Back to Journals » Clinical Ophthalmology » Volume 16
Modified Microneedle for Suprachoroidal Injection of Triamcinolone Acetonide Combined with Intravitreal Injection of Ranibizumab in Branch Retinal Vein Occlusion Patients
Authors Nawar AE
Received 17 February 2022
Accepted for publication 28 March 2022
Published 19 April 2022 Volume 2022:16 Pages 1139—1151
DOI https://doi.org/10.2147/OPTH.S361636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Supplementary video of "Suprachoroidal injection of TA in BRVO patients" [ID 361636].
Views: 2321
Amin E Nawar
Ophthalmology Department, Faculty of Medicine, Tanta University, Tanta, Egypt
Correspondence: Amin E Nawar, Ophthalmology Department, Faculty of Medicine, Tanta University, Tanta, 31516, Egypt, Tel +20 1140095692, Email [email protected]
Purpose: The present study evaluated the efficacy of combined suprachoroidal injection of triamcinolone acetonide (TA) using a modified microneedle with intravitreal injection of ranibizumab in branch retinal vein occlusion (BRVO) patients.
Patients and methods: This is a prospective randomised interventional study that was conducted on 60 eyes of 60 patients with non ischemic BRVO. Patients were divided in two groups, group (1) 30 patients who received intravitreal injection of 0.05 mL (0.5 mg) of ranibizumab, group (2) included 30 patients who received baseline combined intravitreal injection of 0.05 mL (0.5 mg) of ranibizumab and suprachoroidal injection of triamcinolone acetonide (4mg/0.1mL), both groups received monthly injection of ranibizumab on pro-re-nata (PRN) regimen for 1 year duration of the study.
Results: Group 2 received less number of injections (2.47 ± 1.2) as compared to group 1 (4.4 ± 1.5). Both groups achieved significant reduction of central macular thickness (CMT) after 12 months of injection with p value < 0.001. Both groups showed significant improvement of best corrected visual acuity (BCVA) after 12 months with p value < 0.001. Group 2 showed more significant improvement of BCVA after 6 and 12 months. The baseline CMT and the number of injections were the main predictors of the final BCVA in group 1, while the baseline BCVA was the only predictor of final BCVA in group 2.
Conclusion: Combined suprachoroidal injection of TA using this modified microneedle with intravitreal injection of ranibizumab resulted in more significant improvement of BCVA and reduction of CMT compared with ranibizumab monotherapy with no reported ocular or systemic side effects. The study was prospectively registered with clinical trial.gov ID (NCT04690608) in 27-12-2020.
Keywords: branch retinal vein occlusion, suprachoroidal injection, triamcinolone acetonide
Introduction
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder, branch retinal vein occlusion (BRVO) represents about 80% of RVO cases.1,2 BRVO occurs on top of occlusion of a first-or second-order retinal vein at the site of arteriovenous (AV) crossing, 3,4 subsequently, macular edema (ME) and retinal non perfusion develop on top of elevated levels of vascular endothelial growth factor (VEGF).5 Retinal non perfusion results in vitreous hemorrhage if affecting the retinal periphery 6,7 or severe visual deterioration if the macula was affected.8
Nowadays, multiple treatment modalities are present for the management of BRVO. Laser can improve retinal oxygenation, decrease vascular leakage and reduce macular edema.9 In addition, anti-VEGF agents like ranibizumab (Lucentis®, Novartis, Basel, Switzerland) can inhibit angiogenesis and reduce leakage and macular edema, but the main drawback of these agents that they require multiple injections to maintain a stable effect.10
Triamcinolone Acetonide (TA) is a synthetic corticosteroid that can be used for management of various intra-ocular inflammatory disorders in the last years, 11 it can improve visual acuity and decrease macular edema.12 Nowadays, steroid implants are available like Ozurdex® and Iluvein®. Ozurdex® (Allergan, Inc., Irvine, USA) is a dexamethasone implant that can slowly release steroids into the vitreous cavity for 6 months duration, it has less complication rate if compared to intravitreal Triamcinolone Acetonide (IVTA) but still can result in elevation of the intraocular pressure (IOP) and acceleration of cataract progression.13 This raised the need to search for other routes of TA delivery to the eye instead of intravitreal route to minimize side effects of TA.
