Back to Journals » Infection and Drug Resistance » Volume 15
Molecular Characteristics and Gonococcal Genetic Island Carrying Status of Thirty-Seven Neisseria gonorrhoeae Isolates in Eastern China
Authors Zhang D, Hu M, Chi S, Chen H, Lin C, Yu F, Zheng Z
Received 5 August 2022
Accepted for publication 27 October 2022
Published 8 November 2022 Volume 2022:15 Pages 6545—6553
DOI https://doi.org/10.2147/IDR.S385079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Dan Zhang1 *, Mingpeng Hu2 *, Shengying Chi,1 Han Chen,1 Chunchan Lin,1 Fangyou Yu,3 Zhou Zheng1
1Department of Clinical Laboratory, Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Ruian Traditional Chinese Medicine Hospital, Wenzhou, People’s Republic of China; 3Department of Laboratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhou Zheng, Email [email protected]
Purpose: To investigate the carrying situation of N. gonorrhoeae genetic island (GGI), and to understand the existence of GGI of different multilocus sequence types (MLST), so as to provide evidence for epidemiology.
Methods: From January 2018 to December 2020, a total of 37 clinical isolates of N. gonorrhoeae were collected. Resistance to tetracycline, β-lactam, and azithromycin were measured. Genes in GGI (atlA, traG, and traH) were amplified via polymerase chain reaction (PCR). All clinical isolates were subjected to N. gonorrhoeae MLST.
Results: The GGI of N. gonorrhoeae were widespread, and the positive detection rates of atlA, traG and traH were all 81.08% (30/37). In this study, atlA, traG and traH were always detected positive together. No significant difference in the positive rate of the GGI between the azithromycin-sensitive and the resistance groups or between the β-lactam positive and negative groups (P > 0.05) was found; however, there was a significant difference between the high-level tetracycline-resistant group and the non-high-level resistant group (P < 0.05), with the carrier rates being 60.00% and 94.45%, respectively. Among the 37 isolates studied, 12 distinct MLST were determined, while MLST ST8123 occurred most frequently, accounting for 18.91% (7/37), followed by ST1928, ST7367 and ST7822, all 13.51% (5/37).
Conclusion: N. gonorrhoeae typed as ST1928, ST1901, ST1588 and ST7822, the GGI were all positive. These four types are more likely to become highly virulent strains.
Keywords: Neisseria gonorrhoeae, pathogenicity islands, drug resistance, TRNG, MLST
Introduction
Neisseria gonorrhoeae is the only member of the Neisseria genus that parasitizes the human reproductive tract and annually causes an estimated 108 million cases globally. Its pathogenic factors are varied.1 Although N. gonorrhoeae does not produce exotoxins, it can damage host cells by releasing toxin molecules, such as lipo-oligosaccharides and peptidoglycan fragments. Understanding the persistence of gonococci and the evolution of antimicrobial resistance (AMR) is critical for the successful control of gonorrhea.2 Harrison et al, earlier in 2016, noted an association between the presence of gonococcal genetic island (GGI) and predicted resistance to multiple antibiotics.3,4 In 2001, Dillard et al first proposed the theory of PAIs of N. gonorrhoeae in which these authors suggested that the gonococcal genetic island (GGI) are closely related to the virulence of N. gonorrhoeae virulence.5
GGI are virulence gene clusters with large molecular weight (usually >30Kb), G+Cmol%, and code usage on bacterial chromosomes that are significantly different from those of the host bacterial chromosome.6,7 GGI encode various virulence factors that are normally absent in non-pathogenic strains of closely related or the same species. GGI are considered a subclass of genomic islands that are acquired through horizontal gene transfer through transduction, coupling, and transformation, and provide a “quantum leap” in microbial evolution.8–10 Data based on extensive bacterial genome sequencing indicate that GGI are ubiquitous in both Gram-positive and -negative bacterial pathogens of humans, animals, and plants.11,12
