Back to Journals » Risk Management and Healthcare Policy » Volume 16
Modifiable Risk Factors in High-Risk Groups of Colorectal Cancer Screening: A Cross-Sectional Study with Propensity Score Method
Authors Zhong X , Feng N, Ouyang B, Zhao D, Lei L, Peng J, Peng X
Received 18 August 2023
Accepted for publication 16 November 2023
Published 6 December 2023 Volume 2023:16 Pages 2673—2683
DOI https://doi.org/10.2147/RMHP.S435727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gulsum Kubra Kaya
Xuan Zhong,1,* Nongping Feng,2,* Binfa Ouyang,1 Dan Zhao,1 Lin Lei,3 Ji Peng,3 Xiaolin Peng1
1Department of Oncology, Injury and Nutrition, Shenzhen Nanshan Center for Chronic Disease Control, Shenzhen, Guangdong Province, People’s Republic of China; 2Department of Non-Communicable Disease Prevention and Control, Shenzhen Longgang Center for Chronic Disease Control, Shenzhen, Guangdong Province, People’s Republic of China; 3Department of Cancer Prevention and Control, Shenzhen Center for Chronic Disease Control, Shenzhen, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaolin Peng, Department of Oncology, Injury and Nutrition, Shenzhen Nanshan Center for Chronic Disease Control, No. 7, Huaming Road, Nanshan District, Shenzhen, Guangdong Province, People’s Republic of China, Tel +86-0755-26640203, Email [email protected]
Aim: The rising incidence and death rate of colorectal cancer (CRC) have posed a severe danger to the lives and health of residents. Individuals at high risk of CRC are drawing growing attention as the majority of the population impacted by CRC. Hence, it is imperative to examine the detection rates and modifiable factors affecting the populations at high risk for CRC in Shenzhen.
Methods: The multi-stage stratified cluster sampling method was used to select residents aged 45– 74 years old from September 2020 to December 2021. The community-based CRC screening was attended by a total of 30,921 residents from urban and suburb regions. The association between modifiable risk factors and the detection rate of high-risk groups was analyzed using multinomial logistic regression with the inverse probability treatment weighting (IPTW) based on the propensity score.
Results: The cross-sectional analysis included 24,613 people after excluding 6308 people who had missing or invalid fecal occult blood test (FOBT) results. The detection rate for high-risk groups during CRC screening was 28.50%. Higher rate of high-risk groups was detected among those who were male, aged 60 or above, college or above, other marital status, and living in urban (P < 0.05). Demographic characteristics after IPTW showed a weak correlation coefficient with the detection rate of CRC high-risk both in high-risk and general-risk groups (SMD < 0.1), suggesting a balanced group of participants. The results of logistic regression with IPTW indicated that smoking, drinking, obesity, lack of exercise, vegetable or fruit eating infrequently, red meat, processed meat, cereal food and their clustering status were more inclined to be risk indicators of CRC (P < 0.05).
Conclusion: The detection rate for high-risk CRC groups was comparatively high in Shenzhen. The distribution characteristics of lifestyle and dietary risk factors of high-risk groups should be given consideration when adopting individualized intervention measures and comprehensive prevention and control strategies.
Keywords: modifiable risk factors, colorectal cancer, high-risk group, propensity score method
Introduction
With approximately 1.9 million new cases and 0.9 million deaths in 2020, colorectal cancer (CRC) is the third most common cancer diagnosed and the second-leading cause of cancer mortality globally.1 By 2040, the CRC burden is expected to rise to 3.2 million new cases and 1.6 million deaths.2 In China in 2019, there were 607,900 CRC cases and 261,777 CRC deaths, with an age-standardized incidence rate of 30.55/100000 and a mortality rate of 13.86/100000. In China, CRC incidence, death, and disability-adjusted life-year rates have all increased during the last three decades.3 Within one year of diagnosis, nearly 60% of the typical family income in China is spent on CRC therapies.4 CRC not only endangers human health but also puts a massive financial burden on society and families because of its high morbidity and death.
