Back to Journals » Biologics: Targets and Therapy » Volume 15
Monoclonal Antibody Therapies for High Risk Neuroblastoma
Authors Furman WL
Received 28 January 2021
Accepted for publication 10 May 2021
Published 9 June 2021 Volume 2021:15 Pages 205—219
DOI https://doi.org/10.2147/BTT.S267278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shein-Chung Chow
Wayne L Furman
Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, USA
Correspondence: Wayne L Furman
Department of Oncology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN, 38105, USA
Tel +1-901-595-2403
Fax +1– 901-521-9005
Email [email protected]
Abstract: Monoclonal antibodies (mAbs) are part of the standard of care for the treatment of many adult solid tumors. Until recently none have been approved for use in children with solid tumors. Neuroblastoma (NB) is the most common extracranial solid tumor in children. Those with high-risk disease, despite treatment with very intensive multimodal therapy, still have poor overall survival. Results of treatment with an immunotherapy regimen using a chimeric (human/mouse) mAb against a cell surface disialoganglioside (GD2) have changed the standard of care for these children and resulted in the first approval of a mAb for use in children with solid tumors. This article will review the use of the various anti-GD2 mAbs in children with NB, methods that have been or are being evaluated for enhancing their efficacy, as well as review other promising antigenic targets for the therapeutic use of mAbs in children with NB.
Keywords: immunotherapy, neuroblastoma, anti-disialoganglioside, anti-GD2, chimeric, effector cells
Introduction
Monoclonal antibodies (mAbs) have developed into effective therapies for many adult malignancies. The global market for mAbs to treat cancer was estimated to be at more than 40 billion dollars in 2019 and likely to grow to more than 70 billion by 2024 (https://www.marketdataforecast.com/market-reports/global-cancer-monoclonal-antibodies-market). Monoclonal antibodies kill cancer cells in several different ways: inhibiting cancer cell signaling,1–3 stimulating immune effector cells to destroy tumor cells (antibody-dependent cell-mediated cytotoxicity; ADCC),4 by fixing complement (complement-dependent cytotoxicity; CDC), resulting in assembly of a membrane attack complex and cell lysis (Figure 1),4 and by stimulating adaptive immunity.5 Other antibodies can cause changes in the tumor vasculature, resulting in improved treatment response.6 They have also been used as targeting agents by being coupled to toxic payloads such as drugs,7,8 toxins9 or radioisotopes (Figure 1).10,11 More recently mAbs have also been used to target cells in the tumor microenvironment resulting in enhanced anti-tumor immune responses.4,12
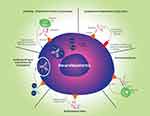 |
Figure 1 Antitumor mechanisms of GD2 antibodies and antibody conjugates. Solid triangle represents disialoganglioside (GD2) on cell surface. |
Neuroblastoma (NB) is the most common extracranial solid tumor in children and is thought to be derived from primitive neural crest cells.13 It can manifest anywhere along the sympathetic nervous system, with an adrenal mass being the most common primary site.14,15 “High-risk” NB is largely defined by patients older than 18 months of age at presentation with widely metastatic disease.16 Despite intensive multimodal treatment, more than half of these patients still die of their disease.17 In the 1980s it was discovered that neuroblasts almost uniformly express disialoganglioside (GD2) on their surfaces and this was used as a target to make several monoclonal antibodies, two of which have now been approved for use by the FDA18–25 (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-naxitamab-high-risk-neuroblastoma-bone-or-bone-marrow) and another approved by the European Medicines Agency (https://www.ema.europa.eu/en/medicines/human/EPAR/qarziba#:~:text=The%20European%20Commission%20granted%20a,Qarziba%20on%2027%20November%202017). The major anti-tumor mechanism of the anti-GD2 mAbs is likely ADCC mediated by NK cells26 and to a lesser extent neutrophils and macrophages.27,28 This article will review the use of the anti-GD2 mAbs in children with NB, methods that have been or are being evaluated for enhancing their efficacy, as well as review other promising antigenic targets for the therapeutic use of mAbs in children with NB.
Immunotherapeutic Targets of Neuroblastoma
Disialoganglioside (GD2)
Characteristics of antigens that make them attractive for mAb based therapy include consistent expression on the target cancer cells and limited expression on normal cells. One such antigen on neuroblasts is the disialoganglioside, GD2.19 While it is uniformly expressed on neuroblasts,19,20,29,30 in normal tissues it is expressed only on peripheral and central nerve fibers,31 mesenchymal stem cells,32,33 melanocytes34 and lymphocytes.31,35,36 It appears to have a role in attachment of tumor cells to the extracellular matrix,37 as well as effects on cell invasion and proliferation.29 Anti-GD2 mAbs that have been used clinically are summarized in Table 1. The first to be evaluated in the clinic were the murine antibodies 3F8 and 14G2a. Common acute toxicities to anti-GD2 mAbs include fever, hypotension, neuropathic pain and capillary leak syndrome (see Table 1). The fever and hypotension are likely related to allergic reactions to murine protein and pain due to mAb binding to GD2 positive peripheral nerves and subsequent complement activation.38,39 Capillary leak was more common during courses administered with interleukin-2 (IL-2) in the large randomized trial of dinutuximab25 and may be mostly related to systemically administered IL-2 or endogenous IL-2 produced in response to anti-GD2 mAb administration.40,41
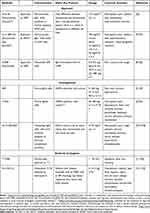 |
Table 1 Clinical Trials of Anti-GD2 Monoclonal Antibodies |
Murine Anti-GD2 Antibodies
3F8
This murine IgG3 mAb specific for GD2,30 kills tumor cells by ADCC42 and by activating complement.30 It has been studied extensively in patients as a single agent21,43 and in combination with other agents used to enhance ADCC, such as GM-CSF42,44,45 and β-glucan (NCT00492167).46 There were some responses in patients with small amounts of bone and/or marrow disease but no responses in those with bulky disease.44,47–49 Human anti-mouse antibodies (HAMA) developed in a majority of patients treated with 3F8.49–52
14G2a
This murine antibody is an isotype switch variant of the murine IgG3 14.18 anti-GD2 mAb. Because it had improved ADCC compared to 14.18, 14G2a was chosen for clinical evaluation.22 Two trials treated 27 patients at dosages from 50 to 400mg/m2.23,53 It was tolerated but with significant side effects (summarized in Table 1) and some modest anti-tumor activity. Human anti-mouse antibodies (HAMA) developed in 25/27 patients.23,53 In an attempt to enhance ADCC it was combined with interleukin-2 in another Phase I study. Thirty-three patients were enrolled, 31 with NB. Pain and allergic reactions were common. Nine of 21 evaluable children developed HAMA.54
Because murine antibodies result in allergic reactions and the development of HAMA, accelerating the clearance of the antibodies and reducing their antitumor effects,55 techniques to make antibodies “more human” have been developed.56 Figure 2 depicts the general structure of antibodies and their engineering modifications which have been utilized to make them more tolerable.
