Back to Journals » Journal of Pain Research » Volume 16
Modulation of Pain Sensitivity by a Hyperventilatory Breathing Exercise and Cold Exposure Training
Authors Zwaag J , Timmerman H , Pickkers P, Kox M
Received 6 December 2022
Accepted for publication 8 May 2023
Published 13 June 2023 Volume 2023:16 Pages 1979—1991
DOI https://doi.org/10.2147/JPR.S400408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Jelle Zwaag,1,2 Hans Timmerman,3,4 Peter Pickkers,1,2 Matthijs Kox1,2
1Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, the Netherlands; 2Radboud Center for Infectious Diseases (RCI), Radboud University Medical Center, Nijmegen, the Netherlands; 3University of Groningen, University Medical Center Groningen, Department of Anesthesiology, Pain Center, Groningen, the Netherlands; 4Department of Anesthesiology, Pain and Palliative Medicine, Radboud University Medical Center, Nijmegen, the Netherlands
Correspondence: Matthijs Kox, Radboud University Medical Center, Department of Intensive Care Medicine (710), Geert Grooteplein Zuid 10, PO Box 9101, Nijmegen, 6500 HB, the Netherlands, Email [email protected]
Background: Evidence indicates that healthy individuals who follow a training program comprised hyperventilatory breathing exercises and cold exposure can voluntarily activate their sympathetic nervous system and attenuate their systemic inflammatory response during experimental endotoxemia (intravenous administration of bacterial endotoxin). Furthermore, trained participants reported less endotoxemia-induced flu-like symptoms. However, it remained to be determined whether the effects on symptoms are due to the mitigated inflammatory response or involve direct analgesic effects of (elements of) the training program.
Methods: In the present study, we used Nijmegen-Aalborg Screening Quantitative sensory testing (NASQ) to objectively map pain sensitivity using non-invasive stimuli to address this question. First, NASQ parameters were evaluated in 20 healthy volunteers before, during, and after the conduct of the hyperventilatory breathing exercise. Second, NASQ measurements were performed before and after 48 healthy volunteers followed different modalities of the training program: breathing exercise training, cold exposure training, the combination of both, or no training. Lastly, NASQ measurements were performed in these 48 subjects during experimental endotoxemia.
Results: Electrical pain detection thresholds increased during the breathing exercise (p = 0.001) as well as four hours afterwards (p = 0.03). Furthermore, cold exposure training resulted in lower VAS scores during hand immersion in ice water (p < 0.001). Systemic inflammation induced by administration of endotoxin nullified the decreased pain perception during the ice water test in subjects trained in cold exposure.
Conclusion: A hyperventilatory breathing exercise decreases pain perception induced by an electrical stimulus. Furthermore, cold exposure training may decrease pain perception induced by hand immersion in ice water.
Keywords: pain thresholds, hyperventilation, breathing, cold exposure, inflammation, endotoxin
Introduction
Healthy volunteers who followed a training program comprising two different breathing exercises, cold exposure training, and meditation are able to activate their sympathetic nervous system voluntarily, reflected by high epinephrine levels.1 This results in attenuation of the inflammatory response during experimental endotoxemia, a standardized in vivo model of systemic inflammation induced by intravenous administration of bacterial endotoxin.1 A recent detailed analysis of the different components in this training program revealed that the combination of cold exposure with one of the breathing exercises is responsible for these effects.2 This training program, or elements of it, could translate into a novel treatment modality for patients with inflammatory conditions.
Strikingly, next to lower levels of pro-inflammatory cytokines, the subjective self-reported flu-like symptoms during endotoxemia were attenuated in trained volunteers.1,2 This is a significant finding, as subjective and patient-reported outcome measures (PROM) are important end-points in clinical studies into inflammatory diseases such as rheumatoid arthritis3 and inflammatory bowel disease (IBD).4 However, it is currently unclear whether the effects on symptoms observed in our previous studies1,2 are either a consequence of the mitigated inflammatory response, or involve direct analgesic effects caused by the training program. Moreover, objectifying signs and symptoms of people (either patients or healthy volunteers) that experience pain and discomfort has proved to be as difficult as it is relevant.5 Complaints of pain are inherently subjective, thus standardizing the manner in which the perception of pain is objectified is of pivotal importance.6
Different efforts to protocolize and standardize pain measurements are reported under the general term `Quantitative Sensory Testing` (QST). The Nijmegen-Aalborg Screening QST (NASQ) was developed to map pain sensitivity at multiple body locations by non-invasive stimuli.7,8 In recent years, this NASQ battery has been optimized and calibrated for use in clinical practice as well as in research, for instance in patients with head and neck pain;9 breast cancer,10 neuropathic pain,11 and in healthy volunteers.12
In the present study, we investigated whether different elements of the aforementioned training program alter pain perception assessed by NASQ measurements. We hypothesized that elements of the training program decrease pain perception.