The suprachoroidal space is a novel route for TA delivery to the posterior segment of the eye, in a study done on rabbits, TA achieved higher concentration in the posterior segment after suprachoroidal injection than intravitreal route with less concentration in the anterior segment and the lens, hence, suprachoroidal route results in less complication rate.14 Multiple recent studies proved the efficacy of suprachoroidal injection of TA in cases of uveitis and RVO with longer duration of action and fewer side effects.15–17
The present study was designed to evaluate the use of a modified inexpensive microneedle for suprachoroidal injection of TA combined with intravitreal injection of ranibizumab in cases of BRVO.
Methods
Study Design
A prospective randomized interventional study that was conducted on 60 eyes of 60 patients with centrally involving macular edema (ME) on top of non-ischemic branch retinal vein occlusion of less than 2 months duration. The study was performed in Ophthalmology department, Tanta University, Egypt. Recruitment of the cases was done in December 2020, the cases were followed up for 12 months duration and the results were collected in December 2021.
Participants
The study included sixty eyes of 60 patients with centrally involving ME on top of recently diagnosed non-ischemic branch retinal vein occlusion of less than 2 months duration with central foveal thickness more than 250 um detected by optical coherence tomography (OCT). Patients were divided in two groups, group (1) involved 30 patients who received intravitreal injection of 0.05 mL (0.5 mg) of ranibizumab, patients were followed up monthly for 12 months and injection of ranibizumab was repeated monthly on pro-re-nata regimen (PRN), group (2) included 30 patients who received baseline combined intravitreal injection of 0.05 mL (0.5 mg) of Ranibizumab and suprachoroidal injection of Triamcinolone Acetonide 4mg/0.1mL followed by monthly injection of ranibizumab on PRN regimen for one year duration of the study. Thorough ophthalmic evaluation was done for all patients including BCVA by Snellen chart that was converted to log MAR for statistical analysis, intraocular pressure (IOP) assessment by applanation tonometry, anterior segment evaluation by slit lamp and fundus examination by slit lamp bimicroscopy using +78 D lens and indirect ophthalmoscopy. Fundus fluorescein angiography was performed to confirm non-ischemic BRVO. Spectral domain optical coherence tomography (SD-OCT) using vertical line scan protocol centered on the fovea was performed for all patients at presentation and monthly for 12 months. All patients with history of previous intraocular surgery, other retinal pathologies like diabetic retinopathy, choroidal neovascular membrane (CNV) and age related macular degeneration were excluded from the study. In addition, patients with history of posterior uveitis and retinal degeneration or dystrophy were not included. History of previous intervention in the form of laser photocoagulation and intravitreal injection of anti-VEGF or Triamcinolone Acetonide were also excluded from the study. Furthermore, patients with advanced glaucoma or IOP more than 21 mmHg, patients with hazy media that interfered with good quality of OCT images and patients who did not complete 12 months follow up were not enrolled in our study. Systemic exclusion criteria included patients with renal failure or under dialysis, patients with history of myocardial infarction, stroke and heart failure, pregnant and lactating females.