As is known to all, individuals at high risk of CRC, such as those have a history of colorectal polyps, CRC, inflammatory bowel disease, familial adenomatous polyposis, or have first-degree relatives are more likely to acquire CRC than the general population.5,6 Effective strategies for preventing CRC include leading a healthy lifestyle, eating a balanced diet, exercising, keeping a healthy body weight, not smoking or quitting, and consuming little or no alcohol.7 Many countries are working to promote a healthy lifestyle for residents. Nevertheless, the association between these modifiable risk factors and CRC among people who are at high risk for the disease is not well understood. What’s more, few studies have looked at cluster status of modifiable risk factors. Through this study, we call attention to the key population at high risk of CRC. Growing data suggested that reducing the development of CRC precancerous events and the premature deaths brought on by them can be accomplished by early identification of high-risk populations and prudent intervention and management for their associated risk factors.8 One of the cities with the heaviest CRC burden is Shenzhen, a developed coastal city in Southern China. The CRC is the third leading cause of cancer in Shenzhen, and the incidence rate continued to rise from 2001 to 2018.9 However, the investigation of populations at higher risk for CRC in Southern China is limited by the lack of extensive epidemiological studies. Geographical environment and dietary habits have an impact on the detection rate of the high-risk groups for CRC in various regions. In order to identify high-risk groups early, implement effective interventions to reduce the incidence of precancerous events in high-risk groups and lay the groundwork for managing and avoiding CRC, this study set up the early screening of high-risk groups of CRC among residents of the community in Shenzhen and evaluated the current situation and modifiable risk factors of high-risk groups.
Materials and Methods
Study Population
The community-based CRC screening program targeted residents of those registered in Shenzhen, Southern China, or those who had lived in Shenzhen for at least 6 months. The current cross-sectional study was completed from September 2020 to December 2021, utilizing a multistage stratified cluster sampling approach. The 10 administrative districts in Shenzhen were divided into two stratums based on the special economic zone division. One district was chosen randomly within each stratum. One urban district and one suburb district were then randomly chosen in each district. The screening involved 30,921 residents from Nanshan District (urban) and Longgang District (suburb). Briefly, the screening strategy employed a two-step screening process. Potential participants aged 45–74 years were first invited to undergo a fecal occult blood test (FOBT) and a high-risk factor questionnaire (HRFQ) by trained staff, while those who tested positive by FOBT or were deemed high risk by HRFQ were referred to a hospital for a colonoscopy examination. Those who met one of the following conditions were excluded: 1) history of CRC; 2) serious heart, lung, cerebral or kidney problems; 3) psychiatric diseases; and 4) pregnant women. After excluding 6308 individuals missing or invalid FOBT results from negative HRFQ group, while the rest of 24,613 individuals had valid FOBT, HRFQ, or both. Figure 1 depicted a comprehensive flowchart of research participants.
 |
Figure 1 Flow diagram of study recruitment and retention. |
Epidemiological Investigation and Definitions
After appointments by residents, in-person investigations were carried out centrally utilizing an electronic collecting information system. Informed consent, object admission, height and weight values, detailed registration (demographic characteristics like name, age, gender, education background and marriage status), and questionnaire survey (controllable factors regarding drinking and smoking habits, dietary preferences, and exercise status) were the main processes and components of the primary screening.
The definitions of modifiable risk factors as follows:10,11 tobacco smoking status: not less than one cigarette per day for at least 6 months continuously or cumulatively; alcohol drinking status: not less than one time per week for at least 6 months continuously or cumulatively; regular consumption of fresh vegetables: 4 days a week and above; Regular consumption of fresh fruit: 4 days a week and above; Regular consumption of fresh red meat: 4 days a week and above; Regular consumption of processed meat: 4 days a week and above; Regular consumption of cereal food: 4 days a week and above. Regular physical activity: 3 times a week and above. The clustering status was defined as the simultaneous presence of 2 or more risk factors in an individual, defined as an aggregation of risk factors.