Methods
This manuscript describes NASQ measurements performed during two studies recently reported on.2 These studies primarily focused on the contribution of the different components of the aforementioned training program on circulating epinephrine levels and inflammatory parameters.2 In the first study (breathing exercises study), NASQ measurements were performed before, during, and after the conduct of one of the breathing exercises described further below (and in).2 In the second study (experimental human endotoxemia study), NASQ parameters were evaluated before and after participants followed a training program involving both a breathing exercise and exposure to cold in four different modalities: participants were randomized to be trained in the breathing exercise, only in cold exposure, the combination of both, or to a control group that did not receive any training (see below).2 Furthermore, in the context of this latter study, NASQ parameters were evaluated during experimental endotoxemia, in which all participants of the four training groups took part (see below).2 Previous data from our group revealed that the systemic inflammatory response induced in this model results in decreased pain thresholds,13 and we explored whether the different training modalities influenced this effect.
Ethics Approval
All procedures were approved by the local ethics committee of the Radboud university medical center (CMO Arnhem-Nijmegen, reference and trial registration numbers are provided in the corresponding sections below) and were conducted in accordance with the declaration of Helsinki including current revisions and Good Clinical Practice guidelines. All participants provided written informed consent to participate in the study and were screened before the start of the experiment to confirm a normal physical examination, electrocardiography, and routine laboratory values. Exclusion criteria were: prior experience with breathing, meditation, or cold exposure exercises, including mindfulness, yoga and exposure to cold showers, frequent visits to sauna facilities (more than once per month), use of any medication, smoking, previous spontaneous vagal collapse, use of recreational drugs within 21 days prior to the start of the training program, surgery or trauma with significant blood loss or blood donation, hospital admission or surgery with general anesthesia, participation in another study within three months prior to the experimental day, or clinically significant acute illness and/or infections within four weeks before the start of the training program.
Breathing Exercises Study
After ethics approval (reference number: 2014–1374/NL51237.091.14), 40 males provided written informed consent to participate in a prospective randomized study registered at https://clinicaltrials.gov/ (NCT02417155). The study was carried out in the research department of the Radboud university medical center from December of 2014 to February of 2015. An extensive description of the methods is described elsewhere.2 A schematic overview of the study procedures is depicted in Figure 1. During the informed consent procedure, all participants were verbally familiarized with the different training procedures that were part of the current study and subsequently gave consent to participate in any of the four groups ahead of randomization. Participants were randomized to four different groups (n = 10 per group) by an independent research nurse using the sealed envelope method: extensive training by the creator of the intervention, extensive training by an independent trainer, short training by the creator of the intervention, and short training by an independent trainer. All participants were trained in the week before the experiment day. NASQ assessments described in the current manuscript were performed in a subset of 20 volunteers who were trained in a breathing exercise that consisted of hyperventilation for an average of 30 breaths using deep and powerful breaths (“hyperventilation phase”) followed by exhalation breath holding for approximately two minutes (“retention phase”). The duration of breath retention was entirely at the discretion of the participant. Breath retention was followed by a deep inhalation breath, that was held for 10 seconds. Subsequently, a new cycle of hyper/hypoventilation began.
A total of five NASQ assessments were performed. The first NASQ assessment was performed after inclusion and randomization but before the start of the training program (NASQPRE, Figure 1). Furthermore, on a separate day, also after randomization but before the start of the training program, two NASQ assessments were performed which were time-matched to those performed on the experiment day (Figure 1). The first NASQ assessment on the control day (NASQCON1) was time-matched with the first NASQ assessment on the experiment day (NASQDuBR, at approximately 10 am), which was performed during execution of the breathing exercise. The second NASQ assessment on the control day (NASQCON2) was time-matched with the second NASQ assessment on the experiment day (NASQAfBR, at approximately 3 pm), which was performed 4 hours after cessation of the breathing exercise. On the experiment day, participants practiced the breathing technique for 1.5 hours and NASQ assessment was started one hour following start of the breathing exercise and lasted 30 minutes. Due to technical issues, the NASQ assessment could not be analyzed in one participant of the breathing exercises studies. Data of all groups were combined, resulting in a total of 19 participants for the breathing exercises study. We analyzed these data together, as the increase in epinephrine levels in the breathing exercises study was similar in all four groups.2 Also, no between-group differences were observed in any of the NASQ parameters (data not shown).
