Back to Journals » Infection and Drug Resistance » Volume 17
Molecular Characteristics and Antimicrobial Susceptibility Profiles of blaKPC-Producing Escherichia Coli Isolated from a Teaching Hospital in Shanghai, China
Authors Cao S, Jiang X, Suo J, Lu Y, Ju M, Zeng Q, Zheng Q, Zhang Z, Tang W
Received 11 October 2023
Accepted for publication 5 January 2024
Published 26 January 2024 Volume 2024:17 Pages 319—327
DOI https://doi.org/10.2147/IDR.S444117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Shuaijun Cao,1,* Xiaoying Jiang,2,* Jinshan Suo,3 Yanyan Lu,2 Mohan Ju,2 Qixiang Zeng,1 Qingru Zheng,1 Zuoyan Zhang,1 Wenqi Tang1
1Department of Critical Care Medicine, Shanghai Sixth People’s Hospital, Shanghai, People’s Republic of China; 2Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Department of Ophthalmology, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zuoyan Zhang; Wenqi Tang, Department of Critical Care Medicine, Shanghai Sixth People’s Hospital, No. 600 Yishan Road, Shanghai, 200000, People’s Republic of China, Tel +86 021-24058331, Email [email protected]; [email protected]
Introduction: Carbapenem-Resistant Enterobacteriaceae (CRE) has posed a significant threat to humans.The aim of this study was to investigate the molecular characteristics of blaKPC-producing Escherichia coli in a university-affiliated tertiary hospital.
Methods: Polymerase chain reaction (PCR) and BLAST+ software were used to detect the prevalence of blaKPC in E. coli and Klebsiella pneumoniae. Whole-genome sequencing was performed for the blaKPC-harboring clinical E. coli isolates. Antimicrobial resistance genes, MLSTs, KPC-carrying plasmid typing and genetic environment of blaKPC were analyzed. A maximum likelihood core single nucleotide polymorphism (SNP)-based phylogeny tree was constructed to determine the evolutionary relationships within this ST131 collection. Conjugation experiments were performed to determine the mobilization of blaKPC. The minimal inhibitory concentrations of the common antimicrobial agents were determined using the broth microdilution method.
Results: The prevalence of blaKPC in 424 clinical E. coli isolates and 1636 E. coli strains from GenBank database were 2.2% (45/2060) whereas the detection rate of blaKPC in K. pneumoniae from the GenBank database was 29.8% (415/1394). The blaKPC-harboring conjugants exhibited resistance to multiple β-lactams, except for cefepime-zidebactam and ceftazidime-avibactam. All blaKPC-carring E. coli isolates were susceptible to tigecycline and polymyxin B. ST131 was the dominant sequence type of blaKPC-carring E. coli, accounting for 40.0% (18/45). Most of the blaKPC-producing ST131 E. coli (89.5%,17/19) belonged to clade C ST131 lineage. Genetic environment analysis revealed that 57.8% (26/45) of blaKPC gene was linked to Tn 4401-associated structure ISKpn6-blaKPC-ISKpn7. IncN was the most common plasmid type in KPC-producing E. coli whereas IncFII was the dominant plasmid type in KPC-producing K. pneumoniae.
Conclusion: The detection rate of blaKPC was lower in E. coli compared with K. pneumoniae. The dominant sequence and plasmid types of blaKPC-harboring isolates differed between E. coli and K. pneumoniae. Further studies about the role of the defense system in acquisition of KPC-plasmids in E. coli will be performed to provide new insights into the low prevalence of blaKPC.