Chimeric Anti-GD2 Antibodies
Dinutuximab (Ch14.18)
To begin to address the immunogenic properties of murine anti-GD2 antibodies, the human Fc constant regions of an IgG1 immunoglobulin was fused with the Fab portion of the murine 14G2a antibody to produce this chimeric mouse/human antibody against GD2 (Figure 2). In vitro, ch14.18 mediated ADCC 50–100 fold more efficiently than 14.G2A,57 and was tested extensively in the clinic as a single agent and with cytokines GM-CSF and IL-2.58–67 In 2010, Yu et al of the Children’s Oncology Group (COG) reported on a randomized Phase 3 trial of ch14.18, in newly diagnosed children with high-risk NB who had achieved at least a partial response to induction chemotherapy. Following myeloablative chemotherapy, in a state of minimal residual disease, patients then received dinutuximab qd x 4 every 28 days given with either GM-CSF (course 1, 3 and 5) or interleukin-2 (IL-2; course 2 and 4) as well as monthly isotretinoin.25 This immunotherapeutic combination resulted in a dramatic improvement in 2-year event-free survival, compared to the group who received isotretinoin alone (66% vs 46% at 2-yrs, respectively; P = 0.01). These data led to NB becoming the first pediatric solid tumor with an approved immunotherapy, using this mAb, now called dinutuximab (Unituxin®), in combination with cytokines and isotretinoin (https://www.cancer.gov/news-events/cancer-currents-blog/2015/dinutuximab-neuroblastoma). These excellent results with dinutuximab provide promise that further improvements in immunotherapy will enhance outcome in children with NB. For example, the use of dinutuximab in combination with irinotecan and temozolomide has shown significant activity in patients with relapsed/refractory disease with objective responses in 22/53 patients (41.5%; 95% CI 28.2–54.8%).68,69 Ongoing studies extending the evaluation of dinutuximab in neuroblastoma include COG studies ANBL1821, a randomized Phase II study of irinotecan, temozolomide and dinutuximab with/without eflornithine (NCT03794349), ANBL07P1, a pilot study in newly diagnosed children adding dinutuximab to induction chemotherapy (NCT03786783) and ANBL19P1, a Pilot study of dinutuximab, GM-CSF and isotretinoin with irinotecan and temozolomide in the post-consolidation setting (NCT04385277). The NANT consortium is also evaluating dinutuximab in combination with Vorinostat and 131I-MIBG (NCT03332667).
Dinutuximab-Beta
A biosimilar to dinutuximab, ch14.18/CHO, now called dinutuximab beta (Qarziba®), has been approved by the European Medicines Agency.70 Ch14.18 was recloned in Chinese hamster ovary (CHO) cells26 and shown to have comparable pharmacokinetics and safety profile to dinutuximab.71 The International Society of Pediatric Oncology Europe Neuroblastoma group (SIOPEN) was evaluating dinutuximab beta in a randomized trial (HR-NBL1), comparing it to isotretinoin alone, until results of COG’s trial with dinutuximab25 became available, after which accrual was halted and the design was modified to investigate the role of dinutuximab beta with/without IL-2.72,73 This randomized study concluded that there were higher rates of fever, pain, allergic reactions and other toxicities when dinutuximab beta was combined with IL-2. Additionally there was no evidence that IL-2 improved outcomes.73 Based on these data, IL-2 is no longer used with dinutuximab in the ongoing COG trial for children with newly diagnosed high-risk NB (ANBL1531; NCT03126916).
To improve the tolerability of dinutuximab beta, a 10-day continuous infusion schedule was developed. Fifty-three patients received 10 mg/m2/day of dinutuximab beta by 24-hr continuous infusion daily x 10 with 6×106 IU/m2 IL-2 (d1–5; 8–12) with oral isotretinoin. They found low pain scores and reduced IV morphine usage with subsequent cycles, allowing mAb infusions as outpatients in more than 90% of cycles after the first course.74 This long-term infusion schedule of dinutuximab beta is being evaluated further in newly diagnosed children by the SIOPEN group, in combination with/without 3×106 IU/m2 IL-2 (d1–5; 8–12), which is 50% of the IL-2 dose used in previous randomization (NCT01704716).73 Dinutuximab beta is also being further evaluated by the Innovative Therapies for Children with Cancer in Europe consortium in a randomized trial in children with relapsed/refractory neuroblastoma in combination with chemotherapy (BEACON-Immuno) and in another consortium, in combination with nivolumab and 131I-MIBG (NCT02914405).
Humanized Anti-GD2 Antibodies
Hu14.18K322A
Humanization of murine mAbs makes them less immunogenic and more tolerable.75 This mAb contains fully human amino acid sequences for the IgG1 kappa light and heavy chains, combined with the complementarity-determining regions of antigen binding of the murine 14.18. The result is an approximately 98% human mAb. Additionally, a single point mutation was introduced to decrease complement activation (K322A)76 in an attempt to ameliorate the severe neuropathic pain39 seen with all anti-GD2 mAbs (see Table 1). As a single agent hu14.18K322A was given in doses of 2–70 mg/m2/d x 4, q 28 days. Toxicities were similar to other anti-GD2 mAbs, including significant pain, especially with the first course.77 However, in a retrospective review comparing pain outcomes of nine newly diagnosed children treated with dinutuximab (25 mg/m2/d x 4, given over 10 hours daily x 4) to nineteen patients with recurrent NB being treated on the Phase 1 trial of hu14.18K322A (dosages of 40, 50, 60 or 70 mg/m2/d x 4, given over 4-hrs daily x 4),77 those receiving hu14.18K322A had lower opioid requirements than the nine who were receiving dinutuximab.78 Furthermore, the differences in median opioid requirements for the overall course were significantly lower (1.57 vs 2.41 mg/kg; p = 0.019).78 This reduction in opioid support for those children receiving hu14.18K322A was despite receiving mAb doses more than 1.5 times the dose of dinutuximab, strongly suggesting that the K322A mutation was effective in reducing, but not eliminating the pain experienced by all anti-GD2 mAbs. Hu14.18K322A was combined with each of six courses of induction chemotherapy in a Phase II study involving 64 patients 19 years or younger with newly diagnosed high-risk NB. Each course of “chemoimmunotherapy” was followed by daily sq GM-CSF and 1 x 106IU/m2 of IL-2 every other day for six doses. Some patients received an additional course of hu14.18K322A along with infusion of parental natural killer (NK) cells during the consolidation phase of treatment. Following recovery from consolidation, minimal residual disease was treated with GM-CSF, IL-2 and isotretinoin, identical to Yu et al,25 with the substitution of hu14.18K322A for dinutuximab.79 In this single center Phase II trial, adding hu14.18K322A to induction chemotherapy produced early partial response (PR) or better in most patients, resulted in no progressions during induction, improved Curie Scores80 at the end of induction, and yielded an encouraging 2-year event-free survival (EFS) of 82.6% (95% CI, 70.1–90.3%).79 These data supported the development of a multi-institutional pilot study of dinutuximab given with induction chemotherapy by the COG (ANBL07P1; NCT03786783) which has just completed accrual.
