Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Molecular Characterization of Eimeria Species in Broiler Chickens, Ethiopia
Authors Chere MA , Melese K, Megerssa YC
Received 25 April 2022
Accepted for publication 18 July 2022
Published 3 August 2022 Volume 2022:13 Pages 153—161
DOI https://doi.org/10.2147/VMRR.S357432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Meskerem Adamu Chere,1 Kasech Melese,2 Yoseph Cherinet Megerssa3
1Bio and Emerging Technology Institute, Addis Ababa, Ethiopia; 2Debre-Zeit Agricultural Research Center, Bishoftu, Ethiopia; 3College of Veterinary Medicine and Agriculture, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Meskerem Adamu Chere, Bio and Emerging Technology Institute, Addis Ababa, Ethiopia, Tel +251935963110, Email [email protected]
Background: Eimeria is a parasitic organism causing coccidiosis, an enteric disease of major economic importance in poultry. The conventional methods for species identification of Eimeria have major limitations.
Methods: Fresh fecal samples were randomly collected from 50 small and large-scale commercial broiler farms located in Adama, Bishoftu, Dukem, and Mojo towns. Polymerase chain reaction (PCR)-based assay was used for the differentiation of Eimeria species circulating among study sites and broiler farms. DNA was extracted from Eimeria oocytes using a DNeasy Tissue Kit. The extracted DNA templates and the genus-specific primers (Invitrogen) were used for the amplification of the ITS-1 region from seven Eimeria species of chicken. Descriptive statistical analysis and t-test were used to summarize and analyze the data.
Results: The PCR result confirms that all the seven species of Eimeria were detected in both small and large-scale broiler farms. Prevalence variation was found among broiler farms and between study sites. The frequency of E. brunetti (P< 0.006) and E. tenella (P< 0.04) in the small-scale broiler farms was significantly higher compared to that of in large-scale farms. A significantly higher frequency of E. acervulina (P< 0.03) and E. brunetti (P< 0.03) was detected in broiler farms of Dukem and Mojo compared to the broiler farms in Bishoftu. The study also revealed that multiple infections of Eimeria species per sample are common in most farms. Among the evaluated small-scale broiler farms of Bishoftu, 80% showed up to 5 mixed species. In addition, about 33% of large-scale and 20% of small-scale broiler farms showed 6– 7 mixed species.
Conclusion: This study characterized all the seven Eimeria species and revealed that multiple infections of Eimeria species per sample are common in most of the evaluated farms. The current findings might be useful for future anticoccidial vaccine development and for effective chemoprophylactic and control strategies.
Keywords: broiler chickens, Eimeria, polymerase chain reaction, Ethiopia
Introduction
Poultry coccidiosis is one of the most important diseases which causes considerable economic loss in the poultry industry. Species of coccidia (Eimeria) which commonly affect poultry are E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella.1,2 All seven valid species of chicken coccidia are ubiquitous.3 Each species attacks a different portion of the intestines or ceca and causes a separate disease exhibiting a characteristic degree of pathogenicity.2 Some are pathogenic eg (E. tenella, E. necatrix), associated with bloody diarrhea, weakness, and death. While other species cause subclinical coccidiosis eg (E. maxima, and E. acervulina). However, even relatively non-pathogenic species are important economically, because their infections can result in markedly reduced production.4,5 Individual birds are frequently infected simultaneously with more than one Eimeria species.6 Despite the introduction of live vaccines, in most countries, prophylactic chemotherapy is still the preferred method for the control of coccidiosis.7 Anticoccidial drugs supplemented to the feed are a good preventive measure and are well adapted to large-scale use; however, due to the prolonged use of these drugs, coccidial resistance has been reported with almost all commercially available drugs.8–10
Conventional diagnosis of coccidian infections can be confirmed by the identification of oocysts in feces or intestinal scrapings or histological sections of affected tissues. In addition, at necropsy, the combination of oocyst size, location in the gut, and appearance of the lesions provide a good guide to differentiate the species of Eimeria present. However, a reliable species diagnosis based on oocyst morphology is not possible, as multiple species of Eimeria can simultaneously infect the host and there can be an “overlap” in the sizes of oocysts and the sites of infection in the intestines of chickens for some species.2,5,6 In recent years various biochemical and molecular methods have been used for the specific diagnosis of coccidiosis as well as the genetic characterization of species of Eimeria. Recent progress demonstrates that some PCR-based methods utilizing genetic markers in nuclear ribosomal DNA provide rapid and powerful complementary diagnostic and/or analytical tools.11 Techniques based on the PCR amplification of DNA have been used for the diagnosis of some parasitic infections. It has also become a routine technology and its application has stimulated rapid progress in the development of reagents that are capable of identifying a single unicellular parasite.4 A species-specific PCR diagnostic assay was developed based on variable sequence regions and specific primers were constructed for the differentiation of Eimeria species.12 The specific diagnosis of infection plays a key role in the prevention, surveillance, and control of coccidiosis. The result of these findings might be useful for future anticoccidial vaccine development and as baseline data for the effective chemo-prophylactic and control strategies. The purpose of this study is to identify and differentiate pathogenic species of Eimeria using a PCR-based assay and to evaluate the prevalence of Eimeria species in the study broiler farms.