Keywords: Escherichia coli, blaKPC, carbapenemases, plasmids typing
Introduction
Escherichia coli, a member of Enterobacteriaceae family, is a prominent cause of many common bacterial infections, including urinary tract infections, bloodstream infections, diarrheal illnesses, and central nervous system infections such as neonatal meningitis.1 The prevalence of multidrug-resistant E. coli poses a significant public health threat. Horizontal gene transfer (HGT), including conjugation by plasmids, transduction by bacteriophages, and natural transformation by extracellular DNA, plays a crucial role in the emergence of antimicrobial resistance.2
Multiple mechanisms including the production of carbapenemases or alterations in outer membrane permeability or upregulation of efflux systems along with hyperproduction of other β-lactamases such as blaAmpC result in carbapenem resistance in E. coli.3 The production of carbapenemases is a major contributor to carbapenem resistance in E. coli. According to Ambler Classification, carbapenemases were classified into 3 groups based on their active sites: Class A (mostly KPC enzymes), Class B (metallo-β-lactamases, MBL such as VIM, NDM and IMP), and Class D (mostly OXA such as OXA-48-like and OXA-23). Class A and D enzymes have a serine-based hydrolytic mechanism, whereas class B metallo-beta-lactamases contain zinc in the active site.4,5
KPC emerged in the late 1990s, was first identified in 1996 in the USA.6 KPC carbapenemases exhibit activity against a wide spectrum of β-lactams, including cephalosporins, cephamycins, aztreonam, carbapenems, and β-lactamase inhibitors.6,7 Since their first description, KPC enzymes have spread internationally. The dissemination of KPC-producing K. pneumoniae is primarily associated with a single multilocus sequence type (ST), ST258, and its related variants. KPCs have been found in many gram-negative species, including Enterobacteriaceae such as E. coli and non-fermenters, such as Pseudomonas aeruginosa.8 A nationwide survey of Carbapenem-Resistant Enterobacteriaceae(CRE) revealed that blaNDM was the dominant carbapenemase genes in E. coli followed by blaKPC-2 carbapenemase.9 It is noteworthy that research in Singapore showed that blaKPC was the predominant carbapenemase type in E. coli, demonstrating greater dissemination potential.10
Given the limited epidemiological data on blaKPC-producing E. coli in China, we investigated the molecular characteristics and antimicrobial susceptibility profiles of blaKPC-harboring E. coli in a university-affiliated tertiary hospital in Shanghai, China.
Materials and Methods
Bacterial Strains
In this study, 424 non-duplicate E. coli isolates were collected from patients at a teaching hospital in Shanghai, China, for the period January to December 2017. The clinical E. coli isolates were identified using the Vitek2 system.
E. coli J53, an azide-resistant laboratory strain, was used as the recipient strain for the conjugation experiments. The complete whole-genome sequences of E. coli (1636 in total, Table S1) and K. pneumoniae (1394 in total, Table S2) were downloaded from the NCBI database.
Screening of blaKPC Gene
Polymerase chain reaction (PCR) was used to detect blaKPC in clinical isolates using the primers KPC-F(5’-TCACTGTATCGCCGTCTA-3’) and KPC-R(5’-CCAACTCCTTCAGCAACA-3’). Amplification was carried out as follows: initial denaturation at 94 °C for 3 min; 35 cycles of 94°C for 30s, 55°C for 30s and 72°C for 50s; and a final elongation step at 72 °C for 5 min. BLAST + software was used to detect blaKPC in the publicly available whole-genome sequences of E. coli and K. pneumoniae (Table S1 and S2).
Extraction of Genomic DNA and Whole-Genome Sequence
Genomic DNA of the clinically isolated blaKPC-producing E. coli was extracted using a bacterial DNA kit (TIANGEN, Beijing, China). Short- and long-read whole-genome sequencing was performed by Shanghai Yuanxu Biotechnology using BGI Genomics (HiSeq X; Illumina, San Diego, CA, USA) and MinION Sequencer (Nanopore, Oxford, UK) respectively. Antimicrobial resistance genes were analyzed at the Center for Genomic Epidemiology (https://cge.cbs.dtu.dk/services/ResFinder/). The information on the completely sequenced blaKPC-producing E. coli is presented in Table S3. The sequences of the 5 blaKPC-positive clinical E. coli were listed in Supplementary data 4–8.