Hu3F8
To circumvent the problem of HAMA development which accelerates antibody clearance, compromises efficacy and in some patients prevents retreatment, a humanized IgG1 form of the murine anti-GD2 mAb 3F8 (Hu3F8) was created.81 Now called naxitamab (DANYELZA®), in a Phase I trial, 57 patients were treated in the outpatient setting. Cohorts of 3 to 6 patients per dose level were enrolled. Naxitamab was given in doses from 0.9 to 9.6 mg/kg/cycle by 30-minute IV infusion on Monday, Wednesday, and Friday as well as GM-CSF, sq from day −5 through the last naxitamab infusion. As with other anti-GD2 mAbs, manageable neuropathic pain was seen in most patients.82 Humanized 3F8, (naxitamab), was granted Breakthrough Therapy designation in August of 2018 for use with GM-CSF in patients refractory to initial therapy or with incomplete response to salvage therapy in patients older than 12 months with persistent, refractory disease limited to bone marrow with or without evidence of concurrent bone involvement (http://www.onclive.com/view/fda-approval-sought-for-naxitamab-in-neuroblastoma). This was followed on November 25, 2020 with accelerated approval, again in combination with GM-CSF for patients older than 1 year with refractory or relapsed high-risk neuroblastoma limited to bone or bone marrow who had achieved at least stable disease to prior therapy. This approval was based on the results of two single-arm trials NCT03363373 and NCT01757626 in which patients received 3 mg/kg naxitamab IV on days 1,3 and 5 every 4-weeks in combination with SQ GM-CSF from day −4 to + 5 (250 µg/m2/d, d – 4 to 0 and then 500 µg/m2/d, d+1 to +5). Of 22 patients treated on NCT03363373 the objective response rate (ORR) was 45% (95% CI: 24–68%). Responses lasted 6 months or longer in 23%. Of 38 patients treated on NCT01757626, 34% had a response (95% CI: 20–50%) and 23% had responses lasting 6 months or more (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-naxitamab-high-risk-neuroblastoma-bone-or-bone-marrow). There is an international Phase 3 randomized trial (NCT04560166) in children with primary refractory disease or in first relapse with irinotecan/temozolomide ± naxitamab that is soon to open.
Pharmacokinetics and Pharmacodynamics of Anti-GD2 Monoclonal Antibodies
As previously noted, these mAbs exert their effects by both ADCC and CDC to varying degrees, depending on the specific antibody. The determinants of mAb pharmacokinetics and pharmacodynamics are dependent on multiple factors including: distribution and density of the target antigen, binding affinity to the antigen, glycosylation pattern of the antibody, immunogenicity of the mAb, rate of antibody penetration into a tumor, and effector cell number and function, among others.83–87 Although the most effective dose is unknown, dinutuximab beta has been shown to be active at concentrations > 1 µg/mL.26,88 Also, as previously noted, the major problem with administration of these antibodies is the induction of severe neuropathic pain, which has limited the dosage that can be given.
There are several different doses and schedules of the various anti-GD2 mAbs currently in use. Dinutuximab is given at a dose of 17.5 mg/m2/day as a 10 to 20 hour infusion, daily x 4.25 In an attempt to ameliorate pain, dinutuximab beta is proceeding with a 10 mg/m2/day dose by continuous infusion x 10 days.89 The humanized antibody naxitamab is given at 3 mg/kg day by 30-minute infusion on M-W-Fri, while hu14.18K322A is given at 40 mg/m2/day by 4-hour infusion daily x 4. Humanization has improved the tolerance, but not eliminated pain. The fact that pain resolves shortly after the mAb infusion is stopped, while the mAb still persists in the circulation suggests that pain may be related to dose-infusion rate rather that AUC.90 A detailed review of the pharmacokinetic/pharmacodynamic parameters of these antibodies is beyond the scope of this review. Optimizing the regimen and dose to maximize tolerability and response still needs some work.
Anti-GD2 Antibody Conjugates
131I-3F8
131I-3F8 has demonstrated specific and sensitive imaging of metastatic NB,91 as have three other anti-GD2 mAbs 131I-14G2a,23 99mTc-ch14.1892 and Cu-p-NH2-Bn-DOTA-hu14.18K322A.93 However 131I-3F8 is the only one that has been used for radioimmunotherapy of patients.94,95 The addition of 131I-3F8 to a multimodality treatment for newly diagnosed children with high-risk NB did not improve their progression free or overall survival, compared to those who did not receive 131I-3F8.50,95
Hu14.18-IL-2
Interleukin-2 (IL-2) improves the ability of effector cells to kill neuroblasts96,97 but has significant systemic side effects.98 The immunocytokine hu14.18-IL-2 was created to augment the antitumor effect of hu14.18 with IL-299 and at the same time limit the toxicity of systemic administration of IL-2.100 In a Phase I trial hu14.18-IL-2 was given to 27 children with recurrent/refractory NB and one with melanoma, IV over 4 hours qd x 3 at various dose levels. Three patients had evidence of tumor responses.101 In a subsequent Phase II study, patients were stratified into those with measurable disease (stratum 1; n = 13) and those with evaluable disease by bone marrow histology and/or 131I-metaiodobenzylguanidine (MIBG) scintigraphy (stratum 2; n = 23). Those with bulky disease (stratum 1) had no responses while 5/23 patients on stratum 2 had marrow CRs.102 On the whole, adverse events were similar to those reported for other anti-GD2 mAbs when given with IL-2. Three patients discontinued treatment because of toxicity, two with acute vascular leak ± hypotension and one with a grade 4 allergic reaction.102 Thus this hu14.18-IL-2 conjugate did not seem to significantly improve the adverse event profile of hu14.18.
Other Antibody Targets in Neuroblastoma
O-Acetyl-GD2
The O-acetyl derivative of the ganglioside GD2 is expressed on neuroblasts but not on peripheral nerves.103 A chimeric O-acetyl anti-GD2 mAb, c.8B6, has been shown to kill neuroblastoma cells without inducing allodynia in an animal model and offers promise that it may be better tolerated than dinutuximab.104,105 This antibody has not yet been clinically evaluated.