High-Risk Groups of CRC
According to the National Comprehensive Cancer Network (NCCN) CRC screening guidelines and Previous consensus on diagnosing and treating of CRC in China,12,13 high-risk groups of CRC indicated that the participant had one of the following: 1) positive FOBT; 2) a past history of cancer; 3) the first-degree relative had a history of CRC; 4) a history of intestinal polyps or adenomas; 5) patients with at least two of the next criteria: chronic constipation, chronic diarrhea, mucinous and bloody stool, a previous experience of chronic appendectomy or appendicitis, and a previous experience of chronic cholecystectomy or cholecystitis. Those without any of the above items were defined as the general risk group.
Statistical Analysis
The high-risk rate of CRC screening was described using frequencies and percentages for different demographic characteristics and modifiable risk factors. The rate of dwellers at high risk of CRC in individuals with various characteristics was compared using the χ2 test. The IPTW method based on propensity score (PS) was implemented to control for demographic characteristics confounding.14 Firstly, logical regression was used to calculate the propensity score for each participant, the different risk groups of CRC as the outcome in the propensity estimation model, and including demographic and socioeconomic factors that included age, gender, education level, marital status and region. The probability weight for high-risk individuals was 1/propensity score, while for general-risk individuals, it was 1/(1-propensity score). By verifying that the model covariates were balanced by different risk groups, the differences between the outcome groups in the model’s pre- and post-weighting covariates were visualized graphically. The criterion we followed was to decrease the standardized differences in means or proportions (SMD) between different risk groups to an absolute value of SMD <0.1.15
Following that, a logistic regression model was used to calculate the associations between risk factors and the detection rate of high-risk groups in the weighted sample. To enhance stability, we performed a conventional regression with covariate adjustment as a sensitivity analysis and contrasted the findings derived from the results of the IPTW. All analyses were performed using R V3.6.3 (R Core Team) and SAS V9.4 (SAS Institute, Cary, NC).
Results
Demographic Characteristics of Participants
Of 24,613 participants of CRC screening, 7014 participants (28.50%) were classified as high-risk groups. The high-risk rate in male was higher than in female (χ2 = 44.00, P < 0.001). The proportion of high-risk participants in the populations of 45~, 50~, 60~ age was 25.12%, 26.79% and 31.96%, respectively (χ2 = 96.69, P < 0.001). People with a college graduate or above were higher rate than high school or less ranked (χ2 = 365.48, P < 0.001). The proportion of high-risk individuals was higher among others marital status than those married (χ2 = 15.85, P < 0.001) and was also higher in urban region than in suburbs region (χ2 = 404.19, P < 0.001). The demographic characteristics of the high-risk group among the CRC screening are detailed in Table 1.
 |
Table 1 The Detection Rate of High-Risk in Colorectal Cancer Screening Among Residents with Different Characteristics |
Comparison of the Exposure Rates of Modifiable Risk Factors Between High-Risk Groups and General-Risk Groups
In terms of the detection rate for CRC high-risk groups, greater consumption of red and processed meat was higher (P < 0.05), while participants who consumed fruit frequently had a lower detection rate than those who did not. There were substantial disparities in detection rates across subjects with different smoking, drinking and BMI (P < 0.05). Statistically significant differences were not observed for exercise, vegetable and cereal food. In addition, the presence of risk factor clustering boosted the detection rate of high-risk groups (P < 0.05). Table 2 shows the detection rates for CRC high-risk groups.
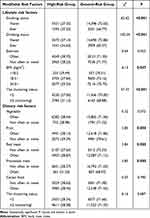 |
Table 2 The Characteristics of Lifestyle and Dietary Factors Among Colorectal Cancer Screening |
Association Between Modifiable Risk Factors and the Rates of High-Risk Groups
Demographic characteristics before and after IPTW adjustment in each group are presented in Figure 2. The correlation coefficients with single modifiable risk factors were weak for all demographic covariates after weighting the participants with inverse probability based on the propensity score (SMD < 0.1), indicating a reasonable balance of participation between groups.