Multilocus Sequence Typing and blaKPC-Carrying Plasmids Typing
The MLSTs of the whole genomes of E. coli and K. pneumoniae were analyzed using the MLST software (https://github.com/tseemann/mlst). Plasmid type blaKPC-harboring isolates were identified using PlasmidFinder (https://cge.food.dtu.dk/services/PlasmidFinder/).
Genetic Environment of BlaKPC-Positive E. Coli
The annotation of the 45 blaKPC-postive E. coli was performed using Prokka software
(https://github.com/tseemann/prokka). Then gggenes (https://github.com/wilkox/gggenes) was used for analyzing the flanking structure of blaKPC gene.
Phylogenomic Analysis
To determine the evolutionary relationships within this ST131 collection, a maximum likelihood core single nucleotide polymorphism (SNP)-based phylogeny tree of 114 E. coli isolates with ST131 and ST131* (adk gene 112 had 99.8% identity, ST may indicate nearest ST) was inferred with RAxML v8.2.12 (PubMed Unique Identifier 24,451,623) after the removal of recombination regions using Gubbins v2.4.1 (PMID 25414349). The resulting tree was rooted at the midpoint and visualized using iTOLs (https://itol.embl.de/).
Conjugation Assay
To determine the mobilization blaKPC, conjugation experiments were performed using clinically isolated blaKPC-producing E. coli as donor strains and E. coli J53 as the recipient strain. Transconjugants were selected on LB agar plates containing 50 mg/L ampicillin and 150 mg/L sodium azide. PCR amplification and sequencing of blaKPC were performed to ensure successful transfer of blaKPC-bearing plasmids.
Antibiotic Susceptibility Testing
Minimal inhibitory concentrations (MICs) of common antimicrobial agents were determined using the broth microdilution method, and interpretation was recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines for 424 clinically isolated E. coli, KPC-positive E. coli and blaKPC-harboring transformants. E. coli ATCC 25922 was used as a quality control strain for antimicrobial susceptibility testing. The interpretation was based on CLSI breakpoints for all antimicrobial agents except tigecycline and polymyxin B.11 Tigecycline and polymyxin B MICs were interpreted using the European Committee for Antimicrobial Susceptibility Testing (EUCAST) criteria.12
Results
Antimicrobial Susceptibility Testing for Common Antibiotics in Clinical Isolated E. Coli
The susceptibility rates of 424 E. coli isolates to quinolone and most β-lactams were less than 40%, including ciprofloxacin, aztreonam, cefotaxime, ceftazidime, cefepime, and piperacillin, while those to imipenem and meropenem were 90.1% and 88.2%, respectively (Table 1). Low susceptibility rates were observed for piperacillin (11.3%), in contrast to the increased susceptibility observed when piperacillin was in combination with β-lactamase inhibitors, namely piperacillin-tazobactam (71.0%). Low resistance rates for amikacin, polymyxin B, and tigecycline were observed (12.0%, 0%, and 5.0%, respectively).
 |
Table 1 Antimicrobial Susceptibilities of Clinical Isolated Escherichia Coli Determined by the Broth Microdilution Method |
Prevalence of blaKPC in E. Coli and Genetic Environment of BlaKPC-Positive E. Coli
The prevalence of blaKPC was 1.2% (5/424) in E. coli clinical isolates and 2.4% (40/1636) in E. coli strains in the GenBank database, whereas the detection rate of blaKPC in K. pneumoniae in the GenBank database was 29.8% (415/1394).
For the 45 KPC-producing E. coli isolates, the dominant sequence type was ST131 (40.0%, 18/45), followed by ST648(8.9%,4/45), ST410(6.7%,3/45), ST10 and ST7358(n=2 for each). In total, 21 different sequence types were identified in the 45 KPC-positive E. coli strains, whereas other sequence types were identified in one strain including ST167, ST216, ST131* and so on.
Genetic environment analysis of blaKPC gene in E. coli revealed that 57.8% (26/45) of blaKPC gene was linked to Tn4401-associated structure ISKpn6-blaKPC-ISKpn7 while blaKPC-ISKpn27 were observed in 15 strains (Figure S1 and Table S4).