Since the discovery and naming of the first GGI in uropathogenic Escherichia coli, UPEC,13 GGI have been successfully found in Staphylococcus,14 Helicobacter pylori,15 Yersinia,16 Salmonella,17 Pseudomonas,18 Vibrio cholerae,19 and others. The existence of more than a dozen GGI has been reported. However, only a few studies addressing the GGI of N. gonorrhoeae are available. The GGI genes currently identified in N. gonorrhoeae mainly include atlA, traH, traG, cspA, exp1, exp2, orf7, and orf8.5 The length of atlA is 543 bp, and its product, AtlA, is a peptidoglycan hydrolase, which strongly resembles the phage glycosyltransferase. Expression of AtlA leads to an increase in levels of toxic peptidoglycan fragments, modulates immune responses, promotes N. gonorrhoeae infection, exacerbates disseminated gonorrhea arthritis, and may cause meningitis. The lengths of traG and traH are 2916 bp and 1503 bp, respectively. These genes are located upstream of atlA and are very similar to the E. coli (ECO) F factor conjugation genes, traG and traH. In the ECO F plasmid, traH and traG are involved in the assembly of F-pili. TraG is required for the stability of conjugation recipient bacteria and is presumed to be part of the conjugation channel. N. gonorrhoeae can secrete DNA through the type IV secretion system encoded by the GGI, which is crucial to the virulence of bacteria, is closely related to its natural transformation, promotes the spread of antigenic variation and drug resistance, and may also be associated with others.20,21
Multi-locus sequence typing (MLST) is used to characterize isolates by amplifying sequences with seven housekeeping loci and is usually used to track the spread of N. gonorrhoeae strains in which genetic variation is indexed over an extended period of time accumulation.22
GGI have been transferred horizontally (laterally) from other microorganisms and are important in the evolution of pathogenesis.23 Such events, which may occur over long periods of time, lead to the evolution of new pathogenic capabilities and/or the emergence of new microbial species.12 The purpose of this study was to investigate the GGI status and genetic characteristics of N. gonorrhoeae from samples obtained from a teaching hospital in Wenzhou, eastern China.
Materials and Methods
Bacterial Strains
From January 2018 to December 2020, a total of 104 N. gonorrhoeae were isolated, out of which 37 non-repetitive isolates were selected by simple random sampling in the First Affiliated Hospital of Wenzhou Medical University, of which 19/104 were from 2018, 14/104 from 2019, 5/104 from 2020; If multiple samples were obtained from the same patient, only the first isolate was retained. All experimental strains were identified by oxidase reaction, sugar fermentation tests, and Gram-staining, and further confirmed as N. gonorrhoeae by the fully automatic rapid microbial mass spectrometry detection system (VITEK MS from bioMérieux, SA, France).
Antimicrobial Susceptibility Testing
The susceptibility of N. gonorrhoeae to β-lactam antibiotics was determined by the disc method (Chongqing Ponton Medical Equipment Co., Ltd.) for qualitative results, the K-B method for tetracycline, and the E-test method for azithromycin (Wenzhou Kangtai Biological Technology Co., Ltd.). All test results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI 2020) guideline standards for all antibiotics.24 An individual N. gonorrhoeae isolate was streaked on Thayer-Martin (T-M) selective medium for isolation and purification. This plate was incubated at 36°C in 5% CO2 for 48 h after which time, the bacteria that reached 0.5 M turbidity were smeared three times in a dense and uniform manner onto 9-cm diameter GC plates. Plates were then incubated for 24 h at 35°C in a 5% CO2 incubator with drug-sensitive paper and E-test strips to determine zone diameter or minimum inhibitory concentrations (MICs). The quality control strain for the antibiotic susceptibility test was N. gonorrhoeae ATCC 49226, which was provided by the Clinical Laboratory of the Ministry of Health of the People’s Republic of China. All strains were kept in glycerol broth and stored at −80℃ until use.
Extraction of Bacterial Genomic DNA
The plasmid DNA of N. gonorrhoeae was extracted using a spin column-type plasmid mini-extraction kit DP103-03 (Tiangen Biochemical Technology Co., Ltd.) after which the supernatant was removed and stored at −20℃ for further use.