Experimental Human Endotoxemia Study
After ethics approval (reference number 2016–2312/NL56686.091.16), 48 males provided written informed consent to participate in this prospective randomized controlled study registered at https://clinicaltrials.gov/ (NCT03240497). The study was carried out in the research department of the Radboud university medical center from April to June of 2016. An extensive description of the methods is described elsewhere.2 A schematic overview of the study is depicted in Figure 1. During the informed consent procedure, all participants were verbally familiarized with the different training procedures that were part of the current study and subsequently gave consent to participate in any of the four groups ahead of randomization. We employed a 2 by 2 design, in which 48 participants were randomized by an independent research nurse using the sealed envelope method to 4 different groups (n = 12 per group): cold exposure (CEX), breathing exercise (BRT), cold exposure and the breathing exercise (CBR), and a control group (CG). Participants of all groups except the control group were trained in the week leading up to the endotoxemia experiment day (further detailed in section `endotoxemia procedures` below). Briefly, the participants in the CEX group followed an intensive 4-day cold exposure training program, consisting of standing in snow with bare feet for up to 30 minutes, lying in snow in shorts for up to 20 minutes, and sitting and swimming in ice-cold water for up to 3 minutes (see video material accompanying our previous publication).2 Furthermore, participants were instructed to end their daily shower with a period of 60 seconds of cold water until the endotoxemia experiment day. Participants in the BRT group were trained in the breathing exercise as described in the previous subsection, but without the prolonged breath retention phase. Instead, participants held their breath for only 10 seconds, during which all body muscles were tightened, after which a new cycle of hyperventilation was initiated. We used this exercise as we showed that it is equally effective in increasing plasma epinephrine levels (the main driver of the anti-inflammatory effects) as the exercise with prolonged retention,2 is easier to learn, and potentially safer. Participants randomized to the CBR group followed both cold and breathing exercise training procedures and participants in the control group did not receive any training.
In total, four NASQ assessments were performed during this study. One NASQ assessment after inclusion but before randomization and before training (BeTR). On the endotoxemia experiment day, the first NASQ assessment was performed one hour before endotoxin administration (T-1). The second NASQ assessment was timed two hours after endotoxin administration (T2), this was during execution of the breathing exercise. The third NASQ assessment was timed six hours after administration of endotoxin (T6), 3.5 hours after cessation of the breathing exercise.
All participants, regardless of the randomization, underwent experimental endotoxemia at the research unit of the Intensive Care department of the Radboud university medical center according to our standard protocol14 also used in our previous studies into this intervention.1,2 Participants refrained from caffeine and alcohol 24 hours before the experiment, and refrained from any intake of food and drinks 10 hours before the experiment. Fasting was maintained until 4.5 hours after administration of endotoxin. A cannula was placed in the antecubital vein of the non-dominant arm for hydration and the radial artery of the same arm was cannulated under local anesthesia using a 20-gauge arterial catheter for continuous arterial monitoring of vital signs. Purified endotoxin (derived from Escherichia coli O:113, Clinical Center Reference Endotoxin) obtained from the Pharmaceutical Development Section of the National Institutes of Health (Bethesda, MD, USA) and supplied as a lyophilized powder, was reconstituted in 5 mL saline 0.9% for injection and vortex-mixed for 20 minutes before being administered as an intravenous bolus at a dose of 2 ng/kg body weight.
Nijmegen-Aalborg Screening Quantitative Sensory Testing Measurements
The measurements of pressure pain threshold (PPT), electrical pain detection threshold (EPDT), electrical pain tolerance threshold (EPTT) and conditioned pain modulation (CPM) test are extensively described7,8 and visualized.11 All measurements were conducted in a stimulus-poor room in our university hospital with a constant temperature (20.5–22°C) and humidity (with a set-point of 6 g/Kg that results in a relative humidity of 45–55%). Measurements were performed by two researchers (HT and JvG), both extensively trained in NASQ measurements. The musculus deltoideus was marked as the training site to help the participants to get used to the assessments via pressure algometry as well as electrical threshold assessment. The following areas were marked bilaterally as test sites: the musculus rectus femoris (15 cm above the patellar ridge), the musculus trapezius (pars medialis, level Th3), the thenar muscle, the musculus abductor hallucis. A description of the specific test sites used for each measurement is provided below.