 |
Figure 2 Standardized differences in means (SMD) of propensity score before and after the inverse probability treatment weighting (IPTW), SMD <0.1 show adequate matching. |
For lifestyle risk factors, after weighting the participants with inverse probability, the positive associations of BMI≥24.0 with high-risk rate turned out to be significantly positive (OR = 1.07, 95% CI = 1.04–1.11, Table 3), and that of BMI <18.5 with high-risk rate was no longer significant (P = 0.085). For smoking, drinking, and exercise less, the risk of high-risk detection increased by 1.14, 1.22, and 1.06 times, respectively (smoke: 95% CI = 1.09–1.19; drink: 95% CI = 1.16–1.27; exercise less: 95% CI = 1.03–1.10; all P <0.05). Despite having boarder confidence intervals for ORs, the traditional adjustment model retained the significant associations shown by the IPTW regression analysis.
 |
Table 3 Association Between the Rates of CRC High-Risk Groups and Modifiable Lifestyle Risk Factors |
Similar findings were obtained in dietary risk factors in IPTW-regression analysis, being consistent with the results from lifestyle factors. IPTW-regression analysis showed association of vegetable, fruit, red meat, processed meat and cereal food with high-risk rate, with statistical significance (P < 0.05; Table 4), whereas the significant associations of vegetable, red meat, and cereal food were removed in traditional logistic regression analysis (P > 0.05). Moreover, the adverse link between the clustering status and high-risk rate in dietary factors seemed to be weaker than that of lifestyle risk factors (diet: OR = 1.08; lifestyle: OR = 1.16).
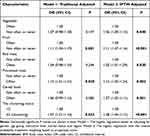 |
Table 4 Association Between the Rates of CRC High-Risk Groups and Modifiable Dietary Risk Factors |
Discussion
This cross-sectional study, which included 24,613 subjects with a positive rate of FOBT or HRFQ of 28.5% in CRC screening, examined the relation between changeable risk factors and the high-risk rate of CRC utilizing the IPTW method based on propensity scores, which produced less biased effect estimates than the conventional correction procedure.16 Compared to other developed cities in China like Shanghai (29%), Guangzhou (16.77%), and Tianjin (5.57%),17–19 as well as developed country like Germany (12.0%) and France (6.2%),20,21 the present results suggest that the detection rate of CRC high-risk in permanent residents of Shenzhen was relatively high, and the prevention of CRC is facing great challenges, and it was also of great significance for improving the health literacy level. The risk variables derived from the IPTW included six well-known modifiable factors (smoking and drinking status, obesity, red and processed meat) that were excluded in the present CRC screening methods. Our findings have the potential to enhance the effectiveness of screening programs by complementing existing CRC screening strategies.
Our regression models showed a similarity to previous work. In this study, there was a significant difference in the detection rate of high-risk CRC groups among male and female respondents, which has been associated to indicate that male gender is certainly a risk factor for CRC. This increase in detection rates is comparable to trends in the age-specific incidence of CRC in the study area and could be explained by a dose–effect relationship between older people’s cumulative lifetime exposure to risk factors and increased detection rates.22 Furthermore, individuals with higher educational levels were more likely to engage in brainwork, have less exercise, experience work-related stress, and engage in sedentary behavior for extended periods of time. These factors have the potential to contribute to disparities in risk detection rates among people with varying educational levels. The study revealed that urban was more likely to detect high-risk groups of CRC than suburb region. The result was partly in accordance with the widespread belief that the food patterns in urban regions have a clear tendency towards “esternization” and should be associated with increased risk.23 A more in-depth investigation was needed into the precise reasons for the distinction between urban and suburb areas.
Few research focused on the lifestyle of people at a high risk of CRC, despite the fact that this group is the majority afflicted by CRC. Many studies have concentrated on the impacts of specific exposure factors on CRC occurrences.24–26 This study explored modifiable risk factors for high-risk groups of CRC using IPTW with strong casual validity to bridge this knowledge gap. This study has several noteworthy research outcomes, based on the traditional logistic regression and IPTW method. These findings indicate that the risk of high-risk detection rate of CRC was associated with lifestyle and dietary modifiable risk factors and their aggregation state.