Amplification of the Pathogenicity Islands Gene
Primers for atlA, traG, and traH genes were synthesized by Beijing Qingke New Industry Biotechnology Co., Ltd. Hangzhou Branch as previously described (Table 1).5 Polymerase chain reaction (PCR) conditions for atlA and traH followed several steps: (1) 10 min at 94℃ for pre-denaturation, (2) 30 cycles of 30 s at 94℃ for denaturation, (3) 30 s at 56℃ for annealing, and (4) extension at 72℃ for 1 min. Conditions for amplification of traG included several steps: (1) 10 min at 94℃, (2) 30 cycles of 30 s each at 94℃, (3) 30 s at 56℃, and (4) 2 min at 72℃.
 |
Table 1 Primer Sequence and Product Length of Target Genes |
DNA samples amplified by PCR were electrophoresed at 110 V for 30 min. All amplified products were then photographed by a gel imager, confirmed as a single band, and the positive strains were recorded.
N. gonorrhoeae Multi-Locus Sequence Typing (MLST)
All N. gonorrhoeae isolates were subject to molecular epidemiologic analysis using MLST in which seven housekeeping genes (adk, abcZ, aroE, fumC, pgm, gdh, and pdhC) were analyzed and the sequencing results submitted to the database system (http://pubmlst.org/neisseria/) to obtain the number of alleles. Strain sequences were then obtained based on the number of alleles (ST). MEGA 7.0 software used the maximum-likelihood method to create a N. gonorrhoeae phylogenetic tree by linking alleles.25
Statistical Analysis
The hospital bacterial resistance monitoring software (WHONET 5.4) provided by WHO and medical statistical software SPSS20.0 were used for data analysis and statistical processing, and P values less than 0.05 were considered statistically significant.
Results
Antimicrobial Susceptibility
Among the 37 N. gonorrhoeae strains that were tested, the positive rate of β-lactam was 62.16%, and the resistance rates of tetracycline and azithromycin were 45.94% and 56.75%, respectively. Among the 21 azithromycin-resistance isolates, two with MIC values of >256 μg/mL exhibited high-level resistance. We published an article in 2019 and found that all nineteen highly tetracycline-resistant N. gonorrhoeae were positive for the Tet-M gene, which is consistent with Allen’s finding that the tetracycline resistance of N. gonorrhoeae is closely related to the Tet-M gene.26,27 Therefore, gonococci with 30ug tetracycline disk zone diameters of =<19mm usually indicate a plasmid-mediated high-level tetracycline-resistant N. gonorrhoeae (TRNG) isolate.24 Of the 17 tetracycline-resistant strains, 15 were TRNGs (Table 2).
 |
Table 2 PAIs and Molecular Typing Results of Clinical Isolates of Neisseria gonorrhoeae |
Detection Results of Pathogenicity Islands Genes and Correlations with Drug Resistance
Using the DNA of 37 strains of N. gonorrhoeae as a template for PCR amplification, it was found that the GGI were widely present, and the positive detection rates of atlA, traG and traH were all 81.08% (30/37). In this study, atlA, traG and traH were always detected positive together. The sequencing diagram is shown in Figure 1. According to whether the MIC value of azithromycin was ≥1 μg/mL,28 37 strains of N. gonorrhoeae could be divided into azithromycin-sensitive and azithromycin-resistant groups, and if the diameter of tetracycline disk zone was ≤ 19mm, the strains could be divided into highly resistant and non-highly resistant groups. A correlation between GGI and antibiotic resistance is shown in Table 3. As can be seen, no significant difference in the positive rate of the GGI between the azithromycin-sensitive and azithromycin-resistance groups was observed (P > 0.05). Similarly, no significant difference between the β-lactam positive and negative group (P > 0.05) was noted; however, a significant difference between the TRNG group and the non-TRNG group (P < 0.05) was observed. Another important finding is that the GGI of the two high-level azithromycin-resistant strains were positive.