Materials and Methods
Samples Collection for Isolation of Eimeria Oocysts
Fresh fecal samples were collected from December to March 2012 from the chicken house of 50 small and large-scale commercial broiler farms. Most of the small-scale broiler farms had only one or two flocks. The flock size ranged from 100 to 2500 and 3000 to 6000 birds per house for small- and large-scale broiler farms, respectively. All broiler chickens in both farms were housed in an intensive deep-litter system Water is provided through an automatic waterer on large and through a plastic waterer on small-scale commercial broiler farms. Some small farms are constructed using local building materials mostly mud bricks, they obtain day-old chicks, feed, and some medicaments from the large farms. In addition, Ethiopian large commercial poultry farms are characterized by moderate to high biosecurity, while small commercial poultry farms the low to minimal biosecurity. All flocks were located in different areas within a radius of 10 to 80 km from Bishoftu town including Adama, Bishoftu, Dukem, and Mojo (Figure 1). The majority of the study farms are located in and near the cities, all chicken houses used deep litter, their flocks were supplemented with anticoccidial products in-feed mainly Coccidiostats, and they were not vaccinated against coccidiosis. Fresh fecal droppings were collected randomly from each flock house. Oocysts were isolated, counted, and purified following a standard salt flotation procedure.13 Thirty-five samples out of 50 were confirmed to have Eimeria oocysts. The ratio of Eimeria oocysts positive flocks from small-scale farms was 57% compared to those in large-scale farms at 43.3%. Samples containing Eimeria oocysts were preserved in a 2% potassium dichromate solution until used.
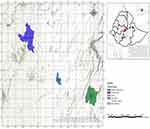 |
Figure 1 Map of study area indicating the cities where farms are located. |
Extraction of Genomic DNA from Eimeria Oocyst
The sample of oocysts was purified by centrifugation at 8000 rpm for 1 min and washed 4× by adding 200 µL of PBS at centrifugation (1400 rpm for 5 min during each wash). Then the oocyst pellet was re-suspended in 200 µL of 5.75% sodium hypochlorite, incubated on ice for 30 minutes, and then centrifuged at 8000 rpm for 1 minute. Each oocyst pellet was suspended in 200 µL distilled water and washed 3× at 1400 rpm for 5 minutes. In order to rupture oocysts, glass beads (2 mm in diameter) were added to the oocyst pellet and vortexed continuously for 10 minutes at maximum speed.
DNA was extracted from Eimeria oocytes using a DNeasy tissue kit (Qiagen) following the manufacturer’s guidelines. The suspension was then transferred to a fresh Eppendorf tube, 180 µL ATL buffer was added to each tube followed by vortex-mixing, and 20 µL of proteinase K was added and incubated at 56°C for 1 hour in a water bath with periodic, gentle mixing. After incubation, 200 µL AL buffer and, 200 µL absolute ethanol were added. The mixture was transferred to the DNeasy Mini spin column and centrifuged at 8000 rpm for 1 min the flow-through was discarded and the DNeasy Mini spin column was transferred to a new 2 mL collection tube. Subsequently, 500 µL of AW1 (wash buffer 1) buffer was added and centrifuged at 8000 rpm for 1 min, and then the DNeasy Mini spin column was placed in a new 2 mL collection tube, and 500 µL of buffer AW2 (wash buffer 2) was added and centrifuged at 13,400 rpm for 3 min. Finally, the DNeasy Mini spin column was placed in a clean 2 mL Eppendorf tube and 100 µL buffer AE was added to the DNeasy membrane and centrifuged at 8000 rpm for 1 min and incubated at room temperature for 1 min and the DNA extract product was preserved at −20°C.