With respect to lifestyle, we found that abnormal BMI, lack of exercise, increased smoking and drinking were associated with increased detection of elevated risk of CRC. These outcomes matched those of other studies on CRC occurrences.27–30 Obesity-related aberrant lipid metabolism, chronic inflammation, adipokines and hormones, disruption of bile acid homeostasis and dysbiosis of the gut microbiota may all play essential roles in the complicated metabolic control of CRC carcinogenesis.31 A growing amount of epidemiological and experimental research have demonstrated exercise has a modulatory influence on CRC initiation and progression. Targeting and modulation of the insulin-like growth factor system, apoptosis, epigenetics, immunity, inflammation, leptin and their signaling pathways are supporting mechanisms significantly underpinning the protective properties of exercise in CRC.32 Through direct ingestion or the circulatory system, the cocktail of chemicals in cigarette smoke can easily enter the colon mucosa and cause genetic and epigenetic abnormalities.33 Acetaldehyde is one of the alcohol’s metabolites that has been classified as carcinogenic since it enhanced the risk of CRC.34 Healthy diet consists of cereal-rich meals, fruits and vegetables, and fewer processed red meats.35 By including sufficient nutrition, abundant dietary fiber, and less fatty and cancer-causing ingredients in this diet pattern, it could lower CRC risk.36 Our findings support the notion that it is critical to offer these persons at high risk of CRC with enough information to fill knowledge gaps and assist them in developing the proper risk perceptions, which is required for executing lifestyle interventions.
Our findings may help high-risk populations understand the value of adopting healthy behaviors in preventing colorectal precancerous and cancerous lesions, which healthy behavior promotion is required to perform in order to lessen the rising burden of CRC. Therefore, encouraging healthy lifestyle recommendations to the population who have undergone screening may help reduce the risk of cancer and other relevant complications, improve prognosis, as well as quality of a lifetime. It would be beneficial to commence with higher risk individuals before providing the intervention to the entire screening population when developing and assessing an action plan for stopping the development of CRC.
Important strengths should be mentioned. Specifically, we applied IPTW regression to address the confusion of demographic characteristics. A single composite score is replaced by an entire set of covariates in the propensity score method, unlike traditional regression. By using the IPTW regression, with a more parsimonious model and higher statistical power, we were able to control for confounders while still allowing for multicollinearity.37 We also ran conventional regression as a sensitivity study in the analytical process, and in comparison to the conventional adjustment approach, the results from the IPTW showed smaller 95% confidence ranges for effect estimations, demonstrating the reliability of our conclusions.
Furthermore, a number of risk-scoring systems have been implemented in different nations for CRC screening;38–40 however, some of the most recent, widely reported changeable risk factors, like tobacco use, drinking alcohol, and BMI, were left out in the HRFQ and may have an impact on the discriminatory power of the screening procedure.41 The risk of detection rate in high-risk groups of CRC was inversely associated with adherence to a healthy lifestyle and dietary habits, as demonstrated by this study. Consequently, for high-risk groups, information on modifiable lifestyle factors makes it possible to formulate preventive health recommendations for those at risk.
Limitations
Our results should be interpreted with several limitations in mind. First, the cross-sectional design of this study cannot determine the causal controllable factors of CRC high-risk groups. However, by employing the IPTW approach, we were able to get a methodologically robust assessment of the relationship. Second, even correcting for various variables, unmeasured confounding (such as socioeconomic status) remains observational studies’ Achilles heel. Likewise, we only enlisted residents of one urban and one suburban location in Shenzhen, China, which may restrict the generalizability of our findings. The under-represented population seems to be less of a concern because this study was aimed at investigating a biological hypothesis. Collectively, further longitudinal design studies continue to be justified. More research is needed to evaluate whether employing genetic risk profiling in addition to recognized risk variables might help identify persons at high risk of CRC.
Conclusion
On balance, this study provided some fresh perspectives to the argument on the controlled risk factor of high-risk groups for CRC via the innovative methodology. These discoveries may have public health implications for designing novel interventions. In the future, to make the most of this advantageous “educational moment” of high-risk period and assist high-risk persons flexibly comprehend the connection between adenomas, CRC, and lifestyle, we should increase training in time management and risk communication skills. Additionally, getting personalized lifestyle guidance from experts is a very effective way to improve one’s ingrained lifestyle behaviors and maintain a healthy lifestyle over the long run.
Ethics Approval and Consent to Participate
All participants gave written informed consent in accordance with the Declaration of Helsinki. The study received approval from the Ethics Review Committee of Shenzhen Nanshan Center for Chronic Disease Control, China (Medical Ethics Review No.: ll20230001).