 |
Table 3 Correlation Analysis Between Antimicrobial Resistance and Pathogenicity Islands of N. gonorrhoeae |
 |
Figure 1 The sequencing results of atlA, traG and traH. |
Molecular Epidemiologic Typing
The 37 N. gonorrhoeae clinical isolates were typed by MLST analysis, and 12 different sequence types (STs) were identified with ST8123 was the most prevalent ST, accounting for 18.91% (7/37), followed by ST1928, ST7367 and ST7822, all 13.51% (5/37). N. gonorrhoeae typed as ST1928, ST1901, ST1588 and ST7822 (STs with values of one were not counted), the GGI genes were all positive.
Based on phylogenetic analysis, four large clusters were identified (Figure 2). Cluster A isolates included four different STs, cluster C isolates included two, and cluster B isolates included only one type, named ST7363. Finally, cluster D contained five different STs, including ST1928, ST1901, ST9899, ST1600 and ST7822, in which three STs GGI genes were all positive.
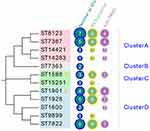 |
Figure 2 Phylogenetic tree constructed using MEGA7.0 for MLST STs of 37 N. gonorrhoeae isolates. Clusters A-D: according to bootstrap, different STs are divided into four clusters, A-D. |
Discussion
N. gonorrhoeae, the causative agent of the sexually transmitted disease gonorrhea, has an estimated annual incidence of 86.9 million adults globally.29 Untreated gonorrhea can lead to serious sequelae, including infertility, pelvic inflammatory disease, neonatal conjunctivitis, and disseminated gonococcal infection. Gonorrhea may also contribute to an increase in HIV transmission.30 Bacterial pathogenicity and antibiotic resistance are two subjects about which people have been generally concerned. Bacterial virulence may be due to acquisition of some different GGI or deletion of some chromosomal DNA under host selection pressure for a long time. In contrast, antibiotic resistance is initiated under the selective pressure of antimicrobial drugs. For N. gonorrhoeae, the study of drug resistance started very early, while the study of GGI was relatively late.
Previous studies have demonstrated that GGI contribute to virulence of bacterial pathogens, and the loss of GGI will lead to decreased virulence.31,32 In this study, atlA, traG, and traH always detected positive as a group with a very high detection rate of 81.08%. It can be seen that GGI are indeed widespread, which is consistent with previous reports.1,11,12 According to Table 3, it can be easily seen that no significant difference between the β-lactam positive and negative groups could be found (P > 0.05), and the same pattern was true for the azithromycin-sensitive and azithromycin-resistance groups (P > 0.05). However, the only two highly resistant azithromycin strains were positive for GGI, which is a finding that needs further study. Previous studies have reported that the mutant with high-resistance to azithromycin (N. gonorrhoeae 23S rRNA A2059G mutant) exhibits enhanced biocompatibility and enhanced epithelial cell invasion during colonization with a selective advantage during colonization in a mouse vaginal infection model.33 Therefore, it is believed that the GGI of the two highly azithromycin-resistant strains found in this study is meaningful since it appears that as azithromycin resistance increases, so does virulence.
The GGI-encoded gene product was found to be predominant in infecting cervical cells in a previous study and was associated with N. gonorrhoeae virulence.34 By comparing the GGI containing the TRNG and non-TRNG groups, it was found that the carrier rate of the non-TRNG group (94.45%) was higher than that of TRNG group (60.00%), and a statistically significant difference (P < 0.05) was found. We therefore inferred that there was a statistically significant association between N. gonorrhoeae virulence negatively correlate and tetracycline resistance, and the lower the tetracycline resistance is, the stronger the virulence is. This finding is quite different from the ongoing concerns about N. gonorrhoeae antibacterial drug resistance.35–37 However, according to the results of this study, it is suggested that both pathogenicity and transmissibility of highly antibiotic-resistant strains are weaker, which means that antibiotic resistance and pathogenicity are determined by different factors, so monitoring and research need to be conducted separately. To our best knowledge, this study is the first report on the relationship between N. gonorrhoeae virulence and tetracycline resistance.