Surgical Technique
Injection was performed by a single experienced surgeon (AEN). Prior to injection, the patients were prepared by topical fluoroquinolone eye drops (Moxifloxacin hydrochloride 0.5% Vigamox, Alcon, USA) 4 times daily for three days. Pupillary dilatation using Mydriacyl eye drops (Tropicamide 1%, Alcon) was performed first, then, topical anesthetic drop of (Benoxinate hydrochloride 0.4%, Benox, Epico, Egypt) was applied to the ocular surface followed by topical instillation of 10%povidone iodine (Betadine) for lids, eye lashes and periocular area and 5%povidone iodine into the conjunctival sac for three minutes before injection. In the operating theatre, intravitreal injection of 0.05 mL (0.5 mg) of ranibizumab was done in the inferotemporal quadrant of the conjunctiva 3.5 mL from the limbus. For suprachoroidal injection, 24 gauge intravenous branula and 30 gauge 1cc insulin syringe (SUNGSHIM MEDICAL CO., LTD, Korea) were used. Needle was removed from branula and the branula was cut in a way that allows only 1000um of insulin syringe to protrude from the branula edge. Suprachoroidal injection of 0.1 mL (4mg) of Triamcinolone Acetonide (Kenakort A by GlaxoSmithKline Brentford, Middlesex, TW8 9GS, United Kingdom) was performed in the superotemporal quadrant 3.5mm from the limbus with bevel pointing backwards. Slight pressure was done after entry to produce slight scleral dimple, if there was no resistance, the plunger was pushed and injection was done followed by use of cotton tipped applicator to minimize drug reflux. Immediate fundus examination was performed following injection and light perception was assured to exclude central retinal artery occlusion. Topical antibiotic drop (Moxifloxacin hydrochloride 0.5% Vigamox, Alcon, USA) was applied after injection and the eye was patched for several hours. The patient was examined the next day after injection to exclude major complications as uveitis, endophthalmitis, elevated IOP, retinal break, retinal detachment and vitreous hemorrhage (a Supplementary Video showing the surgical technique was uploaded with the submission in the Supplementary Material).
PRN Injection of ranibizumab was repeated monthly in case of persistence of intraretinal fluid, intraretinal cysts or subretinal fluid in OCT with central macular thickness more than 250 um or visual loss more than two lines of Snellen chart.
Primary outcomes were improvement of BCVA and reduction of CMT after 12 months of injection while secondary outcomes were to evaluate the safety of suprachoroidal injection of TA and its ability in reducing the number of injections of anti-VEGF and decreasing patient’s injection burden.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 25.0. (IBM Corp, 2017). Categorical variables were presented as number and percentage. Quantitative variables were expressed as mean and standard deviation. Chi-square test was used to compare categorical variables between 2 groups, while Fisher’s exact test was used to compare categorical variables if expected count is less than 5. Student’s t-test was used to compare quantitative data between 2 groups. Repeated measure ANOVA was used to compare quantitative data in each group at baseline, 1, 3, 6 and 12 months. Pearson correlation coefficient was used to assess correlation between quantitative variables. Significant variables were entered into multivariable linear regression analysis to identify significant independent predictors of BCVA. Statistical significance was considered at p value ≤ 0.05.
Results
The mean±SD of age in group 1 is 52.5 ± 7.5 years while that of group 2 is 54.7 ± 9.02 years with no detected statistical significance between the two groups with p value 0.3, no significant difference as regarding the gender between the two groups with p value 0.4, 80% of cases of group 1 and 100% of cases of group 2 were known to be hypertensive, all patients of group 1 needed retreatment, however, 9 patients of group 2 did not need further retreatment after the first injection, group 2 with baseline combined injection needed less number of PRN injections (2.47 ± 1.2) compared to group 1 (4.4 ± 1.5) with reported statistical significance (p value <0.001). These findings are presented in Table 1.
 |
Table 1 Sociodemographic and Clinical Characters of the Studied Groups |
In respect to CMT, both groups showed a significant reduction after 12 months of injection with p value<0.001. CMT decreased from 465 ± 99.5 um at the baseline to 213.5 ±15.1 um after 12 months in group 1, and from 542.6 ± 133.3 at the baseline to 211.9 ±10.7 after 12 months in group 2. Patients of group 2 showed more significant reduction of CMT than group 1 after 1 month of injection with p value 0.008, after 12 months, the CMT was quite similar in both groups, this is demonstrated in Table 2 and Figure 1. Recurrent macular edema was reported to be higher in group 1 compared to group 2, this is well demonstrated by Kaplan-Meier curve that shows longer recurrence interval in group 2 with a significant log rank test (p=0.003) (Figure 2).