Polymerase Chain Reaction (PCR) Assay
The extracted DNA templates were extracted from Eimeria collected from the different farms and the seven pairs of genus-specific primers were used for the amplification of the ITS-1 region from seven pathogenic Eimeria spp. of chicken. The PCR amplifications were conducted separately for each primer pair (Table 1). Amplification of the ITS-1 sequences of genomic DNA was carried out in 50 μL reaction volumes containing 5 μL of DNA template, 1 μL of each primer (reverse and forward), and 43 μL of Mastermix (DEPC H2O, 10× Dream Taq buffer, 25 mM MgCl2, 10 mM dNTP Mix, Taq DNA Polymerase). A Negative sample from the flotation method was used as a negative control in each PCR run. The PCR tube containing the mixture was labeled and loaded into the thermocycler and the thermal program of PCR was formulated at 95°C for 5 min for initial denaturation, followed by 35 cycles each of denaturation at 94°C for 30 secs, annealing at 68°C for 2 min, extension step at 72°C for 30 secs and the final extension was performed at 72°C for 7min. To verify the results, 10 μL of each PCR product and 2 μL of loading buffer were electrophoresed in a 3% agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator.
 |
Table 1 Primers Used for the Identification and Differentiation of Prevalent Eimeria Species of Chicken (Invitrogen) PETERSBURG, VA, USA |
Data Analysis
Collected data were coded, recorded, and analyzed in a Microsoft Excel spreadsheet. Descriptive statistical analysis (mean and percentage) was used to summarize and present the data, and t-test: Two-Sample Assuming Equal Variances was used to evaluate the prevalence association of each Eimeria species among broiler farms and study sites. P-value<0.05 was considered significant.
Results
The DNA samples from seven species of Eimeria oocysts were amplified using the primers shown in Table 1, and the PCR result shows that all seven species of Eimeria: E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella were detected in both small and large- scale broiler farms. The prevalence of E. tenella, was higher in the small-scale broiler farms, 76.2(%), followed by E. mitis 68.3(%), E. maxima 61.6(%), E. acervulina 58.7(%), E. brunetti 47.5(%), E. necatrix 28.7(%), and E. praecox 26.2(%). The prevalence of E. mitis, was higher in the large-scale broiler farms, 39.5(%), followed by E. tenella 39.0(%), E. acervulina 38.0(%), E. maxima 32.0(%), E. praecox 32.0(%), E. brunetti 31.0(%), and E. necatrix 23.5(%). A statistically significant difference was found in the prevalence of some Eimeria species among broiler farms. The frequency of E. brunetti (P<0.006) and E. tenella (P<0.04) in the small-scale broiler farms was significantly higher compared to the large-scale farms (Table 2).
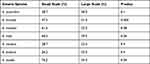 |
Table 2 Prevalence of Eimeria Species Among Different Broiler Farms |
Prevalence variation was also identified between study sites. A significantly higher frequency of E. acervulina (P<0.03) and E. brunetti (P<0.03) was detected in broiler farms of Dukem and Mojo compared to the broiler farms in Bishoftu. However, E. necatrix was absent in the broiler farms of Adama and Mojo, while E. praecox was absent in Dukem and Mojo (Table 3). The prevalence of E. tenella, E. acervulina, E. brunetti, E. maxima, E.mitis, and E. necatrix was 87.5, 75.0, 75.0, 62.5, 62.5, and 37.5 (%) in Dukem and Mojo (DK & MO), respectively, while the occurrence of E. tenella, E. mitis, E. praecox, E. acervulina, E. brunetti, E. maxima, and E. necatrix In Bishoftu farms Was 52.8, 52.8, 48.0, 45.3, 39.2, 32.1, and 29.2(%), respectively.
 |
Table 3 Prevalence of Eimeria Species Among Chickens of Different Study Sites |
Furthermore, the result of this study revealed that multiple infections of Eimeria species per sample are common in 90% of the evaluated farms (Figure 2). Accordingly, the data showed that about 25% of Mojo and 14% of Bishoftu large-scale broiler farms has 2–3 mixed species. The occurrence of 4–5 mixed-species was detected in 80% of small-scale broiler farms of Bishoftu, followed by 75% Mojo, 50% Adama, 50% Dukem, and 16% and 28% of large-scale farms of Bishoftu, 2 and Bishoftu, 3 respectively. In addition, 6–7 mixed species were also recorded in 33% of large-scale broiler farms of Bishoftu, followed by Dukem at 25% and 20% in the small-scale broiler farms of Bishoftu (Figure 3).