B7-H3 (CD276)
This is a membrane protein involved in the regulation of T and NK cells and is overexpressed on many solid tumors, including neuroblastoma.106–108 Expression on a neuroblast cell line protected neuroblasts from NK-cell killing106 and overexpression on tumors often correlates with faster tumor progression and poor outcome.109 Omburtamab (8H9), a murine antibody that recognized B7-H3,110,111 has been linked to 131I (Burtomab) and used to treat children with Central Nervous System NB. In a phase 1 study, 80 patients were treated with burtomab in combination with intraventricular compartmental chemotherapy with irinotecan, temozolomide and carboplatin. Improvement on imaging was seen in 36% of children with measurable disease with a median duration of response of 49 weeks (range, 2.6 to 586 weeks).10,112,113 Based on these data burtomab was granted Breakthrough Therapy designation for metastatic NB (https://www.cancertherapyadvisor.com/home/cancer-topics/pediatric-cancer/burtomab-granted-breakthrough-therapy-designation-for-metastatic-neuroblastoma/). A humanized anti-B7-H3 mAb, enoblituzumab, has been evaluated in a Phase I trial in children with various solid tumors, including NB [NCT02982941]. Results are not yet available.114
ALK (Anaplastic Lymphoma Kinase)
Mutations of ALK are observed in about 8% of all neuroblastomas115 and small molecule tyrosine kinase inhibitors of ALK are being used clinically in these patients [NCT03126916]. The native ALK protein is expressed on the majority of NB cells and not on normal cells,116 making treatment with an antibody targeting this protein a possibility. In human derived NB cell lines an ALK antibody inhibits growth in the absence of immune effector cells and is also able to mediate ADCC.116 Anti-ALK antibodies are not yet available for clinical testing.
PD-1/PD-L1 (Programmed Cell Death-1; Programmed Death-Ligand 1)
PD-1 and its ligands, PD-L1 and PD-L2, are molecules involved in the regulation of the immune system and part of multiple pathways called immune checkpoint pathways.12 Several cancers, including NB,117 make use of these pathways to inhibit tumor cell killing by immune effector cells. In a preclinical model of NB, when treated with dinutuximab beta, PD-L1 expression on neuroblasts was upregulated. When a murine anti-PD-1 mAb was combined with dinutuximab beta a synergistic antitumor response was seen.118 These data prompted the use of nivolumab, an antibody specific for PD-1, in combination with dinutuximab beta for the treatment of two heavily pretreated refractory patients, leading to a CR in one and VGPR in another.119 This report suggests that immune checkpoint inhibitors combined with anti-GD2 mAbs may be a promising approach to treat NB.
GPC2 (Glycosylphosphatidylinositol Anchored Signaling Co-Receptor Glypican 2)
Glypicans are a group of cell-surface glycoproteins linked to heparan sulfate glycosaminoglycan chains and regulate a number of growth and survival functions during embryogenesis.120 GPC2 is expressed at high levels on most NB, but not on normal tissues and is required for neuroblast cell proliferation.121 An antibody against GPC2 conjugated to pyrrolobenzodiazepine, a DNA crosslinking agent, induced cytotoxicity, in seven human NB cell lines.121 These data suggest that GPC2 may be a promising new target for an anti-GPC2 mAb antibody drug conjugate.122
CD47
Integrin-associated protein (IAP) or CD47 is a transmembrane glycoprotein that is expressed on the surface of many types of cancer cells,123 including NB.124 It is an immune checkpoint on macrophages that functions as a “don’t eat me” signal and has been co-opted by many types of cancer to prevent tumor cell killing.123,125,126 Paraffin embedded tumor tissues of 66 NB patients were tested for expression of CD47 and 28/66 (42.4%) were positive, significantly more of these patients were high-risk, compared to low risk (P = 0.049).124 Since macrophages frequently infiltrate metastatic NB tumors127 and since CD47 is frequently expressed on high-risk tumors, the combination of dinutuximab with an anti-CD47 antibody was evaluated in pediatric xenograft models of NB and the combination was found to be synergistic.128 This combination will soon be tested in children.128
Bispecific Monoclonal Antibodies (bsAbs)
Bispecific antibodies are constructed with two different antigen-binding sites129 and for NB have been used to redirect activated T-cells to GD2 expressing neuroblast cell lines.130 These data supported the development of a clinical trial using the humanized 3F8 anti-GD2 mAb combined with CD3 (Hu3F8-BsAb; Nivatrotamab) which is currently recruiting patients [NCT03860207]. Another bsAb, coupling an anti-GD2 antibody with a B7-H3 antibody has been developed with the goal of limiting off-target binding to GD2+/B7-H3 negative cells and reducing neuropathic pain.131 This bsAb, INV721, binds to tumors that express both GD2 and B7H3 but minimally to cells that do not express both antigens and is capable of ADCC.132
Discussion
Despite intensive multimodal therapy, many children with high-risk neuroblastoma still have poor outcomes. GD2, because of its consistent expression on neuroblasts, has been targeted by several different mAbs to improve the outcome of these patients (Table 1). As previously described, the use of dinutuximab with GM-CSF, IL-2 and isotretinoin, has dramatically improved the outcome of these patients leading to the first approved immunotherapy for children with solid tumors.25 Although these results are very promising, significant challenges remain to optimize these results for more children with this aggressive and deadly cancer. Significant toxic effects of dinutuximab included pain, hypersensitivity reactions, capillary leak and hypotension.25 Next generation antibodies were humanized (hu14.18K322A), or fully human (naxitamab) to improve tolerability and reduce the development of neutralizing antibodies. Hu14.18K322A was further modified to reduce complement activation,76 to ameliorate pain, an on-target, off-tumor effect of antibody binding to peripheral nerves and activating complement.39 Although this modification appears to improve the tolerability of hu14.18K322A, when compared to dinutuximab,78 this is not the whole answer. Is it possible that targeting the O-acetyl derivative of GD2, which is expressed on neuroblasts but not on peripheral nerves will alleviate this problem? Antibodies to this target are not yet available for clinical use. The humanization of hu14.18K322A has allowed significant dose escalation (40 mg/m2/dose),79 when compared to dinutuximab (17.5 mg/m2/dose) and the fully human naxitamab is able to be administered in an outpatient setting.82 Another structural modification to enhance ADCC is incorporated in the production of hu14.18K322A, and that is it is produced in a cell line that results in decreased fucosylation. Absence of fucose can improve antibody affinity to effector cells by up to 50-fold.133,134
Another augmentation to treatment with anti-GD2 mAbs has been to add cytokines such as GM-CSF42 and interleukin-2,135 because of their ability to enhance ADCC. Recent data have cast doubt on the added value of IL-2 because of significant added toxicities73 and its possible role in suppression of anti-tumor immune response136 through induction of regulatory T cells.137 It is possible that other cytokines such as Interleukin-15138 or IL-21139 will further enhance the effectiveness of anti-GD2 mAbs, without the significant toxicities of IL-2.