The N. gonorrhoeae typing method, MLST, assists in understanding of gonorrhea spread. In this study, a total of 12 MLST types were identified, of which 33.33% (4/12) of the STs were represented by only a single isolate, suggesting that these clinical isolates of N. gonorrhoeae exhibit considerable genetic diversity. According to the phylogenetic tree, four different STs with all positive GGI were found, three of them belonged to cluster D, namely ST1901, ST1928, and ST7822, and ST1588 was found in cluster C. These four STs share the same housekeeping genes, adk and pgm, while MLST 1901, MLST1928, and MLST7822 have four identical housekeeping genes (adk, fumC, pdhC, and pgm). This pattern indicates that the highly virulent N. gonorrhoeae strains are mainly concentrated in groups C and D, and the relationship is very close. It is worth mentioning that the highly virulent type ST1901 found in this study is consistent with the multidrug-resistant and widely disseminated N. gonorrhoeae identified in the Harrison report,1 which explains that the presence of the gonococcal genetic island (GGI) may be a significant factor in the Western hemisphere expansion of gonococcus belonging to ST1901. Although the four ST1901 strains in the present research are not highly resistant strains, this type has spread in the Northern hemisphere, which is concerning. Furthermore, the spread of ST1901 as a highly virulent strain may lead to widespread dissemination of GGI as a mobile genetic element. This finding must also be a cause for concern.
Recent studies on GGI have not only led to the identification of many new virulence factors used by these species during infection of their respective hosts and have also dramatically changed the way researchers think about the evolution of bacterial virulence.8,11 Timely and in-depth studies of GGI not only helps us understand the complex microbial world and the mechanism of the emergence of new pathogenic microorganisms but also provide new ideas for the study of new pathogenic bacteria, re-emerging pathogenic microorganisms, and development of effective attenuated vaccines.38
Conclusion
Although the sample number of this research is limited, it is impossible to explain whether the GGI carrier rate of the highly azithromycin resistant strains is higher, but it is of great significance to study the GGI and molecular characteristics of N. gonorrhoeae in Wenzhou, Eastern China. Measures should be conducted to monitor the spread of MLST ST1901, ST1928, ST7822, and ST1588 N. gonorrhoeae clones, especially ST1901, which exhibit high virulence in Eastern China.
Ethics Statement
This study was approved by the Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University (Issuing No. KY2022-R118). No informed consent was required due to the observational nature of the study, which focused on bacteria and did no interventions on patients. All the patient information was anonymized and de-identified. Therefore, the Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University waived the need for consent. All experiments were performed in compliance with the relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki.
Acknowledgments
This work was supported by the Science and Technology Bureau of Wenzhou City, China (No. Y2020939) and Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province (2022E10022).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Harrison OB, Clemence M, Dillard JP, et al. Genomic analyses of Neisseria gonorrhoeae reveal an association of the gonococcal genetic island with antimicrobial resistance. J Infect. 2016;73(6):578–587. doi:10.1016/j.jinf.2016.08.010
2. Harrison OB, Cehovin A, Skett J, et al. Neisseria gonorrhoeae population genomics: use of the gonococcal core genome to improve surveillance of antimicrobial resistance. J Infect Dis. 2020;222(11):1816–1825. doi:10.1093/infdis/jiaa002
3. Shaskolskiy B, Kravtsov D, Kandinov I, et al. Comparative whole-genome analysis of Neisseria gonorrhoeae; isolates revealed changes in the gonococcal genetic island and specific genes as a link to antimicrobial resistance. Front Cell Infect Microbiol. 2022;12:831336. doi:10.3389/fcimb.2022.831336