 |
Figure 3 Prevalence of Multiple infections of Eimeria species per farm across different study sites. Bishoftu 1: small scale broiler farm; Bishoftu (2 and 3): large scale broiler farms. |
Discussion
In Ethiopia, studies regarding chicken coccidiosis epidemiology have already been carried out based on the conventional diagnostic method on exotic chickens reared in deep litter from different places of Ethiopia.14–18 However, a reliable species-specific diagnosis based on oocyst morphology is difficult, as multiple species of Eimeria can simultaneously infect the host and there can be an overlap in the sizes of oocysts and the sites of infection in the intestines for some species.2,6 In the current study, all the seven Eimeria species were molecularly detected in both small and large-scale broiler farms. Our result was in agreement with the report of,19 who have detected seven species of Eimeria by real-time PCR from fecal samples of village chickens in Ethiopia,20 who have detected five species of Eimeria by real-time qPCR from fecal samples of commercial chicken farms in Nigeria, and21 who have detected seven species of Eimeria by multiplex PCR technique from fecal samples of broiler chicken farms in Brazil. However, there is no documented report related to the prevalence of Eimeria species in the intensively reared broiler or layer chickens, based on the molecular findings in the country. The molecular characterization of pathogenic Eimeria species using a PCR-based assay infection plays a key role in the prevention, surveillance, and control of coccidiosis.
Prevalence variation was detected among broiler farms and study sites. The frequency of E. brunetti and E. tenella in the small-scale broiler farms was significantly higher compared to those in the large-scale farms. Moreover, a significantly higher frequency of E. acervulina and E. brunetti was detected in the small-scale broiler farms of Dukem and Mojo compared to the small and large-scale broiler farms in Bishoftu, on the other hand, Eimeria necatrix was absent in the broiler farms of Adama and Mojo while E. praecox was absent in Dukem and Mojo. The discrepancy in Eimeria prevalence among broiler farms could be due to the differences in bio-security practices. The poor bio-security status is practiced in the majority of small-scale intensive poultry farms in Ethiopia.18,22,23
Multispecies infections of Eimeria per each farm were identified on 90% of the farms. For instance, up to 5 mixed species were detected in the 80, 75, 50, and 50 (%) of small-scale broiler farms of Bishoftu, Mojo, Adama, and Dukem, respectively, and these were higher compared to 28 (%) in the large-scale farms. Our results showed the wide distribution of multispecies Eimeria infections in the study broiler farms. This finding was in agreement with those of,19,20 who found up to seven and five multispecies infections of Eimeria respectively. The higher prevalence of multi-species infections in the present study might be associated with a subclinical form of coccidia infection. This is similar to the report of,6 who reported that this type of disease tends to be chronic and may be associated with several species of Eimeria infection, in this case, mortality may not be heavy but morbidity may retard the growth of broilers significantly. Even though all study flocks were supplemented with anticoccidial products in-feed, the poor management practiced under the small-scale broiler farms; and the coccidial resistance reported with almost all commercially available drugs,8–10 may be related to the higher prevalence of multi-species infections. Since no vaccination is used for the coccidiosis control and prevention of broiler production in Ethiopia, the current findings might be useful for future anticoccidial vaccine development and for effective chemoprophylactic and control strategies.
Conclusions
This study characterized all the seven Eimeria species based on molecular diagnostic techniques and determined the prevalence association of each Eimeria species among broiler farms and study sites for a better understanding of the epidemiology and dynamics of the disease. Prevalence variation of some Eimeria species was detected among broiler farms and study sites. The frequency of E. brunetti and E. tenella in the small-scale broiler farms was significantly higher compared to those in the large-scale farms. A significantly higher frequency of E. acervulina and E. brunetti was detected in the small-scale broiler farms of Dukem and Mojo. Multiple Eimeria species infections were identified on the majority of the farms. The current findings might be useful for future anticoccidial vaccine development and as baseline data for the effective chemo-prophylactic and control strategies.
Abbreviations
AW2, wash buffer 2; DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; ITS-1, internal transcribed spacers; qPCR, quantitative polymerase chain reaction; DECP, diethyl pyrocarbonate; MAC, Meskerem Adamu Chere; KM, Kasech Melesse; YCM, Yoseph Cherinet Megerssa.
Ethics Statement
The study was conducted with permission from the institutional animal research ethics review committee (Guideline VM/ERC/2013) at the College of Veterinary Medicine and Agriculture the Addis Ababa University. The purpose of the study was well explained to the farm owners before taking the samples, and the research informed consent was obtained to take the appropriate sample through verbal consent.
Acknowledgments
The authors would like to thank the Rural Capacity Building Project for the financial support, the National Veterinary Institute, and the technical staff for their support in allowing us to use their laboratory. We thank Professor Wondi Mersie, Associate Dean and Director of Agricultural Research, Virginia State University, for providing us with the primers. Small and large-scale commercial broiler farms in the study area are also acknowledged for allowing us for sample collection.