 |
Table 2 Comparison of CMT, BCVA and IOP Changes Between the 2 Groups and Within Each Group |
 |
Figure 1 Central macular thickness at baseline, 1, 3, 6 and 12 months in the two groups. |
 |
Figure 2 Kaplan–Meier curve of macular edema recurrence in the two groups. |
Regarding BCVA, both groups showed significant improvement after 12 months with p value <0.001, the BCVA improved from 1.2 ±0.2 to 0.4±0.1 and from 1.2 ± 0.2 to 0.3±0.09 in groups 1, 2 respectively, group 2 showed more significant improvement in BCVA as compared to group 1 after 6 and 12 months with p values 0.01 and 0.02 respectively, 19 patients in group 1 and 25 patients of group 2 gained two or more lines of Snellen chart after 12 months, this is well explained in Table 2 and Figure 3.
 |
Figure 3 BCVA by log MAR at baseline, 1, 3, 6 and 12 months in the two group. |
Concerning changes in IOP after injection, IOP reached its highest level after injection in both groups after 1 month of injection and returned gradually to the normal level at the end of the study duration, the IOP was slightly higher in group 2 (12.7± 0.6) after 12 months with p value 0.03 as shown in Table 2.
In addition, statistically significant positive correlation between the number of injections, baseline CMT and baseline BCVA with the final BCVA in group 1 with p values 0.001, 0.01 and 0.007 respectively. In group 2, the number of injections and baseline BCVA showed statistically significant positive correlation with the final BCVA with p values 0.03 and 0.01 respectively. Furthermore, the number of injections showed statistically significant positive correlation with the final CMT in group 1 only with p value 0.02 (Table 3).
 |
Table 3 Correlation of Age, Number of Injections, Baseline CMT and BCVA with Final CMT and Final BCVA |
Regarding the independent predictors of the final BCVA in both groups, it was detected that the baseline CMT and the number of injections were the main predictors of the final BCVA in group 1 with p values 0.01 and 0.001 respectively, in group 2 the baseline BCVA was the only predictor of the final BCVA with p value 0.01, this is demonstrated in Table 4.
 |
Table 4 Multivariable Linear Regression Analysis of Independent Predictors of Final BCVA in the Two Groups |
Negative correlations were detected between age, number of injections, baseline BCVA, baseline CMT and the CMT change (the difference between CMT at the baseline and CMT after 12 months) in group 1, the results were statistically significant only as regarding age, baseline BCVA and baseline CMT with p values 0.05, <0.001 and <0.001 respectively. Similar results were obtained in group 2 with detected statistical significance concerning baseline BCVA and baseline CMT with p values <0.001. Furthermore, negative correlations were reported between the age, baseline BCVA, baseline CMT and the BCVA change (the difference between BCVA at the baseline and BCVA after 12 months) in groups 1, 2 with statistical significance regarding baseline BCVA and baseline CMT in group 1 with p values <0.001 and 0.01 respectively and p values of <0.001 in group 2. Furthermore, strong positive correlation was detected between BCVA change and CMT change after 12 months with p values 0.005 and <0.001 in groups 1, 2 respectively. All these changes are presented in Table 5.
 |
Table 5 Correlation of Age, Number of Injections, Baseline BCVA, Baseline CMT with CMT and BCVA Change |
No cases were transformed into ischemic BRVO during the follow-up period of the study. No cases of cataract progression, endophthalmitis, retinal detachment or serious systemic adverse effects were reported throughout the study period.
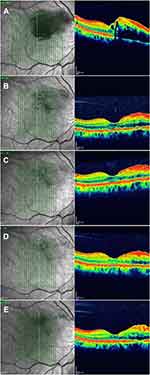
Figure 4 is an example of a female patient aged 55 years old presented with right upper temporal BRVO, the CMT was 295 um, the BCVA was 1.2 by log MAR (Figure 4A), the patient received single combined intravitreal injection of ranibizumab (0.5mg) and suprachoroidal injection of TA (4 mg), the CMT declined to 195 um and BCVA improved to 0.3 after 1 month (Figure 4B), after 3 months of the first injection, the CMT was 187 um and BCVA was 0.2 (Figure 4C), after 6 months of the first injection, the CMT was 192 um and BCVA was 0.2 (Figure 4D), after 12 months of the first injection, the CMT was 185 um and BCVA was 0.2 (Figure 4E).