The initial evaluation of dinutuximab focused on treatment of patients after recovery from consolidation, in a state of minimal residual disease. This is because traditionally chemotherapy has been thought to be too immunosuppressive to combine with monoclonal antibodies. However recent studies suggest, even in the setting of “bulky” solid tumors, the combination of chemotherapy with monoclonal antibodies can be synergistic.140–143 In other preclinical studies chemotherapy can increase the efficacy of immunotherapy by depleting immunosuppressor cells such as regulatory T-cells which are known to suppress NK cell-mediated immunotherapy,144–146 the presumed major effector cells of anti-GD2 mAb induced ADCC in neuroblastoma.24,147 Also chemotherapy-induced tumor cell death can trigger tumor antigen release, uptake by antigen processing cells and an enhanced antitumor immune response.140,145,148 For these reasons we combined hu14.18K322A with induction chemotherapy in newly diagnosed children with high-risk NB in a single institution pilot Phase II study. All patients had clinical benefit with a near doubling of early responses, compared to a group of patients who received identical chemotherapy without hu14.18K322A, improvement in Curie Scores and no progressions during induction.79 These results suggest that anti-GD2 mAbs can have significant activity in bulky disease if utilized with the “right” combinations. More work needs to be done to determine how best to integrate these antibodies into standard treatment. The impressive improvement in event free survival with the use of dinutuximab has provided a “proof of principle”149 for further refining therapy using antibodies to other targets such as those described above.
Conclusion
The use of the chimeric anti-GD2 mAb following consolidation in newly diagnosed children with high-risk disease has revolutionized the treatment of these patients. The resulting improvements in EFS has led to enthusiasm that optimization of antibody design, addition of additional cytokines to further enhance ADCC and or mAbs to other targets will lead to more cures with less toxicity. Vaccines or cellular therapies such as chimeric antigen receptor (CAR) T cells150–152 or adoptive NK cells,153–156 although beyond the scope of this review, could further improve the treatment of these challenging patients.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Yoshida S, Kawaguchi H, Sato S, Ueda R, Furukawa K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Jpn J Cancer Res. 2002;93(7):816–824. doi:10.1111/j.1349-7006.2002.tb01324.x
2. Kowalczyk A, Gil M, Horwacik I, Odrowaz Z, Kozbor D, Rokita H. The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Lett. 2009;281(2):171–182. doi:10.1016/j.canlet.2009.02.040
3. Doronin II, Vishnyakova PA, Kholodenko IV, et al. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer. 2014;14:295. doi:10.1186/1471-2407-14-295
4. Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi:10.1038/nri2744
5. Shuptrine CW, Surana R, Weiner LM. Monoclonal antibodies for the treatment of cancer. Semin Cancer Biol. 2012;22(1):3–13. doi:10.1016/j.semcancer.2011.12.009
6. Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–3950. doi:10.1158/1078-0432.CCR-07-0278
7. Sano R, Krytska K, Larmour CE, et al. An antibody-drug conjugate directed to the ALK receptor demonstrates efficacy in preclinical models of neuroblastoma. Sci Transl Med. 2019;11:483. doi:10.1126/scitranslmed.aau9732
8. Brown BS, Patanam T, Mobli K, et al. Etoposide-loaded immunoliposomes as active targeting agents for GD2-positive malignancies. Cancer Biol Ther. 2014;15(7):851–861. doi:10.4161/cbt.28875
9. Tur MK, Sasse S, Stocker M, et al. An anti-GD2 single chain Fv selected by phage display and fused to Pseudomonas exotoxin A develops specific cytotoxic activity against neuroblastoma derived cell lines. Int J Mol Med. 2001;8(5):579–584. doi:10.3892/ijmm.8.5.579
10. Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97(3):409–418. doi:10.1007/s11060-009-0038-7
11. Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25(34):5465–5470. doi:10.1200/JCO.2007.11.1807
12. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
13. Hoehner JC, Gestblom C, Hedborg F, Sandstedt B, Olsen L, Pahlman S. A developmental model of neuroblastoma: differentiating stroma-poor tumors’ progress along an extra-adrenal chromaffin lineage. Lab Investig. 1996;75(5):659–675.
14. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–2120. doi:10.1016/S0140-6736(07)60983-0
15. Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi:10.1038/nrdp.2016.78
16. Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17(1):7–13. doi:10.1097/01.mop.0000150631.60571.89
17. Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–2211. doi:10.1056/NEJMra0804577
18. Schultz LM, Majzner R, Davis KL, Mackall C. New developments in immunotherapy for pediatric solid tumors. Curr Opin Pediatr. 2018;30(1):30–39. doi:10.1097/MOP.0000000000000564
19. Mujoo K, Cheresh DA, Yang HM, Reisfeld RA. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 1987;47(4):1098–1104.
20. Wu ZL, Schwartz E, Seeger R, Ladisch S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986;46(1):440–443.
21. Cheung NK, Lazarus H, Miraldi FD, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5(9):1430–1440. doi:10.1200/JCO.1987.5.9.1430
22. Mujoo K, Kipps TJ, Yang HM, et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49(11):2857–2861.
23. Murray JL, Cunningham JE, Brewer H, et al. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J Clin Oncol. 1994;12(1):184–193. doi:10.1200/JCO.1994.12.1.184
24. Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51(1):144–149.
25. Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi:10.1056/NEJMoa0911123
26. Zeng Y, Fest S, Kunert R, et al. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol. 2005;42(11):1311–1319. doi:10.1016/j.molimm.2004.12.018
27. Horta ZP, Goldberg JL, Sondel PM. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy. 2016;8(9):1097–1117. doi:10.2217/imt-2016-0021
28. Alvarez-Rueda N, Leprieur S, Clemenceau B, et al. Binding activities and antitumor properties of a new mouse/human chimeric antibody specific for GD2 ganglioside antigen. Clin Cancer Res. 2007;13(18):5613s–5620s. doi:10.1158/1078-0432.CCR-07-1057
29. Nazha B, Inal C, Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. 2020;10:1000. doi:10.3389/fonc.2020.01000
30. Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45(6):2642–2649.
31. Lammie G, Cheung N, Gerald W, Rosenblum M, Cordoncardo C. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas - an immunohistochemical study. Int J Oncol. 1993;3(5):909–915. doi:10.3892/ijo.3.5.909
32. Jin HJ, Nam HY, Bae YK, et al. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol Life Sci. 2010;67(11):1845–1858. doi:10.1007/s00018-010-0292-z
33. Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109(10):4245–4248. doi:10.1182/blood-2006-08-039347
34. Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets. 2015;1–14.