4. Callaghan MM, Heilers JH, van der Does C, Dillard JP. Secretion of chromosomal DNA by the Neisseria gonorrhoeae type IV secretion system. Curr Top Microbiol Immunol. 2017;413:323–345. doi:10.1007/978-3-319-75241-9_13
5. Dillard JP, Seifert HS. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol. 2001;41(1):263–277. doi:10.1046/j.1365-2958.2001.02520.x
6. Jores J, Rumer L, Kiessling S, Kaper JB, Wieler LH. A novel locus of enterocyte effacement (LEE) pathogenicity island inserted at pheV in bovine Shiga toxin-producing Escherichia coli strain O103:H2. FEMS Microbiol Lett. 2001;204(1):75–79. doi:10.1111/j.1574-6968.2001.tb10866.x
7. Carniel E. The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect. 2001;3(7):561–569. doi:10.1016/S1286-4579(01)01412-5
8. Morschhäuser J, Köhler G, Ziebuhr W, et al. Evolution of microbial pathogens. Philos Trans R Soc Lond B Biol Sci. 2000;355(1397):695–704. doi:10.1098/rstb.2000.0609
9. Arnold DL, Pitman A, Jackson RW. Pathogenicity and other genomic islands in plant pathogenic bacteria. Mol Plant Pathol. 2003;4(5):407–420. doi:10.1046/j.1364-3703.2003.00187.x
10. Jackson RW, Athanassopoulos E, Tsiamis G, et al. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc Natl Acad Sci U S A. 1999;96(19):10875–10880. doi:10.1073/pnas.96.19.10875
11. Gal-Mor O, Finlay BB. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell Microbiol. 2006;8(11):1707–1719. doi:10.1111/j.1462-5822.2006.00794.x
12. Rivas LA, Mansfield J, Tsiamis G, Jackson RW, Murillo J. Changes in race-specific virulence in Pseudomonas syringae pv. phaseolicola are associated with a chimeric transposable element and rare deletion events in a plasmid-borne pathogenicity island. Appl Environ Microbiol. 2005;71(7):3778–3785. doi:10.1128/AEM.71.7.3778-3785.2005
13. Hacker J, Bender L, Ott M, et al. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb Pathog. 1990;8(3):213–225. doi:10.1016/0882-4010(90)90048-U
14. Novick RP, Fischetti VA, Novick RP. Pathogenicity islands and their role in staphylococcal biology. Microbiol Spectr. 2019;7(3). doi:10.1128/microbiolspec.GPP3-0062-2019
15. Noto JM, Peek RM. The helicobacter pylori cag pathogenicity island. Methods Mol Biol. 2012;921:41–50.
16. Schubert S, Rakin A, Heesemann J. The Yersinia high-pathogenicity island (HPI): evolutionary and functional aspects. Int J Med Microbiol. 2004;294(2–3):83–94. doi:10.1016/j.ijmm.2004.06.026
17. Lou L, Zhang P, Piao R, Wang Y. Salmonella pathogenicity island 1 (SPI-1) and its complex regulatory network. Front Cell Infect Microbiol. 2019;9:270. doi:10.3389/fcimb.2019.00270
18. Harrison EM, Carter ME, Luck S, et al. Pathogenicity islands PAPI-1 and PAPI-2 contribute individually and synergistically to the virulence of Pseudomonas aeruginosa strain PA14. Infect Immun. 2010;78(4):1437–1446. doi:10.1128/IAI.00621-09
19. Murphy RA, Boyd EF. Three pathogenicity islands of Vibrio cholerae can excise from the chromosome and form circular intermediates. J Bacteriol. 2008;190(2):636–647. doi:10.1128/JB.00562-07
20. van Ulsen P, Tommassen J. Protein secretion and secreted proteins in pathogenic Neisseriaceae. FEMS Microbiol Rev. 2006;30(2):292–319. doi:10.1111/j.1574-6976.2006.00013.x
21. Callaghan MM, Klimowicz AK, Shockey AC, Kane J, Pepperell CS, Dillard JP. Transcriptional and translational responsiveness of the Neisseria gonorrhoeae type IV secretion system to conditions of host infections. Infect Immun. 2021;89(12):e0051921. doi:10.1128/IAI.00519-21
22. Mavroidi A, Tzelepi E, Siatravani E, et al. Analysis of emergence of quinolone-resistant gonococci in Greece by combined use of Neisseria gonorrhoeae multiantigen sequence typing and multilocus sequence typing. J Clin Microbiol. 2011;49(4):1196–1201. doi:10.1128/JCM.02233-10
23. Yoon SH, Park YK, Lee S, et al. Towards pathogenomics: a web-based resource for pathogenicity islands. Nucleic Acids Res. 2007;35:D395–D400. doi:10.1093/nar/gkl790