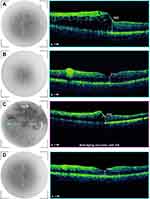
Figure 5 is an example of a male patient aged 48 years old presented with right upper temporal BRVO, the CMT was 393 um, the BCVA was 1.3 by log MAR (Figure 5A), the patient received 3 consecutive intravitreal injection of ranibizumab (0.5 mg), the CMT declined to 211 um and BCVA improved to 0.4 after 3 months (Figure 5B), after 6 months of the first injection, recurrent ME occurred with CMT 332 um and the BCVA declined to 1.2 by log MAR (Figure 5C), the patient received two additional injections of ranibizumab, after 12 months of the first injection, the CMT was 194 um and BCVA improved to 0.3 (Figure 5D).
Discussion
BRVO represents one of the commonest retinal vascular diseases. 1Macular edema (ME) on top of BRVO is the main cause of visual loss in these cases.18 Long standing macular edema more than 8 months can result in photoreceptor damage with subsequent irreversible loss of visual acuity and permanent visual disability, hence it is better to control ME as early as possible to prevent visual loss.2,18,19 Other complications like epimacular membrane and macular hole can occur on top of long term ME due to BRVO that also can result in severe visual loss and may require surgical intervention.20
Steroids can target the inflammatory cascade in BRVO by variable mechanisms, first, they are able to modify the integrity of the tight junctions, inhibit different molecules and mediators involved in vascular permeability and inflammation process, such as interleukin-6, Intercellular adhesion molecule-1 (ICAM-1), stroma-derived factor-1 and vascular endothelial growth-factor (VEGF).21 The use of intraocular steroids in the management of RVO cases was reported in multiple studies. In a study conducted by Campochiaro et al, 2015, the use of Ozurdex in RVO cases not responding to previous anti-VEGF agents injection resulted in marked reduction of ME, nevertheless, recurrence of ME occurred after several months.21 Intraocular steroids can achieve more prolonged effect than anti-VEGF drugs, so it is reasonable to combine steroids to anti-VEGF agents to enhance their effect and maintain their action, but unfortunately, cataract progression and increased IOP were the main problems associated with steroid use.
The suprachoroidal space (SCS) is a novel route for administration of various drugs to the posterior segment of the eye with minimal side effects regarding cataract progression and IOP elevation, but the method to approach this space remained the main obstacle. Various microneedles have been manufactured to deliver drugs into the SCS in a safe and a reliable manner. A microneedle is a hollow-bore needle that is modified in length to prevent deeper penetration than the SCS and to prevent inadvertent intravitreal injection.22,23 Marashi et al, 2022 introduced a new microneedle for suprachoroidal injection of TA made of 30 gauge needle with a rubber stopper to permit only 1000 um of the needle to penetrate the sclera for the treatment of a case of pseudophakic cystoid macular edema with marked anatomical and visual improvement.24 The present study evaluated the efficacy of a modified inexpensive microneedle for suprachoroidal injection of TA combined with intravitreal injection of ranibizumab in the management of BRVO cases.