Author Contributions
MAC, KM, and YCM designed the study. MAC and YCM participated in sample collection and molecular biology laboratory (DNA extraction, PCR work). MAC and KM analyzed the results; MAC, KM, and YCM drafted and revised the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest to declare in this work.
References
1. Ballweber LR. Veterinary parasitology. In: The Practical Veterinarian. Butterworth–Heinemann; 2001:191–197.
2. Conway DP, McKenzie ME. Poultry Coccidiosis Diagnostic and Testing Procedures.
3. Williams RB. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol. 1998;28:1089–1098. doi:10.1016/S0020-7519(98)00066-6
4. Kaufmann J. Parasitic infection of domestic animals. In: Diagnostic Manual. Postfach 133, CH-4010 Basel, Schweiz: Birkhauser Verlag; 1996:1–348.
5. Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Veterinary helminthology. In: Veterinary Parasitology.
6. Taylor MA, Coop RL, Wall RL, et al. Parasites of poultry and game birds. In: Taylor MA, Coop RL, Wall RL, editors. Veterinary Parasitology.
7. Chapman HD. Practical use of vaccines for the control of coccidiosis in the chicken. Worlds Poult Sci J. 2000;56:7–20. doi:10.1079/WPS20000002
8. Chapman HD. Resistance of field isolates of Eimeria species to anticoccidial drugs. Avian Pathol. 1976;5:283–290. doi:10.1080/03079457608418197
9. Chapman HD. Biochemical, genetic, and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi:10.1080/03079459708419208
10. Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15:58–65. doi:10.1128/CMR.15.1.58-65.2002
11. Morris GM, Gasser RB. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol Adv. 2006;24:590–603. doi:10.1016/j.biotechadv.2006.06.001
12. Su YC, Fei AC, Tsai FM, et al. Differential diagnosis of five avian Eimeria species by polymerase chain reaction using primers derived from the internal transcribed spacer 1 (ITS-1) sequence. Vet Parasitol. 2003;117:221–227. doi:10.1016/j.vetpar.2003.07.028
13. Permin A, Hansen JW. The Epidemiology, Diagnosis, and Control of Poultry Parasites. Rome, Italy: FAO Animal Health Manuals; 1998:155.
14. Lobago F, Worku N, Wossene A, et al. Study on coccidiosis in Kombolcha poultry farm, Ethiopia. Trop Anim Health Prod. 2005;37:245–251. doi:10.1023/B:TROP.0000049302.72937.12
15. Mersha C, Tamiru N, Samuel BT, et al. Occurrence of concurrent infectious diseases in broiler chickens is a threat to commercial poultry farms in central Ethiopia. Trop Anim Health Prod. 2009;41:1309–1317. doi:10.1007/s11250-009-9316-9
16. Getachew GJ. Studies on Poultry Coccidiosis in Tiyo Wereda, Arsi Zone, Oromia Regional State [Thesis]. Ethiopia: Addis Ababa University; 2004.
17. Amare A, Worku N, Negussie H, et al. Coccidiosis prevailing in parent stocks: a comparative study between growers and adult layers in Kombolcha poultry breeding and multiplication center, Ethiopia. Glob Vet. 2012;8:285–291.
18. Adamu M. Evaluation of risk factors associated with coccidiosis in broiler farms of selected towns of Ethiopia. African J Basic Appl Sci. 2015;7:34–40.
19. Luu L, Bettridge J, Christley RM, et al. Prevalence and molecular characterization of Eimeria species in Ethiopian village chickens. BMC Vet Res. 2013;9:208. doi:10.1186/1746-6148-9-208
20. Adeyemi OS, Olatoye IO, Oladele DO, et al. Morphometric and molecular identification of Eimeria species from commercial chickens in Nigeria. J Dairy Vet Anim Res. 2020;9:
21. Moraes JC, Franca M, Sartor AA, et al. Prevalence of Eimeria spp. in broilers by multiplex PCR in the Southern Region of Brazil on two hundred and fifty farms. Avian Dis. 2015;59:277–281. doi:10.1637/10989-112014-Reg
22. Wossene A. Poultry bio-security study in Ethiopia. A consultancy report. Ministry of Agriculture and Rural Development Animal Health Department and Food and Agriculture Organization of the United Nations, April 2006, Addis Ababa, Ethiopia. 2006.
23. Ayele G, Rich KM. Poultry value chains and HPAI in Ethiopia, Africa/Indonesia team working paper; 2010. Available from: http://www.hpai-research.net.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