PPT
Pressure pain threshold (PPT) was measured on both the left and the right side of the body at the m. deltoideus, m. rectus femoris, thenar and m. abductor hallucis. The measurements were first performed on the training site (m.deltoideus), and secondly on the study sites (directly on the specific muscle). Pressure was manually delivered with the pressure algometer (Wagner instruments, Force TENTM Digital Force Gage FDX 50, Greenwich, CT, USA) with a 1.0 cm2 probe under a 90° angle. A ramping rate of ~5 Newton(N)/s was used by manually adjusting the applied pressure based on visual feedback using the display of the pressure algometer. Pressure was started at 0 N and applied up to a maximum of 250 N for safety purposes. The participants were instructed to say “stop” when they felt a burning, painful or stitching sensation alongside the feeling of pressure. The participants were asked to rate the associated pain on a VAS scale using a 10 cm line printed on A4 paper. Test pressure values and VAS scores were noted on a sheet while making sure the participants could not read the values of the measurements during the execution of the tests. The PPT measured with a pressure algometer showed a good test–retest (r = 0.88) and interobserver reliability (r = 0.84).15 In healthy individuals, the ICC values showed excellent reliability (ICC = 0.74) on the thenar.16 In another study including healthy volunteers, intra-rater reliability was shown to be excellent (ICC > 0.9).17 In a recently conducted systematic review, test-retest ICC for the VAS scale was 0.77–0.90.18
EPDT
Electrical pain detection threshold (EPDT) was measured on the m. rectus femoris and m. trapezius test sites. A QST stimulator (QST-III; JNI Biomedical ApS, Klarup Denmark) was used to obtain the electrical pain detection threshold. The QST stimulator delivers tetanic stimulation at 100 Hz with 0.2 ms square waves. The ramping rate was set to 1 mA/s. The initial current was set to 0 mA, the maximum current was automatically set to 50 mA for safety purposes. The participants were instructed to press the power button to start the flow of current and to release the button at the moment the sensation started to be painful and annoying. At each site, three measurements were taken, allowing at least 15 seconds in between measurements to avoid windup effects. Electrical values and VAS scores were noted on a sheet while making sure the participants could not read the values of the measurements during the execution of the tests. The mean value for each EPDT test location was calculated. Data on the reliability of EPDT testing in healthy volunteers are, to our knowledge, not available. In patients with painful chronic pancreatitis, the test-reliability was poor in pancreatic viscerotomes (ICC 0.15–0.43).19
EPTT
Electrical pain tolerance threshold (EPTT) was measured on the m. rectus femoris of the non-dominant leg. The same QST stimulator was used as described for the EPDT measurement above. The participants were instructed to press the power button to start the flow of current and release the button at the moment the feeling was the maximum tolerable pain. Again, electrical values and VAS scores were noted on a sheet while making sure the participants could not read the values of the measurements during the execution of the tests. In patients with painful chronic pancreatitis, the ICC was shown to be fair: 0.48–0.49.19
CPM (`Ice Water Test`)
Conditioned pain modulation (CPM) was measured using a bucket of water with melting ice with a target temperature range of one to four degrees Celsius. Throughout the experiment, a temperature probe was used to check if the water was at the intended target temperature. Ice was added if necessary. The PPT and the EPTT measurements described above were used as a preconditioning test stimulus. The participants were asked to immerse one hand into the ice water in the bucket until the wrist and with the fingers spread without touching the wall or bottom of the bucket. The participants were told to remove their hand from the water after three minutes of immersion or sooner if the pain became intolerable. During the immersion, the participants were asked to rate the pain on a 0 to 100 scale on a 10 cm line printed on A4 paper every 10 seconds, in which 0 represents no pain and 100 unbearable pain. Finally, the PPT and EPTT measurements were performed again directly after taking the hand out of the ice water bucket to determine test stimulus post-conditioning. CPM was calculated by using the difference of the pre- and postconditioning measurements as a proportion of the preconditioning measurement expressed as a percentage (post-conditioning minus preconditioning/preconditioning)*100%. Using the combination of the PPT handheld algometer and ice water stimuli to assess the CPM, is one of the most reliable methods described, with a modest test–retest reliability (ICC = 0.49; coefficient of variation = 63.6%).20
Statistical Analysis
As this manuscript describes secondary endpoints of two studies that primarily focused on the contribution of the different components of the aforementioned training program on circulating epinephrine levels and inflammatory parameters,2 the study was not formally powered for the NASQ endpoints.
For PPT and EPDT data, the median value of pain pressure threshold in Newton or electrical stimulus threshold in milliampere (mA) was calculated from the values obtained at each measurement site for each participant. During all ice water tests in both studies, the majority of participants reached the maximum time of 180 seconds immersion in ice water, rendering comparisons between average times of little value. As such, the proportions of participants reaching the maximum time were compared. For ice water VAS, the highest levels of pain reported by each individual participant during the ice water test were used for analysis. Group data are presented as median [interquartile range], means ± standard error of the mean (SEM) or number (%). Differences were analyzed using Fisher exact tests, paired t-tests, repeated measures one-way analysis of variance (ANOVA) followed by Tukey post-hoc tests, or Kruskal–Wallis tests. Pearson correlation was used. Bonferroni correction was applied to adjust for multiple testing. Analyses were performed using Graphpad Prism V5.03 (Graphpad Software, San Diego, CA, USA) and SPSS V25.0.0.1 (IBM Corp, Armonk, New York, USA).
Results
Participant Characteristics
Baseline characteristics of participants who were included in the breathing exercises and endotoxemia studies are listed in Table 1. Flow diagrams of both studies are provided in Supplemental Figures 1 and 2. Due to technical issues, the NASQ assessment could not be analyzed in one participant of the breathing exercises study. Data of all randomization groups in the breathing exercise study were combined, resulting in a total of 19 participants. We analyzed data of all groups together, as the increase in epinephrine levels in the breathing exercises study was similar in all four groups.2 Also, no between-group differences were observed in any of the NASQ parameters (data not shown).
 |
Table 1 Demographic Characteristics |
Learning Effects
Learning effects were assessed in the breathing exercise study by comparing NASQPRE with NASQCON1 (Figure 1). No significant differences between these measurements for any of the NASQ parameters were present (Table 2).
 |
Table 2 Learning Effects During Breathing Exercises Study |
Acute and Prolonged Effects of the Breathing Exercise on NASQ Parameters
The effects of acute and prolonged effects of the breathing exercise on pain perception in the absence of systemic inflammation were evaluated in the breathing exercise study by comparing NASQCON1 with NASQDuBR and NASQCON2 with NASQAfBR, respectively (Figure 1).