Plasmids Typing
Among the 45 KPC-positive E. coli, most blaKPC genes were found on the plasmids, except for E. coli strain 3385 and HS3555, which was located on the chromosome. The plasmids were IncC (n = 2), IncFIA and IncP1 (n = 1 each) among the four clinical isolates, whereas IncN was the most common plasmid type (11/40, 27.5%), followed by IncC, IncR, and IncFIB (n=3 each) in KPC-producing E. coli from the GenBank database, as shown in Figure 1.
 |
Figure 1 Plasmid typing composition of Klebsiella pneumoniae carbapenemase-producing Escherichia coli strains. |
Among the blaKPC-producing K. pneumoniae, 379 strains carried only one KPC-harboring plasmid while 5 strains carried only one blaKPC located on the chromosome (Table S5). Six strains were observed with two blaKPC genes located on plasmid and the chromosome while 23 strains carried two KPC-positive plasmids. IncFII was the dominant plasmid type (43.3%,190/439), followed by IncFIB (11.6%,51/439), IncR (10.7,47/439), and repB (6.2%,27/439) as shown in Figure 2.
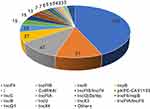 |
Figure 2 Plasmid typing composition of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae strains. |
Phylogenomic Analysis
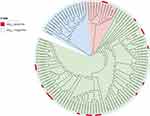
A core-genome alignment and maximum likelihood (SNP)-based phylogeny was obtained for all 114 E. coli isolates with ST131 and ST131* genomes which revealed a 3-clade structure identical to those previously described (Figure 3). ST131 clade A contains the previously-sequenced ERR161235 strain.13 ST131 clade B containing previously-sequenced ERR161305 strain was very similar to clade C. ST131 clade C strains make up 59% (85/114) of the ST131 strains. Most of the blaKPC-producing ST131 E. coli (89.5%,17/19) belonged to clade C ST131 lineage except for Ecol_AZ159(Accession number GCF_002012145.1) and Ecol_244(Accession number GCF_002012305.1) which belonged to clade B ST131 lineage. Two blaKPC-postive clinical isolated E. coli HS1496 and HS2039 were in clade C ST131 lineage.
Antimicrobial Susceptibility Testing for Common Antibiotics in Clinical Isolated E. Coli and Its blaKPC-Harboring Conjugants
The E. coli clinical isolates and their blaKPC-harboring conjugants exhibited resistance to multiple β-lactams, including piperacillin-tazobactam, meropenem, imipenem, and ceftolozane-tazobactam, but susceptibility to ceftazidime-avibactam and cefepime-zidebactam. The MICs of cefepime-tazobactam ranged from 4 to 16 mg/L in blaKPC-carriers, in contrast to the increased susceptibility when cefepime was in combination with another β-lactamase inhibitor, namely cefepime-zidebactam (0.125 to 1 mg/L). All the isolates were susceptible to tigecycline and polymyxin B (Table 2).
 |
Table 2 Susceptibility of blaKPC-Positive Clinical Isolated Escherichia Coli, Their Conjugants and Recipient to Antimicrobial Agents |
Discussion
Antimicrobial resistance (AMR) has been identified as a major health threat and anticipated to cause 10 million deaths annually by 2050.14 The World Health Organization (WHO) listed Carbapenem-Resistant Enterobacteriaceae (CRE) as critical pathogens and the highest prioritization of pathogens due to increasing antibiotic resistance and significant threat to humans.15 Successful expansion of resistance clonal groups and frequent horizontal gene transfer (HGT) of carbapenemases harboring plasmids are causing increasing carbapenem resistance.16
Globally distributed in many bacterial genera, certain carbapenemases are associated with specific bacterial species and clonal groups. The international spread of KPC-producing K. pneumoniae has been linked to clonal complex 258(CC258) while blaNDM was the most prevalent carbapenemase gene in E. coli. Studies on blaKPC-harboring E. coli is limited in China. Interestingly, research in Singapore showed that blaKPC was the predominant carbapenemase type in E. coli whereas the prevalence of blaKPC was less than 3% in this study. The distribution of KPC-producing E. coli in our study, consistent with previous studies, was significantly different from that in Singapore, which may be related to geographic differences. ST131 was the dominant sequence type among the KPC-producing E. coli isolates in our study. Similar to the international spread of ST258 and its related variants in K. pneumoniae, ST131, the predominant E. coli lineage among extraintestinal E. coli isolates, was commonly reported to produce extended-spectrum β-lactamases and resistant to fluoroquinolones.17 It is noteworthy that the prevalence of E. coli ST131 among KPC-producing E. coli strains demonstrated greater dissemination potential, making it possible to become the predominant carbapenemase type in China in the future.