Funding
The study was supported by The Nanshan Science and Technology Innovation Bureau (NS2023062) and Nanshan Medical Key Discipline Construction Funding (Noninfectious Chronic Disease).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siege RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–344. doi:10.1136/gutjnl-2022-327736
3. Li Q, Wu H, Cao M, et al. Colorectal cancer burden, trends and risk factors in China: a review and comparison with the United States. Chin J Cancer Res. 2022;34(5):483–495. doi:10.21147/j.issn.1000-9604.2022.05.08
4. Sun D, Li H, Cao M, et al. Cancer burden in China: trends, risk factors and prevention. Cancer Biol Med. 2020;17(4):879–895. doi:10.20892/j.issn.2095-3941.2020.0387
5. Wong M, Rerknimitr R, Lee GK, et al. Development and validation of the Asia-Pacific proximal colon neoplasia risk score. Clin Gastroenterol Hepatol. 2021;19(1):119–127.e1. doi:10.1016/j.cgh.2019.12.031
6. Chen H, Li N, Ren J, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68(8):1450–1457. doi:10.1136/gutjnl-2018-317124
7. Clinton SK, Giovannucci EL, Hursting SD. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–671. doi:10.1093/jn/nxz268
8. Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ. 2021;374:n1855. doi:10.1136/bmj.n1855
9. Liu FJ, Wang YR, Cai WC, et al. The incidence and trend of colorectal cancer in Shenzhen city from 2001 to 2018. China Cancer. 2021;30(09):671–677. doi:10.11735/j.issn.1004-0242.2021.09.A006
10. Ng R, Sutradhar R, Yao Z, et al. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int J Epidemiol. 2020;49(1):113–130. doi:10.1093/ije/dyz078
11. Wang YF, Sun MX, Xue H, et al. Understanding the China blue paper on obesity prevention and control and policy implications and recommendations for obesity prevention and control in China [J]. Chin J Prev Vet Med. 2019;53(9):875–884. doi:10.3760/cma.j.issn.0253-9624.2019.09.003
12. Provenzale D, Ness RM, Llor X, et al. NCCN guidelines insights: colorectal cancer screening, version 2.2020. J Natl Compr Canc Netw. 2020;18(10):1312–1320. doi:10.6004/jnccn.2020.0048
13. Digestive NC, Prevention NE, Alliance TC, Chinese consensus of early colorectal cancer screening (2019, Shanghai). Zhonghua Nei Ke Za Zhi. 2019;58(10):736–744. doi:10.3760/cma.j.issn.0578-1426.2019.10.004
14. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi:10.1002/sim.6607
15. M S A, Prieto-Alhambra D, Lopes LC, et al. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front Pharmacol. 2019;10:973. doi:10.3389/fphar.2019.00973
16. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
17. Zhang M, Zhao L, Zhang Y, et al. Colorectal cancer screening with high risk-factor questionnaire and fecal immunochemical tests among 5, 947, 986 asymptomatic population: a population-based study. Front Oncol. 2022;12:893183. doi:10.3389/fonc.2022.893183
18. Li Y, Liu HZ, Liang YR, et al. Analysis of community colorectal cancer screening in 50-74 years old people in Guangzhou, 2015-2016. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(1):81–85. doi:10.3760/cma.j.issn.0254-6450.2018.01.017
19. Zhu Y, Zhao G, Ma W, et al. Real-world application of a fast stool DNA test for colorectal cancer screening in primary screening positive population. Cancer Med. 2023;12(7):7689–7698. doi:10.1002/cam4.5521
20. Amitay EL, Cuk K, Niedermaier T, et al. Factors associated with false-positive fecal immunochemical tests in a large German colorectal cancer screening study. Int, J, Cancer. 2019;144(10):2419–2427. doi:10.1002/ijc.31972
21. Raginel T, Puvinel J, Ferrand O, et al. A population-based comparison of immunochemical fecal occult blood tests for colorectal cancer screening. Gastroenterology. 2013;144(5):918–925. doi:10.1053/j.gastro.2013.01.042