35. Cavdarli S, Groux-Degroote S, Delannoy P. Gangliosides: the double-edge sword of neuro-ectodermal derived tumors. Biomolecules. 2019;9(8):311. doi:10.3390/biom9080311
36. Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets. 2010;10(2):200–209. doi:10.2174/156800910791054167
37. Cheresh DA, Pierschbacher MD, Herzig MA, Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol. 1986;102(3):688–696. doi:10.1083/jcb.102.3.688
38. Yuki N, Yamada M, Tagawa Y, Takahashi H, Handa S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. J Neurol Sci. 1997;149(2):127–130. doi:10.1016/S0022-510X(97)05390-2
39. Sorkin LS, Otto M, Baldwin WM, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149(1):135–142. doi:10.1016/j.pain.2010.01.024
40. Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37(2–3):117–132. doi:10.1016/S0162-3109(97)00041-6
41. Matthay KK. Interleukin 2 plus anti-GD2 immunotherapy: helpful or harmful? Lancet Oncol. 2018;19(12):1549–1551. doi:10.1016/S1470-2045(18)30627-2
42. Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73(7):1936–1941. doi:10.1182/blood.V73.7.1936.1936
43. Cheung NK, Kushner BH, Cheung IY, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16(9):3053–3060. doi:10.1200/JCO.1998.16.9.3053
44. Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19(22):4189–4194. doi:10.1200/JCO.2001.19.22.4189
45. Cheung IY, Hsu K, Cheung NK. Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2012;30(4):426–432. doi:10.1200/JCO.2011.37.6236
46. Cheung NK, Modak S. Oral (1–>3),(1–>4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8(5):1217–1223.
47. Cheung NK, Kushner BH, Yeh SD, Larson SM. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: a phase II study. Int J Oncol. 1998;12(6):1299–1306. doi:10.3892/ijo.12.6.1299
48. Johnson E, Dean SM, Sondel PM. Antibody-based immunotherapy in high-risk neuroblastoma. Expert Rev Mol Med. 2007;9(34):1–21. doi:10.1017/S1462399407000518
49. Cheung NK, Cheung IY, Kramer K, et al. Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer. 2014;135(9):2199–2205.
50. Cheung NK, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30(26):3264–3270. doi:10.1200/JCO.2011.41.3807
51. Kushner BH, Ostrovnaya I, Cheung IY, et al. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with anti-GD2 immunotherapy and isotretinoin: a prospective Phase II study. Oncoimmunology. 2015;4(7):e1016704. doi:10.1080/2162402X.2015.1016704
52. Cheung IY, Kushner BH, Modak S, Basu EM, Roberts SS, Cheung NV. Phase I trial of anti-GD2 monoclonal antibody hu3F8 plus GM-CSF: impact of body weight, immunogenicity and anti-GD2 response on pharmacokinetics and survival. Oncoimmunology. 2017;6(11):e1358331. doi:10.1080/2162402X.2017.1358331
53. Handgretinger R, Baader P, Dopfer R, et al. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35(3):199–204. doi:10.1007/BF01756188
54. Frost JD, Hank JA, Reaman GH, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children’s Cancer Group. Cancer. 1997;80(2):317–333. doi:10.1002/(SICI)1097-0142(19970715)80:2<317::AID-CNCR21>3.0.CO;2-W
55. Cheung NK, Cheung IY, Canete A, et al. Antibody response to murine anti-GD2 monoclonal antibodies: correlation with patient survival. Cancer Res. 1994;54(8):2228–2233.
56. Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1.
57. Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144(4):1382–1386.
58. Handgretinger R, Anderson K, Lang P, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. EurJ Cancer. 1995;31(2):261–267. doi:10.1016/0959-8049(94)00413-Y
59. Yu AL, Uttenreuther-Fischer MM, Huang CS, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–2180. doi:10.1200/JCO.1998.16.6.2169
60. Murray JL, Kleinerman ES, Jia SF, et al. Phase Ia/Ib trial of anti-GD2 chimeric monoclonal antibody 14.18 (ch14.18) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in metastatic melanoma. J Immunother Emphasis Tumor Immunol. 1996;19(3):206–217. doi:10.1097/00002371-199605000-00005
61. Saleh MN, Khazaeli MB, Wheeler RH, et al. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum Antibodies Hybridomas. 1992;3(1):19–24. doi:10.3233/HAB-1992-3104
62. Albertini MR, Gan J, Jaeger P, et al. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J Immunother Emphasis Tumor Immunol. 1996;19(4):278–295.
63. Albertini MR, Hank JA, Schiller JH, et al. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3(8):1277–1288.
64. Yu AL, Batova A, Alvarado C, et al. Usefulness of a chimeric anti-GD2 (ch14.18) and GM-CSF for refractory neuroblastoma: a POG phase II study. PROC ASCO. 1997;16:1846.
65. Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J Clin Oncol. 2000;18(24):4077–4085. doi:10.1200/JCO.2000.18.24.4077
66. Simon T, Hero B, Faldum A, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22(17):3549–3557. doi:10.1200/JCO.2004.08.143
67. Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27(1):85–91. doi:10.1200/JCO.2006.10.3564
68. Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, Phase 2 trial. Lancet Oncol. 2017;18(7):946–957. doi:10.1016/S1470-2045(17)30355-8
69. Mody R, Yu AL, Naranjo A, et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the Children’s Oncology Group. J Clin Oncol. 2020;38(19):2160–2169. doi:10.1200/JCO.20.00203
70. EMA approval of dinutuximab beta; 2020. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/qarziba.
71. Ladenstein R, Weixler S, Baykan B, et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: a SIOPEN Phase 1 study. mAbs. 2013;5(5):801–809. doi:10.4161/mabs.25215
72. Ladenstein R, Potschger U, Valteau-Couanet D, et al. Investigation of the role of dinutuximab beta-based immunotherapy in the SIOPEN high-risk neuroblastoma 1 trial (HR-NBL1). Cancers. 2020;12(2):309. doi:10.3390/cancers12020309
73. Ladenstein R, Potschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1617–1629. doi:10.1016/S1470-2045(18)30578-3
74. Mueller I, Ehlert K, Endres S, et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD2 antibody ch14.18/CHO. mAbs. 2018;10(1):55–61. doi:10.1080/19420862.2017.1402997
75. Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. mAbs. 2010;2(3):256–265. doi:10.4161/mabs.2.3.11641
76. Thommesen JE, Michaelsen TE, Loset GA, Sandlie I, Brekke OH. Lysine 322 in the human IgG3 C(H)2 domain is crucial for antibody dependent complement activation. MolImmunol. 2000;37(16):995–1004.
77. Navid F, Sondel PM, Barfield R, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32(14):1445–1452. doi:10.1200/JCO.2013.50.4423
78. Anghelescu DL, Goldberg JL, Faughnan LG, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr Blood Cancer. 2015;62(2):224–228. doi:10.1002/pbc.25280
79. Furman WL, Federico SM, McCarville MB, et al. A phase II Trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res. 2019;25(21):6320–6328. doi:10.1158/1078-0432.CCR-19-1452
80. Ady N, Zucker JM, Asselain B, et al. A new 123I-MIBG whole body scan scoring method–application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31(2):256–261. doi:10.1016/0959-8049(94)00509-4
81. Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology. 2012;1(4):477–486. doi:10.4161/onci.19864
82. Kushner BH, Cheung IY, Modak S, Basu EM, Roberts SS, Cheung NK. Humanized 3F8 Anti-G D2 monoclonal antibody dosing with granulocyte-macrophage colony-stimulating factor in patients with resistant neuroblastoma. JAMA Oncol. 2018;4:1729. doi:10.1001/jamaoncol.2018.4005
83. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–558. doi:10.1038/clpt.2008.170
84. Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometr Syst Pharmacol. 2017;6(9):576–588. doi:10.1002/psp4.12224
85. Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52(2):83–124.