Transmission of the KPC carbapenemase can be mediated by the mobility of transposons, horizontal transfer of plasmids, and clonal spread.18 Bacteria and archaea possess defense systems such as CRISPR-Cas and restriction-modification (R-M) systems to protect microbes from infection by phages and other invading DNA such as plasmids. The CRISPR-Cas system in K. pneumoniae could effectively perturb the transfer of KPC plasmids, and the scarcity of the CRISPR-Cas system is a potential factor leading to the propagation of high-risk AMR linkages in K. pneumoniae CC258.19,20 Interestingly, in E. coli, CRISPR-Cas systems are unable to target blaKPC-producing plasmids and instead, it appears that the type I R-M system could impede blaKPC-carrying plasmid conjugation in E. coli. The absence of the type I R-M system in ST131 contributed to the dissemination of blaKPC plasmids in this clonal group.21
The global dissemination of KPC-producing K. pneumoniae CC258 is closely related to the epidemic IncF plasmids, contributing to the success of the high-risk linkage CC258–IncF, while IncN was the dominant plasmid type in KPC-producing E. coli. IncN plasmids are important drivers for the transmission of blaKPC between multiple bacterial species co-colonizing individual patients and environmental surfaces in several genomic studies.22–24 Compared with IncF plasmids, blaKPC-harboring IncN plasmids were more frequently identified in multiple bacteria from the same patients, demonstrating that IncN plasmids contributed to the inter-genera dissemination of the blaKPC genes. Interestingly, anti-restriction proteins encoded by ardA and ardB were identified in all or almost all sublineages of IncN plasmids, which may further facilitate their dissemination by inhibiting the function of the R-M system.25
This study collected E. coli isolates in 2017, and more strains isolated during different periods will be included to dynamically monitor the prevalence of blaKPC in E. coli. Novel defense systems, such as the BREX system, have been discovered against phages.26 Further studies will be performed to determine whether these novel defense systems could block blaKPC plasmid acquisition in E. coli.
Conclusion
The detection rate of blaKPC is lower in E. coli compared with K. pneumoniae. The dominant sequence and plasmid types of blaKPC-producing isolates differed between E. coli and K. pneumoniae. Further studies about the role of the defense system in acquisition of KPC-plasmids in E. coli will be performed to provide new insights into the low prevalence of blaKPC.
Ethics Approval
Clinically isolated E. coli was obtained from the biological sample and strain bank of the Institute of Antibiotics, Huashan Hospital, Shanghai, China. The ethics committee of Huashan Hospital approved this study. This study would not do harm to rights, benefits, or health of the participants.