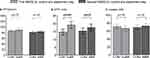
For pressure pain thresholds (PPT), no differences were observed (Figure 2A). However, for electrical pain detection thresholds (EPDT), a significant increase in EPDT was observed both between NASQCON1 (14.6±1.9 mA) and NASQDuBR (19.0±2.2 mA, p = 0.001, Figure 2B), and between NASQCON2 (15.2±2.0 mA) and NASQAfBR (17.5±2.2 mA, p = 0.03, Figure 2B). To explore possible mechanisms behind this effect, we correlated the sharp increases in plasma epinephrine levels and pH observed during the breathing exercises2 with the increases in EPDT. No such relationships were identified (data not shown). For the ice water test, no significant differences were found in the proportion of participants that reached the maximum time of 180 seconds neither during the breathing exercise (NASQcon1 84% vs NASQDuBR 74%, p = 1.00), nor after the breathing exercise (NASQcon2 89% vs NASQAfBR 79%, p = 1.00). The peak pain score (VAS) during the ice water test was also similar between the different measurements (Figure 2C). Finally, conditioned pain modulation (CPM) parameters showed no change between the measurements, both for pressure and electrical stimulation (data not shown).
Influence of the Different Training Regimens Employed in the Human Endotoxemia Study on NASQ Parameters
The influence of the different training modalities used in the endotoxemia study (ie, cold exposure [CEX], breathing exercise [BRT], cold exposure and breathing exercise [CBR], and the control group [CG]) on NASQ parameters were evaluated. NASQBeTR and NASQT-1 measurements were compared, reflecting changes in pain perception caused by the training program, before induction of human endotoxemia (Figure 1).
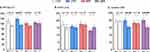
For PPT, significantly lower PPT values post-training were observed in the CEX group (118.6±6.7 N/cm2 vs 94.8±6.8 N/cm2, p = 0.01), whereas no significant differences were found in the other groups (Figure 3A). For EPDT, no differences were observed in any of the groups (Figure 3B). Likewise, for the ice water test, no significant differences were found in the proportion of participants who reached the maximum time (CG: 33.3% vs 16.7%, p = 1.00; CEX: 66.7% vs 81.8%, p = 1.00; BRT: 41.7% vs 50.0%, p = 1.00; CBR: 58.3% vs 83.3%, p = 0.71). No effects of the training were observed for the highest reported pain score (VAS) during the ice water test in the control group (76.7±4.7 vs 77.2±4.1, p = 1.00) or the BRT group (72.0±7.5 vs 70.6±8.3, p = 1.00, Figure 3C). However, significantly lower VAS scores were reported in both cold exposure groups after training (CEX group: 78.9±6.8 vs 61.0±5.8, p = 0.04; CBR group: 79.6±5.6 vs 59.8±5.7, p < 0.001, Figure 3C). CPM parameters again showed no change between the measurements, both for pressure and electrical stimulation (data not shown).
NASQ Parameters During Experimental Endotoxemia in the Different Training Groups
To assess the effects of endotoxemia on pain perception as well as the possible modulating effects of the different training modalities and, for the BRT and CBR groups, performing the learned breathing exercise, NASQT-1, NASQT2 and NASQT6 measurements were compared within the different groups (Figure 1).
In all groups, statistically significant lower values of PPT were found during human endotoxemia (CG p < 0.001, CEX p = 0.03, BRT p < 0.001, CBR p < 0.001, Figure 4A). Post-hoc testing revealed significant differences in all groups at time-points 2 and 6 hours (Figure 4A). For EPDT, no statistically significant changes were observed over time in the CG (p = 0.52) or BRT (p = 0.32) groups. However, significant changes were found in the CEX (p = 0.04, post-hoc: increase at 6 hours post-endotoxin) as well as the CBR group (p = 0.004, post-hoc: decrease at 2 hours post-endotoxin, Figure 4B).
For the ice water test, no differences in the percentage of participants that reached the maximum time of 180 seconds were observed in all groups across timepoints T-1, T2 and T6 (CG: 16.7%, 0%, 8.3%, p = 0.34; CEX: 75.0%, 58.3%, 75.0%, p = 0.59; BRT 50.0%, 25.0%, 50.0%, p = 0.36; CBR: 83.3%, 66.7%, 83.3%, p = 0.53). Across all timepoints, 8.3%, 69.4%, 41.7%, and 77.8% of participants reached 180 seconds in the CG, CEX, BRT, and CBR group, respectively (p = 0.005). Nevertheless, peak VAS increased significantly over time in the CEX (p = 0.01, post-hoc: increase at both 2 and 6 hours post-endotoxin) and CBR groups (p = 0.02, post-hoc: increase at 2 hours post-endotoxin), whereas no significant changes were observed in the CG (p = 1.00) or BRT (p = 1.00) groups (Figure 4C). Again, CPM parameters showed no significant changes over time throughout the endotoxemia experiment (data not shown).