86. Deng R, Jin F, Prabhu S, Iyer S. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamic considerations for drug development? Expert Opin Drug Metab Toxicol. 2012;8(2):141–160. doi:10.1517/17425255.2012.643868
87. Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–2668. doi:10.1002/jps.20178
88. Siebert N, Eger C, Seidel D, et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. mAbs. 2016.
89. Ladenstein RL, Poetschger U, Valteau-Couanet D, et al. Randomization of dose-reduced subcutaneous interleukin-2 (scIL2) in maintenance immunotherapy (IT) with anti-GD2 antibody dinutuximab beta (DB) long-term infusion (LTI) in front line high-risk neuroblastoma patients: early results from the HR-NBL1/SIOPEN trial. J Clin Oncol. 2019;37(15).
90. Desai AV, Fox E, Smith LM, Lim AP, Maris JM, Balis FM. Pharmacokinetics of the chimeric anti-GD2 antibody, ch14.18, in children with high-risk neuroblastoma. Cancer Chemother Pharmacol. 2014;74(5):1047–1055. doi:10.1007/s00280-014-2575-9
91. Yeh SD, Larson SM, Burch L, et al. Radioimmunodetection of neuroblastoma with iodine-131-3F8: correlation with biopsy, iodine-131-metaiodobenzylguanidine and standard diagnostic modalities. J Nucl Med. 1991;32(5):769–776.
92. Reuland P, Geiger L, Thelen MH, et al. Follow-up in neuroblastoma: comparison of metaiodobenzylguanidine and a chimeric anti-GD2 antibody for detection of tumor relapse and therapy response. J Pediatr Hematol Oncol. 2001;23(7):437–442. doi:10.1097/00043426-200110000-00009
93. Vavere AL, Butch ER, Dearling JL, et al. 64Cu-p-NH2-Bn-DOTA-hu14.18K322A, a PET radiotracer targeting neuroblastoma and melanoma. J Nucl Med. 2012;53(11):1772–1778. doi:10.2967/jnumed.112.104208
94. Modak S, Cheung NK. Antibody-based targeted radiation to pediatric tumors. J Nucl Med. 2005;46(Suppl 1):157S–163S.
95. Cheung NK, Kushner BH, LaQuaglia M, et al. N7: a novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001;36(1):227–230. doi:10.1002/1096-911X(20010101)36:1<227::AID-MPO1055>3.0.CO;2-U
96. Sondel PM, Hank JA. Combination therapy with interleukin-2 and antitumor monoclonal antibodies. Cancer J Sci Am. 1997;3(Suppl 1):S121–127.
97. Nguyen R, Houston J, Chan WK, Finkelstein D, Dyer MA. The role of interleukin-2, all-trans retinoic acid, and natural killer cells: surveillance mechanisms in anti-GD2 antibody therapy in neuroblastoma. Cancer Immunol Immunother. 2018;67(4):615–626. doi:10.1007/s00262-017-2108-6
98. Ribeiro RC, Rill D, Roberson PK, et al. Continuous infusion of interleukin-2 in children with refractory malignancies. Cancer. 1993;72(2):623–628. doi:10.1002/1097-0142(19930715)72:2<623::AID-CNCR2820720248>3.0.CO;2-S
99. Yang RK, Kalogriopoulos NA, Rakhmilevich AL, et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J Immunol. 2012;189(5):2656–2664. doi:10.4049/jimmunol.1200934
100. Yamane BH, Hank JA, Albertini MR, Sondel PM. The development of antibody-IL-2 based immunotherapy with hu14.18-IL2 (EMD-273063) in melanoma and neuroblastoma. Expert Opin Investig Drugs. 2009;18(7):991–1000. doi:10.1517/13543780903048911
101. Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12(6):1750–1759. doi:10.1158/1078-0432.CCR-05-2000
102. Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–4975. doi:10.1200/JCO.2009.27.8861
103. Alvarez-Rueda N, Desselle A, Cochonneau D, et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PLoS One. 2011;6(9):e25220. doi:10.1371/journal.pone.0025220
104. Terme M, Dorvillius M, Cochonneau D, et al. Chimeric antibody c.8B6 to O-acetyl-GD2 mediates the same efficient anti-neuroblastoma effects as therapeutic ch14.18 antibody to GD2 without antibody induced allodynia. PLoS One. 2014;9(2):e87210. doi:10.1371/journal.pone.0087210
105. Fleurence J, Fougeray S, Bahri M, et al. Targeting O-Acetyl-GD2 ganglioside for cancer immunotherapy. J Immunol Res. 2017;2017:5604891. doi:10.1155/2017/5604891
106. Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101(34):12640–12645. doi:10.1073/pnas.0405025101
107. Gregorio A, Corrias MV, Castriconi R, et al. Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology. 2008;53(1):73–80. doi:10.1111/j.1365-2559.2008.03070.x
108. Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22(14):3425–3431. doi:10.1158/1078-0432.CCR-15-2428
109. Du H, Hirabayashi K, Ahn S, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35(2):221–237 e228. doi:10.1016/j.ccell.2019.01.002
110. Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61(10):4048–4054.
111. Ahmed M, Cheng M, Zhao Q, et al. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J Biol Chem. 2015;290(50):30018–30029. doi:10.1074/jbc.M115.679852
112. Kramer K, Kushner B, Modak S, et al. A curative approach to central nervous system metastases of neuroblastoma. Pediatr Blood Cancer. 2019;66:S56–S56.