24. CLSI. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100.
25. Wan C, Li Y, Le W, et al. Increasing resistance to azithromycin in Neisseria gonorrhoeae in Eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from Nanjing. Antimicrob Agents Chemother. 2018;62(5). doi:10.1128/AAC.02499-17
26. Allen VG, Farrell DJ, Rebbapragada A, et al. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob Agents Chemother. 2011;55(2):703–712. doi:10.1128/AAC.00788-10
27. Zheng Z, Chen H, Deng T, et al.Drug resistance mechanism and molecular characteristics of tetracycline-resistant Neisseria gonorrhoeae clinical isolates from a tertiary hospital. Chin J Microbiol Immunol. 2019;39(2):2.
28. Zheng Z, Liu L, Shen X, et al. Antimicrobial resistance and molecular characteristics among Neisseria gonorrhoeae Clinical isolates in A Chinese Tertiary Hospital. Infect Drug Resist. 2019;12:3301–3309. doi:10.2147/IDR.S221109
29. Unemo M, Seifert HS, Hook EW, et al. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. doi:10.1038/s41572-019-0128-6
30. Unemo M. Current and future antimicrobial treatment of gonorrhoea – the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis. 2015;15(1):364. doi:10.1186/s12879-015-1029-2
31. Hochhut B, Dobrindt U, Hacker J. Pathogenicity islands and their role in bacterial virulence and survival. Contrib Microbiol. 2005;12:234–254.
32. Nieto PA, Pardo-Roa C, Salazar-Echegarai FJ, et al. New insights about excisable pathogenicity islands in Salmonella and their contribution to virulence. Microbes Infect. 2016;18(5):302–309. doi:10.1016/j.micinf.2016.02.001
33. Zhang J, van der Veen S. Neisseria gonorrhoeae 23S rRNA A2059G mutation is the only determinant necessary for high-level azithromycin resistance and improves in vivo biological fitness. J Antimicrob Chemother. 2019;74(2):407–415. doi:10.1093/jac/dky438
34. Zola TA, Strange HR, Dominguez NM, Dillard JP, Cornelissen CN. Type IV secretion machinery promotes ton-independent intracellular survival of Neisseria gonorrhoeae within cervical epithelial cells. Infect Immun. 2010;78(6):2429–2437. doi:10.1128/IAI.00228-10
35. Ortiz Á, Santander M, E. P, Lugo PJ. Neisseria gonorrhoeae: un patógeno díscolo. Conceptos microbiológicos, resistencia a antimicrobianos y su vigilancia epidemiológica en Chile [Neisseria gonorrhoeae: a wayward pathogen. Microbiological concepts, antimicrobial resistance and its epidemiological surveillance in Chile]. Rev Chilena Infectol. 2021;38(4):512–522. Spanish. doi:10.4067/S0716-10182021000400512
36. Aitolo GL, Adeyemi OS, Afolabi BL, Owolabi AO. Neisseria gonorrhoeae antimicrobial resistance: past to present to future. Curr Microbiol. 2021;78(3):867–878. doi:10.1007/s00284-021-02353-8
37. Unemo M, Golparian D, Eyre DW. Antimicrobial Resistance in Neisseria gonorrhoeae and Treatment of Gonorrhea. Methods Mol Biol. 2019;1997:37–58.
38. Tang C, Holden D. Pathogen virulence genes--implications for vaccines and drug therapy. Br Med Bull. 1999;55(2):387–400. doi:10.1258/0007142991902448
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