Discussion
In this study, we first investigated the effects of a breathing exercise which was previously shown to result in profoundly increased plasma epinephrine levels2 on pain perception in healthy volunteers. Our results indicate that the electrical pain threshold is increased during and several hours after this exercise, which is characterized by cycles of vigorous hyperventilation and prolonged breath retention. In the second part of this study, we evaluated the effects of different training regimens involving combinations of a similar breathing exercise and cold exposure on pain perception before and during experimental human endotoxemia, a standardized controlled model of systemic inflammation. Both training regimens that involve cold exposure resulted in lower pain perception during an ice water test before induction of endotoxemia. Systemic inflammation lowered the pressure pain threshold, whereas it nullified the decreased pain perception during the ice water test in participants trained in both regimens involving cold exposure.
No learning effects on any of the measured parameters were observed when performing repeated NASQ measurements in the absence of an intervention in the “breathing exercise study” part of the current investigation. These results are in line with earlier studies on the short-term test-retest reliability of specific QST parameters.21 Also, the absence of a learning effect is further substantiated by the lack of differences between the first two NASQ measurements in the control group of the endotoxemia study, in which no intervention was applied as well. Collectively, these findings indicate that the results of our analyses into the effects of the different interventions can be interpreted without considering a relevant test-retest effect. This is an important finding, as not every QST battery has this property. For instance, in male patients with chronic pain, reproducibility of QST parameters was found to be insufficiently stable over a period of 10 days to be used in a clinical setting.22
In the breathing exercise study, an increase in electrical pain threshold was observed during the conduct of the breathing exercise. Furthermore, this effect persisted for several hours after cessation of the breathing exercise. These findings are in line with an earlier study on the effects of voluntary breathing, in which an increase in electrical pain threshold was found in a group of healthy volunteers that was instructed to take effortful deep and fast inhalations.23 The mechanism behind this analgesic effect may involve pathways that are generally attributed to exercise-induced hypoalgesia:24 activation of the endogenous opioid system and the autonomic nervous system. First, a direct effect on the endogenous opioid system, specifically an increase in the analgesic nociceptin/orphanin levels, has been documented during vigorous hyperventilation.25 However, in the present study, we found no relationship between increased blood pH levels (as a measure of the extent of hyperventilation) and the increase in electrical pain thresholds. Second, activation of the autonomic nervous system may be involved; indeed, breathing exercise was previously shown to result in a strong increase in blood levels of epinephrine.2 Epinephrine and other catecholamines are associated with an analgesic effects in the complex network of central pathways in the brain26 and pain modulation effects.27 Furthermore, in the above described study on the effects of vigorous hyperventilation, a relationship between norepinephrine and nociceptin/orphanin levels was identified.25 However, similar as for pH, we found no correlation between increased epinephrine levels and changes in electrical pain thresholds. The absence of such correlations could also be due to a relatively low group size or a saturation effect (eg, every subject was able to reach high pH and epinephrine levels).
When assessing the effects of the different training regimens used in the endotoxemia study, we observed a decrease in pressure pain threshold (PPT) before induction of endotoxemia in the group that was trained only in cold exposure. We do not have a clear explanation for this effect; furthermore, it was not observed in the group that was trained in both cold exposure and the breathing technique. Nevertheless, a consistent decrease in pain perception (VAS score) induced by cold exposure during the ice water test was found post-training in both cold exposure groups, whereas no change in the proportion of participants reaching the maximum immersion time of 180 seconds was present. This is in line with studies showing cold acclimation effects after repeated exposure to cold on several other parameters, including less discomfort and cold sensation as well as greater heat retention and possible improvements in cognitive performance.28–30
In all groups, the pressure pain detection threshold decreased during human endotoxemia, indicating a hyperalgesic change of pain perception during systemic inflammation. These results corroborate earlier work of our group, in which pressure and electrical pain thresholds were significantly decreased two hours after endotoxin administration.13 No effects of any of the training regimens on pressure pain threshold (PPT) during endotoxemia were observed. Furthermore, the increase in electrical pain threshold (EPDT) observed in the breathing exercise study could not be recapitulated during the endotoxemia study, as both groups that were trained in the breathing exercise (ie, the BRT and CBR groups) did not display an increase during endotoxemia. We speculate that the hyperalgesic effects of endotoxemia as found in the current as well as our previous study13 override the potential analgesic effects elicited by the hyperventilation exercise. This may be supported by the fact that clinical effects of endotoxemia are very dominant, for instance represented by a development of fever and significant flu-like symptoms.13 Alternatively, a small difference in the breathing exercise employed between the studies (ie, the lack of prolonged breath retention in the endotoxemia study) may be involved, which could implicate a role for hypoxia in modulation of the electrical pain threshold, as epinephrine induction by both exercises was similar.2 Along these lines, hypoxia itself has been linked to hyperalgesia either through hormonal or inflammatory mediators,31 for instance in patients with nocturnal hypoxia due to sleep apnea.32
In both groups that underwent cold exposure training, several changes in electrical pain thresholds were observed during endotoxemia. However, the two groups showed a contradicting pattern. After administration of endotoxin, in the CEX group, EPDT values were higher after 6 hours, whereas values in the CBR group were lower after 2 hours. As such, it is difficult to draw conclusions on the effects of cold exposure training on electrical pain thresholds. Also, in these two `cold exposure` groups, the reported VAS score during the ice water test increased following endotoxin administration. Conceivably, the systemic inflammatory response nullified the training-induced effects observed before endotoxin administration (ie, lower VAS scores compared with the pre-training measurement in these two groups, see above), resulting in a significant increase afterwards. Despite this increase in VAS, a larger proportion of participants of these two groups reached the maximum ice water immersion period of 180 seconds across all timepoints on the endotoxemia experiment day, possibly indicating that participants in these groups were able to endure these higher VAS scores for a longer period of time.