113. Kramer K, Kushner BH, Modak S, et al. A curative approach to central nervous system metastases of neuroblastoma. J Clin Oncol. 2017;35:10545. doi:10.1200/JCO.2017.35.15_suppl.10545
114. Desantes K, Maris JM, McDowell K, et al. A phase 1, open-label, dose escalation study of enoblituzumab (MGA271) in pediatric patients with B7-H3-expressing relapsed or refractory solid tumors. J Clin Oncol. 2017;35:TPS2596–TPS2596. doi:10.1200/JCO.2017.35.15_suppl.TPS2596
115. Bresler SC, Weiser DA, Huwe PJ, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014;26(5):682–694. doi:10.1016/j.ccell.2014.09.019
116. Carpenter EL, Haglund EA, Mace EM, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012;31(46):4859–4867. doi:10.1038/onc.2011.647
117. Dondero A, Pastorino F, Della Chiesa M, et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology. 2016;5(1):e1064578. doi:10.1080/2162402X.2015.1064578
118. Siebert N, Zumpe M, Juttner M, Troschke-Meurer S, Lode HN. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD2 antibody ch14.18/CHO. Oncoimmunology. 2017;6(10):e1343775. doi:10.1080/2162402X.2017.1343775
119. Ehlert K, Hansjuergens I, Zinke A, et al. Nivolumab and dinutuximab beta in two patients with refractory neuroblastoma. J Immunother Cancer. 2020;8(1):e000540. doi:10.1136/jitc-2020-000540
120. Li N, Gao W, Zhang YF, Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018;4(11):741–754. doi:10.1016/j.trecan.2018.09.004
121. Bosse KR, Raman P, Zhu Z, et al. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell. 2017;32(3):295–309 e212. doi:10.1016/j.ccell.2017.08.003
122. Theocharopoulos C, Lialios PP, Gogas H, Ziogas DC. An overview of antibody-drug conjugates in oncological practice. Ther Adv Med Oncol. 2020;12:1758835920962997. doi:10.1177/1758835920962997
123. Zhang W, Huang Q, Xiao W, et al. Advances in anti-tumor treatments targeting the CD47/SIRPalpha axis. Front Immunol. 2020;11:18. doi:10.3389/fimmu.2020.00018
124. Aktas S, Ozdemir A, Serinan E, Altun Z, Olgun N. “Don’t Eat Me” signals of neuroblastoma by CD47 for immune escape: a novel prognostic biomarker. Proceedings. 2018;2(25):1538. doi:10.3390/proceedings2251538
125. Oronsky B, Carter C, Reid T, Brinkhaus F, Knox SJ. Just eat it: a review of CD47 and SIRP-alpha antagonism. Semin Oncol. 2020;47(2–3):117–124. doi:10.1053/j.seminoncol.2020.05.009
126. Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi:10.1016/j.cell.2009.05.046
127. Asgharzadeh S, Salo JA, Ji L, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30(28):3525–3532. doi:10.1200/JCO.2011.40.9169
128. Theruvath J, Smith B, Linde MH, et al. Abstract PR07: GD2 is a macrophage checkpoint molecule and combined GD2/CD47 blockade results in synergistic effects and tumor clearance in xenograft models of neuroblastoma and osteosarcoma. Cancer Res. 2020;80(14Supplement):PR07.
129. Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. doi:10.1038/s41573-019-0028-1
130. Yankelevich M, Kondadasula SV, Thakur A, Buck S, Cheung NK, Lum LG. Anti-CD3 x anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer. 2012;59(7):1198–1205. doi:10.1002/pbc.24237
131. Hernandez R, Erbe A, Gerhardt D, et al. GD2/B7-H3 bispecific antibodies for next-generation neuroblastoma treatment</strong>. J Nucl Med. 2020;61(supplement1):376.
132. Erbe A, Gerhardt D, Hernandez R, et al. Improving specific targeting of tumors through bispecific sniper antibodies. J Immunother Cancer. 2020;8:A280–A280. doi:10.1136/jitc-2020-SITC2020.0461
133. Mori K, Iida S, Yamane-Ohnuki N, et al. Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology. 2007;55(2–3):109–114. doi:10.1007/s10616-007-9103-2
134. Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi:10.1074/jbc.M202069200
135. Hank JA, Robinson RR, Surfus J, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50(17):5234–5239.
136. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118. doi:10.1038/cr.2016.151
137. Lode H, Siebert N, Troschke-Meurer S, et al. Administration of subcutaneous IL-2 is associated with strong induction of regulatory T cells in high-risk neuroblastoma patients treated with dinutuximab beta, a SIOPEN study. Pediatr Blood Cancer. 2019;66:S55–S56.
138. Nguyen R, Moustaki A, Norrie JL, et al. Interleukin-15 enhances anti-GD2 antibody-mediated cytotoxicity in an orthotopic PDX model of neuroblastoma. Clin Cancer Res. 2019;25(24):7554–7564. doi:10.1158/1078-0432.CCR-19-1045
139. Heinze A, Grebe B, Bremm M, et al. The synergistic use of IL-15 and IL-21 for the generation of NK cells from CD3/CD19-depleted grafts improves their ex vivo expansion and cytotoxic potential against neuroblastoma: perspective for optimized immunotherapy post haploidentical stem cell transplantation. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.02816
140. Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63(15):4490–4496.
141. Lake RA, Robinson BW. Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer. 2005;5(5):397–405. doi:10.1038/nrc1613
142. Buhtoiarov IN, Sondel PM, Wigginton JM, et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132(2):226–239. doi:10.1111/j.1365-2567.2010.03357.x
143. Holden SA, Lan Y, Pardo AM, Wesolowski JS, Gillies SD. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin Cancer Res. 2001;7(9):2862–2869.
144. Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–344. doi:10.1002/eji.200324181
145. Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57(11):1579–1587. doi:10.1007/s00262-008-0505-6
146. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi:10.1038/nri2216
147. Raffaghello L, Marimpietri D, Pagnan G, et al. Anti-GD2 monoclonal antibody immunotherapy: a promising strategy in the prevention of neuroblastoma relapse. Cancer Lett. 2003;197(1–2):205–209. doi:10.1016/S0304-3835(03)00100-9
148. Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170(10):4905–4913. doi:10.4049/jimmunol.170.10.4905
149. Park JA, Cheung NV. Targets and antibody formats for immunotherapy of neuroblastoma. J Clin Oncol. 2020;38(16):1836–1848. doi:10.1200/JCO.19.01410
150. Richards RM, Sotillo E, Majzner RG. CAR T cell therapy for neuroblastoma. Front Immunol. 2018;9:2380. doi:10.3389/fimmu.2018.02380
151. Heczey A, Louis CU. Advances in chimeric antigen receptor immunotherapy for neuroblastoma. Discov Med. 2013;16(90):287–294.
152. Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi:10.1182/blood-2011-05-354449
153. Oh S, Lee JH, Kwack K, Choi SW. Natural killer cell therapy: a new treatment paradigm for solid tumors. Cancers. 2019;11(10):1534. doi:10.3390/cancers11101534
154. Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283. doi:10.3389/fimmu.2018.00283
155. Modak S, Le Luduec JB, Cheung IY, et al. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: results of a phase I study. Oncoimmunology. 2018;7(8):e1461305. doi:10.1080/2162402X.2018.1461305
156. Barry WE, Jackson JR, Asuelime GE, et al. Activated natural killer cells in combination with anti-GD2 antibody dinutuximab improve survival of mice after surgical resection of primary neuroblastoma. Clin Cancer Res. 2018;25:325–333. doi:10.1158/1078-0432.CCR-18-1317
157. Uttenreuther-Fischer MM, Huang CS, Reisfeld RA, Yu AL. Pharmacokinetics of anti-ganglioside GD2 mAb 14G2a in a phase 1 trial in pediatric cancer patients. Cancer Immunol Immunother. 1995;41:29–36.
158. Kramer K, Cheung NK, Humm JL, et al. Targeted radioimmunotherapy for leptomeningeal cancer using (131)I-3F8. Med Pediatr Oncol. 2000;35:716–8.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