Regarding our study, no significant changes between the two groups were detected concerning age and gender. In addition, group 2 with combined injection received less number of injections (2.47 ± 1.2) if compared to group 1 (4.4 ± 1.5) within 12 months of follow up denoting the efficacy of suprachoroidal injection of TA in reducing the number of intravitreal injection of anti-VEGF in BRVO cases. This is quite similar to the Tanzanite study which compared combined suprachoroidal injection of TA and intravitreal injection of aflibercept with intravitreal injection of aflibercept alone, this study reported reduced number of aflibercept retreatment in the combined injection group (23 vs 9), furthermore, the percentage of patients required no retreatment was markedly increased (78% vs 30%).15
The current study supports the hypothesis that suprachoroidal injection of TA in BRVO cases is a safe maneuver as IOP was slightly elevated after 1 month in both groups and gradually returns to its normal level till the end of the study and no patients needed anti-glaucoma medication. In addition, no cases developed significant cataract during the period of the study. This is quite similar to Tanzanite study that documented the safety of suprachoroidal injection of TA, the IOP was not significantly elevated after suprachoroidal injection and only two cases who are known to be glaucomatous needed additional anti-glaucoma medication, furthermore, one case developed cataract progression which was mostly unrelated to the injected drug.15
In the present study, marked visual and anatomical improvement was achieved in both groups. The rationale of achieving better anatomical and functional improvement in BRVO cases with combined suprachoroidal injection of TA with intravitreal injection of ranibizumab with lower reinjection frequency with no elevated IOP and cataract progression is well documented in the present study which can reduce the economical burden to the patient. The RETAIN study illustrates this hypothesis in which, the mean number of anti-VEGF injections was 14.0 for patients of BRVO and 19.5 for patients of CRVO through 4 years follow up duration, also half of these cases required additional treatment after 4 years. In addition, patients who required injections for 4 years have marked visual loss due to recurrent edema with subsequent macular damage.25
Another study in consistence with ours reported marked anatomical and functional improvement in BRVO cases of less than 8 weeks duration after combined intravitreal injection of Bevacizumab (1.25 mg) and TA (2 mg), the BCVA improved from (1.08 ± 0.35) at presentation to (0.55 ± 0.17), (0.56 ± 0.21), and (0.38 ± 0.1) after 1, 3, 6 months respectively. The CMT declined from (482 ± 107) to (319 ± 53), (344 ± 89), and (241 ± 29) after 1, 3, 6 months respectively. IOP transiently increased after 1 month in 6 patients and gradually returned to its normal level with topical beta blocker drops. The number of injections was markedly reduced with mean number of re‐injections of 0.35 ± 0.48 per each patient. No cases of cataract, retinal detachment, endophthalmitis or systemic side effects were recorded.26
Concerning ranibizumab monotherapy in BRVO cases, BRIGHTER study that was performed on 455 eyes detected significant superiority of ranibizumab over laser treatment alone with no detected ocular side effects.27 Furthermore, BRAVO study that was conducted on 397 eyes reported more significant improvement of BCVA and more significant reduction of CMT with ranibizumab therapy (0.3 and 0.5 mg) as compared to sham injection.28 Nevertheless, recurrent edema that required additional injections was the main problem and represented a significant burden to the patient.29 Both BRIGHTER and BRAVO studies evaluated the efficacy of ranibizumab monotherapy which is different from our study.
Another recent study evaluated the efficacy of ranibizumab monotherapy in BRVO patients younger than 50 years old detected significant improvement of both CMT and BCVA. The CMT declined after injection from (361 ± 80 μm) to (285 ± 57 μm) after 12 months follow up, the mean BCVA change from baseline is 7.0 ± 7.1 letters but patients needed 4.4 ± 2.4 injections that is more than the number of combined injection in our study 2.47 ± 1.2 confirming the efficacy of combined injection in reducing injection burden.30
In the present study, increased number of injections, more baseline CMT and worse baseline BCVA before injection are associated with worse final BCVA after 12 months in both groups of patients. A similar study to ours performed on ranibizumab injection in RVO patients younger than 50 years old detected worse final BCVA after 12 months with worse baseline BCVA before injection, also, the study reported no correlation between the number of injections and the final visual acuity which is different from our study that detected significant correlation in both groups.30
In contrast to our study, Zhang et al detected other predictors of better response to intravitreal injection of TA including younger age, ME of recent onset, the presence of serous retinal detachment, BRVO of non ischemic type and coexistent systemic diseases.31
The study has some limitations including the small sample size, short duration of follow up, absence of control group and absence of comparison with other anti-VEGF drugs other than ranibizumab, so larger number of patients with longer follow-up period is recommended for more evaluation of the efficacy and safety of the suprachoroidal route by this inexpensive modified microneedle in BRVO cases and other retinal disorders.