22. Xia L, Xu YJ, Xu XJ, et al. Cancer incidence and mortality in pearl river delta area, 2009-2013. Zhonghua Zhong Liu Za Zhi. 2019;41(5):393–397. doi:10.3760/cma.j.issn.0253-3766.2019.05.014
23. Wen D, Zou W, Wen X, et al. Urban-rural disparity in colorectal cancer incidence and increasing trend in relation to socioeconomic development and urbanization in China. J Int Med Res. 2018;46(10):4181–4196. doi:10.1177/0300060518791090
24. Hori M, Sawada N, Kito K, et al. Vegetable and fruit intake and colorectal cancer risk by smoking status in adults: the Japan public health center-based prospective study. Eur J Clin Nutr. 2023;77(2):255–263. doi:10.1038/s41430-022-01214-2
25. Mayen AL, Viallon V, Botteri E, et al. A longitudinal evaluation of alcohol intake throughout adulthood and colorectal cancer risk. Eur J Epidemiol. 2022;37(9):915–929. doi:10.1007/s10654-022-00900-6
26. Vallis J, Wang PP. The role of diet and lifestyle in colorectal cancer incidence and survival. Gastrointestinal Cancers. 2022. doi:10.36255/exon-publications-gastrointestinal-cancers-diet-colorectal-cancer
27. Botteri E, Borroni E, Sloan EK, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol. 2020;115(12):1940–1949. doi:10.14309/ajg.0000000000000803
28. Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study. Gastroenterology. 2020;159(1):129–138.e9. doi:10.1053/j.gastro.2020.03.016
29. Cho YA, Lee J, Oh JH, et al. Genetic risk score, combined lifestyle factors and risk of colorectal cancer. Cancer Res Treat. 2019;51(3):1033–1040. doi:10.4143/crt.2018.447
30. Choi J, Jia G, Wen W, et al. Healthy lifestyles, genetic modifiers, and colorectal cancer risk: a prospective cohort study in the UK biobank. Am J Clin Nutr. 2021;113(4):810–820. doi:10.1093/ajcn/nqaa404
31. Ye P, Xi Y, Huang Z, et al. Linking obesity with colorectal cancer: epidemiology and mechanistic insights. Cancers. 2020;12(6):1408. doi:10.3390/cancers12061408
32. Amirsasan R, Akbarzadeh M, Akbarzadeh S. Exercise and colorectal cancer: prevention and molecular mechanisms. Cancer Cell Int. 2022;22(1):247. doi:10.1186/s12935-022-02670-3
33. Giovannucci E, Martinez ME. Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst. 1996;88(23):1717–1730. doi:10.1093/jnci/88.23.1717
34. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
35. Zhou E, Rifkin S. Colorectal cancer and diet: risk versus prevention, is diet an intervention? Gastroenterol Clin N Am. 2021;50(1):101–111. doi:10.1016/j.gtc.2020.10.012
36. Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020;49(1):246–258. doi:10.1093/ije/dyz064
37. D’Agostino RJ. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17((19):2265–2281. doi:10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b
38. Sekiguchi MMT, Kakugawa Y, Matsumoto M, Matsuda T. A scoring model for predicting advanced colorectal neoplasia in a screened population of asymptomatic Japanese individuals. J Gastroenterol. 2018;53(10):1109–1119. doi:10.1007/s00535-018-1433-7
39. Park CH, Kim NH, Park JH, et al. Individualized colorectal cancer screening based on the clinical risk factors: beyond family history of colorectal cancer. Gastrointest Endosc. 2018;88(1):128–135. doi:10.1016/j.gie.2018.02.041
40. Zhao S, Wang S, Pan P, et al. FIT-based risk-stratification model effectively screens colorectal neoplasia and early-onset colorectal cancer in Chinese population: a nationwide multicenter prospective study. J Hematol Oncol. 2022;15(1):162. doi:10.1186/s13045-022-01378-1
41. Wu WM, Gu K, Yang YH, et al. Improved risk scoring systems for colorectal cancer screening in Shanghai, China. Cancer Med. 2022;11(9):1972–1983. doi:10.1002/cam4.4576
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