A strength of the current work is that, compared with other QST studies that employed specific tests/elements, we used NASQ, a comprehensive battery of QST measurements used extensively in other studies.9–12 Our study is limited by the fact that we only included male participants. This may be of relevance, because within the spectrum of QST parameters, the largest effect sizes for differences in gender were found in cold-induced pain and pain to blunt pressure.33 However, as human endotoxemia experiments are very costly and labor-intensive studies, and for ethical reasons (we want to expose as few volunteers as possible to endotoxemia), we only include male participants in virtually all of these studies. Another limitation is the potential to extrapolate the findings to clinical practice, as we studied healthy volunteers. For instance, exercise-induced hypoalgesia observed in pain-free adults may have opposing hyperalgesic effects in patients with chronic pain.24 However, we feel this work has important value in the translation to clinical practice.
Conclusions
In conclusion, a breathing exercise characterized by cycles of vigorous hyperventilation and prolonged breath retention decreases pain perception induced by an electrical stimulus. Furthermore, training in cold exposure may decrease pain perception induced by hand immersion in ice water. Whether these effects translate to beneficial effects in patients, for instance those suffering from autoimmune diseases, remains to be determined.
Data Sharing Statement
Data will be shared by the corresponding author Dr Matthijs Kox upon reasonable request and in accordance with the General Data Protection Regulation (GDPR).
Acknowledgments
The authors would like thank Jacky van Gemert for help with the QST measurements.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was internally funded by Department of Intensive Care Medicine, Radboud university medical center, Nijmegen, the Netherlands.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kox M, van Eijk LT, Zwaag J, et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A. 2014;111(20):7379–7384. doi:10.1073/pnas.1322174111
2. Zwaag J, Naaktgeboren R, van Herwaarden AE, Pickkers P, Kox M. The effects of cold exposure training and a breathing exercise on the inflammatory response in humans: a pilot study. Psychosom Med. 2022;84(4):457–467. doi:10.1097/PSY.0000000000001065
3. Hiligsmann M, Rademacher S, Kaal KJ, Bansback N, Harrison M. The use of routinely collected patient-reported outcome measures in rheumatoid arthritis. Semin Arthritis Rheum. 2018;48(3):357–366. doi:10.1016/j.semarthrit.2018.03.006
4. de Jong MJ, Huibregtse R, Masclee AAM, Jonkers D, Pierik MJ. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16(5):648–663 e3. doi:10.1016/j.cgh.2017.10.019
5. Cowen R, Stasiowska MK, Laycock H, Bantel C. Assessing pain objectively: the use of physiological markers. Anaesthesia. 2015;70(7):828–847. doi:10.1111/anae.13018
6. Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi:10.1093/bja/aen103
7. Wilder-Smith O. A paradigm-shift in pain medicine: implementing a systematic approach to altered pain processing in everyday clinical practice based on quantitative sensory testing [DoctoralThesis]. Aalborg University; 2013. https://vbn.aau.dk/files/103432008/DoctoralThesis_Oliver_Wilder_Smith.pdf.