Acknowledgments
We thank Dan Li and Pei Li at the Institute of Antibiotics for their assistance with the laboratory work. This work was supported by the Science and Technology Commission of Baoshan District (No. 20-E-2) and the Foundation of Shanghai Sixth People’s Hospital (grant number 20212511).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi:10.1038/nrmicro818
2. Roer L, Aarestrup FM, Hasman H, Gourse RL. The ecoki type I restriction-modification system in Escherichia coli affects but is not an absolute barrier for conjugation. J Bacteriol. 2015;197(2):337–342. doi:10.1128/JB.02418-14
3. Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi:10.1016/j.molmed.2012.03.003
4. Potter RF, Aw D, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat. 2016;29:30–46. doi:10.1016/j.drup.2016.09.002
5. Martínez-Martínez L, González-López JJ. Carbapenemases in Enterobacteriaceae: types and molecular epidemiology. Enfermedades Infecciosas y Microbiologia Clinica. 2014;32(Suppl 4):4–9. doi:10.1016/S0213-005X(14)70168-5
6. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161. doi:10.1128/AAC.45.4.1151-1161.2001
7. Papp-Wallace KM, Bethel CR, Distler AM, Kasuboski C, Taracila M, Bonomo RA. Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A beta-lactamase. Antimicrob Agents Chemother. 2010;54(2):890–897. doi:10.1128/AAC.00693-09
8. Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi:10.1016/j.tim.2014.09.003
9. Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. doi:10.1016/j.ebiom.2017.04.032
10. Marimuthu K, Venkatachalam I, Khong WX, et al. Clinical and molecular epidemiology of carbapenem-resistant Enterobacteriaceae among adult inpatients in Singapore. Arch Clin Infect Dis. 2017;64(suppl_2):S68–s75.
11. CLSI. Performance standards for antimicrobial susceptibility testing: twenty-ninth edition; 2019:M100.
12. European Committee on Antimicrobial Susceptibility Testing.EUCAST clinical breakpoint table. Available from: https://www.eucast.org/clinical_breakpoints/.
13. Petty NK, Ben Zakour NL, Stanton-Cook M, et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci USA. 2014;111(15):5694–5699. doi:10.1073/pnas.1322678111
14. The Review on Antimicrobial Resistance. Chaired by Jim O’Neill;2016.
15. De Oliveira DMP, Forde BM, Kidd TJ, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33(3): doi:10.1128/CMR.00181-19
16. Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Arch Clin Infect Dis. 2018;66(8):1290–1297. doi:10.1093/cid/cix893
17. Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27(3):543–574. doi:10.1128/CMR.00125-13
18. Munoz-Price LS, Quinn JP. The spread of Klebsiella pneumoniae carbapenemases: a tale of strains, plasmids, and transposons. Arch Clin Infect Dis. 2009;49(11):1739–1741. doi:10.1086/648078
19. Tang Y, Fu P, Zhou Y, et al. Absence of the type I-E CRISPR-cas system in Klebsiella pneumoniae clonal complex 258 is associated with dissemination of IncF epidemic resistance plasmids in this clonal complex. J Antimicrob Chemother. 2020;75(4):890–895. doi:10.1093/jac/dkz538
20. Zhou Y, Tang Y, Fu P, et al. The type I-E CRISPR-cas system influences the acquisition of bla(KPC)-IncF plasmid in Klebsiella pneumonia. Emerging Microbes Infect. 2020;9(1):1011–1022. doi:10.1080/22221751.2020.1763209
21. Li D, Li P, Peng M, et al. Transmission barrier of the blaKPC plasmid mediated by type I restriction-modification systems in Escherichia coli. J Antimicrob Chemother. 2022;77(4):952–956. doi:10.1093/jac/dkab489
22. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41(3):252–275. doi:10.1093/femsre/fux013
23. Weingarten RA, Johnson RC, Conlan S, et al. Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. mBio. 2018;9(1): doi:10.1128/mBio.02011-17
24. Hazen TH, Mettus R, McElheny CL, et al. Diversity among bla(KPC)-containing plasmids in Escherichia coli and other bacterial species isolated from the same patients. Sci Rep. 2018;8(1):10291. doi:10.1038/s41598-018-28085-7
25. Gomez-Simmonds A, Annavajhala MK, Tang N, et al. Population structure of blaKPC-harbouring IncN plasmids at a New York city medical centre and evidence for multi-species horizontal transmission. J Antimicrob Chemother. 2022;77(7):1873–1882. doi:10.1093/jac/dkac114
26. Goldfarb T, Sberro H, Weinstock E, et al. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 2015;34(2):169–183. doi:10.15252/embj.201489455
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.