
43. Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32(12):2813–2820.
44. Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011;408(1):1–5.
45. Ganjali S, AM G, Ruscica M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. 2018;233(12):9237–9246.
46. Canpolat U, Cetin EH, Cetin S, et al. Association of Monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost. 2016;22(5):476–482.
47. Acikgoz SK, Acikgoz E, Sensoy B, Topal S, Aydogdu S. Monocyte to High-Density Lipoprotein Cholesterol Ratio Is Predictive of in-Hospital and Five-Year Mortality in ST-Segment Elevation Myocardial Infarction. Cardiol J. 2016;23(505):–12.
48. Chen SA, Zhang MM, Zheng M, et al. The Preablation Monocyte/ High Density Lipoprotein Ratio Predicts the Late Recurrence of Paroxysmal Atrial Fibrillation After Radiofrequency Ablation. BMC Cardiovasc Disord. 2020;20:401.
49. Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, Mechanisms, and Consequences. Immunity. 2019;51(1):27–41.
50. Yu X, Mi S, Ye J, Lou G. Aberrant lipid metabolism in cancer cells and tumor microenvironment: the player rather than bystander in cancer progression and metastasis. J Cancer. 2021;12(24):7498–7506.
51. Long J, Zhang CJ, Zhu N, et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. 2018;8(5):778–791.
52. Paul B, Lewinska M, Andersen JB. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022;4(6):100479.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