8. Timmerman H, Wilder-Smith O, van Weel C, Wolff A, Vissers K. Detecting the neuropathic pain component in the clinical setting: a study protocol for validation of screening instruments for the presence of a neuropathic pain component. BMC Neurol. 2014;14:94. doi:10.1186/1471-2377-14-94
9. Chua NHL, Timmerman H, Vissers KC, W-S O. Multi-modal quantitative sensory testing in patients with unilateral chronic neck pain: an exploratory study. J Musculoskelet Pain. 2012;20(4):292–299. doi:10.3109/10582452.2012.733803
10. Timmerman H, Wilder-Smith OH, Steegers MA, Vissers KC, Wolff AP. The added value of bedside examination and screening QST to improve neuropathic pain identification in patients with chronic pain. J Pain Res. 2018;11:1307–1318. doi:10.2147/JPR.S154698
11. van Helmond N, Timmerman H, Olesen SS, et al. A quantitative sensory testing paradigm to obtain measures of pain processing in patients undergoing breast cancer surgery. J Vis Exp. 2018;131. doi:10.3791/56918
12. Van Boekel RLM, Timmerman H, Bronkhorst EM, Ruscheweyh R, Vissers KCP, Steegers MAH. Translation, cross-cultural adaptation, and validation of the pain sensitivity questionnaire in Dutch healthy volunteers. Pain Res Manag. 2020;2020:1050935. doi:10.1155/2020/1050935
13. de Goeij M, van Eijk LT, Vanelderen P, et al. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One. 2013;8(12):e84159. doi:10.1371/journal.pone.0084159
14. van Lier D, Geven C, Leijte GP, Pickkers P. Experimental human endotoxemia as a model of systemic inflammation. Biochimie. 2018;159:99–106. doi:10.1016/j.biochi.2018.06.014
15. Geber C, Klein T, Azad S, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152(3):548–556. doi:10.1016/j.pain.2010.11.013
16. Marcuzzi A, Wrigley PJ, Dean CM, Adams R, Hush JM. The long-term reliability of static and dynamic quantitative sensory testing in healthy individuals. Pain. 2017;158(7):1217–1223. doi:10.1097/j.pain.0000000000000901
17. Jayaseelan DJ, Cole KR, Courtney CA. Hand-held dynamometer to measure pressure pain thresholds: a double-blinded reliability and validity study. Musculoskelet Sci Pract. 2021;51:102268. doi:10.1016/j.msksp.2020.102268
18. Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: a systematic review. J Pain. 2019;20(3):245–263. doi:10.1016/j.jpain.2018.07.009
19. Olesen SS, van Goor H, Bouwense SA, Wilder-Smith OH, Drewes AM. Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg Anesth Pain Med. 2012;37(5):530–536. doi:10.1097/AAP.0b013e3182632c40
20. Imai Y, Petersen KK, Morch CD, Arendt Nielsen L. Comparing test-retest reliability and magnitude of conditioned pain modulation using different combinations of test and conditioning stimuli. Somatosens Mot Res. 2016;33(3–4):169–177. doi:10.1080/08990220.2016.1229178
21. Gehling J, Mainka T, Vollert J, Pogatzki-Zahn EM, Maier C, Enax-Krumova EK. Short-term test-retest-reliability of conditioned pain modulation using the cold-heat-pain method in healthy subjects and its correlation to parameters of standardized quantitative sensory testing. BMC Neurol. 2016;16:125. doi:10.1186/s12883-016-0650-z
22. Martel MO, Wasan AD, Edwards RR. Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Med. 2013;14(11):1757–1768. doi:10.1111/pme.12220
23. Li S, Berliner JC, Melton DH, Li S. Modification of electrical pain threshold by voluntary breathing-controlled electrical stimulation (BreEStim) in healthy subjects. PLoS One. 2013;8(7):e70282. doi:10.1371/journal.pone.0070282
24. Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain. 2019;20(11):1249–1266. doi:10.1016/j.jpain.2019.03.005
25. Fontana F, Pizzi C, Bernardi P, Pich EM, Bedini A, Spampinato S. Plasma nociceptin/orphanin FQ levels in response to the hyperventilation test in healthy subjects. Peptides. 2010;31(4):720–722. doi:10.1016/j.peptides.2010.01.005
26. Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi:10.1016/s0301-0082(02)00009-6
27. Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience. 2016;338:93–113. doi:10.1016/j.neuroscience.2016.05.057
28. Jones DM, Bailey SP, De Pauw K, et al. Evaluation of cognitive performance and neurophysiological function during repeated immersion in cold water. Brain Res. 2019;1718:1–9. doi:10.1016/j.brainres.2019.04.032
29. Brazaitis M, Eimantas N, Daniuseviciute L, Baranauskiene N, Skrodeniene E, Skurvydas A. Time course of physiological and psychological responses in humans during a 20-day severe-cold-acclimation programme. PLoS One. 2014;9(4):e94698. doi:10.1371/journal.pone.0094698
30. Castellani JW, Young AJ. Human physiological responses to cold exposure: acute responses and acclimatization to prolonged exposure. Auton Neurosci. 2016;196:63–74. doi:10.1016/j.autneu.2016.02.009
31. Kaczmarski P, Karuga FF, Szmyd B, et al. The role of inflammation, hypoxia, and opioid receptor expression in pain modulation in patients suffering from obstructive sleep apnea. Int J Mol Sci. 2022;23(16):9080. doi:10.3390/ijms23169080
32. Terzi R, Yilmaz Z. Evaluation of pain sensitivity by tender point counts and myalgic score in patients with and without obstructive sleep apnea syndrome. Int J Rheum Dis. 2017;20(3):340–345. doi:10.1111/1756-185X.12629
33. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi:10.1016/j.pain.2006.01.041
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.