Conclusions
Combined suprachoroidal injection of TA with intravitreal injection of ranibizumab resulted in significant improvement of BCVA and reduction of CMT in BRVO patients after 12 months of injection with less number of PRN injections if compared to intravitreal injection of ranibizumab alone with no recorded ocular or systemic side effects. The baseline CMT and the number of injections were the main predictors of the final BCVA in patients with ranibizumab monotherapy, in patients with combined injection, the baseline BCVA was the only predictor of final BCVA. The author recommends the use of this inexpensive microneedle designed for suprachoroidal injection of TA in these cases to reduce the financial burden of anti-VEGF injection.
Data Sharing Statement
All individual deidentified participant data used during the current study including clinical data, figures, video of the surgical technique and follow-up data are available at any time from the corresponding author on a reasonable request.
Ethical Approval and Consent to Participate
The study was approved by the Ethical Committee of the Faculty of Medicine, Tanta University, Egypt (approval code 34328/12/20). All procedures were performed under the tenets of the 1964 Helsinki Declaration. Informed consent was obtained from every patient after discussing the procedure, possible advantages and harmful side effects. The study was prospectively registered with clinical trial.gov ID (NCT04690608) in 27-12-2020.
Consent to Publish
The author certifies that he has obtained all appropriate patient consent forms to publish their clinical data in this journal without showing their name or initials.
Acknowledgments
The author would like to dedicate this study to all members of Ophthalmology Department, Tanta University, Egypt who offered great help and facilities for this work. The author would like to acknowledge Department of Ophthalmology, Tanta University in which the whole study was performed.
Funding
No funding, financial support, or sponsorship was received in this study.
Disclosure
The author has no relevant financial or non-financial conflicts of interest for this work to disclose.
References
1. Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–319. doi:10.1016/j.ophtha.2009.07.017
2. Noma H, Yasuda K, Shimura M. Cytokines and the pathogenesis of macular edema in branch retinal vein occlusion. J Ophthalmol. 2019;2019:1–9. doi:10.1155/2019/5185128
3. Weinberg D, Dodwell DG, Fern SA. Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am J Ophthalmol. 1990;109(3):298–302. doi:10.1016/s0002-9394(14)74554-4
4. Feist RM, Ticho BH, Shapiro MJ, Farber M. Branch retinal vein occlusion and quadratic variation in arteriovenous crossings. Am J Ophthalmol. 1992;113(6):664–668. doi:10.1016/s0002-9394(14)74791-9
5. Hayreh SS, Zimmerman MB. Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 2014;132(1):13–22. doi:10.1001/jamaophthalmol.2013.5515
6. Jonas J, Paques M, Mones J, Glacet-Bernard A. Retinal vein occlusions. Dev Ophthalmol. 2010;47:111–135. doi:10.1159/000320076
7. Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25. doi:10.1016/j.preteyeres.2014.04.001
8. Kadomoto S, Muraoka Y, Uji A, Tamiya R, Oritani Y, Kawai K. Nonperfusion area quantification in branch retinal vein occlusion: a Widefield optical coherence tomography angiography study. Retina. 2021;41(6):
9. Gawęcki M. Subthreshold diode micropulse laser combined with intravitreal therapy for macular edema—A systematized review and critical approach. J Clin Med. 2021;10(7):1394. doi:10.3390/jcm10071394
10. Barquet LA. Papel del factor de crecimiento del endotelio vascular en las enfermedades de la retina [Role of VEGF in diseases of the retina]. Arch Soc Esp Oftalmol. 2015;90:3–5. doi:10.1016/S0365-6691(15)30002-2
11. Nozik RA. Periocular injection of steroids. Transactions-American Academy of Ophthalmology and Otolaryngology. Am Acad Ophthalmol Otolaryngol. 1972;76(3):695–705. PMID: 4677452.
12. Munk MR, Bolz M, Huf W, et al. Morphologic and functional evaluations during development, resolution, and relapse of uveitis-associated cystoid macular edema. Retina. 2013;33(8):1673–1683. doi:10.1097/IAE.0b013e318285cc52
13. Urbancic M, Gardasevic Topcic I. Dexamethasone implant in the management of diabetic macular edema from clinician’s perspective. Clin Ophthalmol. 2019;13:829–840. doi:10.2147/OPTH.S206769
14. Edelhauser HF, Verhoeven RS, Burke B, Struble CB, Patel SR. Intraocular distribution and targeting of triamcinolone acetonide suspension administered into the suprachoroidal space. Invest Ophthalmol Vis Sci. 2014;55(13):5259.
15. Campochiaro PA, Wykoff CC, Brown DM, et al. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the tanzanite study. Ophthalmol Retina. 2018;2(4):320–328. doi:10.1016/j.oret.2017.07.013
16. Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54(4):2483–2492. doi:10.1167/iovs.13-11747
17. Yeh S, Kurup SK, Wang RC, et al. Suprachoroidal injection of triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis: a randomized, Phase 2 study (DOGWOOD). Retina. 2019;39(10):1880–1888. doi:10.1097/IAE.0000000000002279
18. Coscas G, Cunha-Vaz J, Soubrane G. Macular edema: definition and basic concepts. Dev Ophthalmol. 2010;47:1–9. doi:10.1159/000320070
19. Augustin A, Loewenstein A, Kuppermann BD. General pathophysiology. Dev Ophthalmol. 2010;47:10–26. doi:10.1159/000320071
20. Cimolai N. Comment on “Insights into the pathogenesis of cystoid macular edema: leukostasis and related cytokines”. Int J Ophthalmol. 2020;13(8):1343–1344. doi:10.18240/ijo.2020.08.25
21. Campochiaro PA, Hafiz G, Mir TA, et al. Pro-permeability factors after dexamethasone implant in retinal vein occlusion; the Ozurdex for retinal vein occlusion (ORVO) study. Am J Ophthalmol. 2015;160(2):313–321. doi:10.1016/j.ajo.2015.04.025
22. Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433–4441. doi:10.1167/iovs.12-9872
23. Park SH, Lee KJ, Lee J, et al. Microneedle-based minimally- invasive measurement of puncture resistance and fracture toughness of sclera. Acta Biomater. 2016;44:286–294. doi:10.1016/j.actbio.2016.08.011
24. Marashi A, Zazo A. A manually made needle for treating Pseudophakic cystoid macular edema by injecting triamcinolone acetonide in the suprachoroidal space: a case report. Am J Ophthalmol Case Rep. 2022;25:101254. doi:10.1016/j.ajoc.2021.101254
25. Campochiaro PA, Sophie R, Pearlman J, Brown DM, Boyer DS, Heier JS. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN Study. Ophthalmology. 2014;121(1):209–219. doi:10.1016/j.ophtha.2013.08.038
26. Ali RI, Kapoor KG, Khan AN, Gibran SK. Efficacy of combined intravitreal bevacizumab and triamcinolone for branch retinal vein occlusion. Indian J Ophthalmol. 2014;62(4):396. doi:10.4103/0301-4738.120227
27. Tadayoni R, Waldstein SM, Boscia F, Gerding H, Pearce I, Priglinger S. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology. 2016;123(6):1332–1344. doi:10.1016/j.ophtha.2016.02.030
28. Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a Phase III study. Ophthalmology. 2010;117(6):1102–1112. doi:10.1016/j.ophtha.2010.02.021
29. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594–1602. doi:10.1016/j.ophtha.2011.02.022
30. Battaglia Parodi M, Romano F, Arrigo A, Mercuri S, Franceschi A, Bandello F. Ranibizumab for macular edema secondary to central and branch retinal vein occlusion in patients younger than 50 years of age. Biomed Res Int. 2020;2020:1–7.
31. Zhang S, An N, Ha W, et al. Factors correlated with the resolution of macular oedema after one dose injection of intravitreal triamcinolone acetonide treatment in branch retinal vein occlusion. J Int Med Res. 2016;44(3):685–697. doi:10.1177/0300060515617386
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.