Back to Journals » Cancer Management and Research » Volume 10
Modern management for brain metastasis patients using stereotactic radiosurgery: literature review and the authors’ gamma knife treatment experiences
Authors Higuchi Y , Yamamoto M , Serizawa T , Aiyama H, Sato Y, Barfod BE
Received 18 January 2018
Accepted for publication 23 April 2018
Published 5 July 2018 Volume 2018:10 Pages 1889—1899
DOI https://doi.org/10.2147/CMAR.S116718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Yoshinori Higuchi,1,* Masaaki Yamamoto,2,* Toru Serizawa,3 Hitoshi Aiyama,2 Yasunori Sato,4 Bierta E Barfod2
1Department of Neurological Surgery, Chiba University Graduate School of Medicine, Chiba, Japan; 2Katsuta Hospital Mito Gamma House, Hitachi-Naka, Japan; 3Tokyo Gamma Unit Center, Tsukiji Neurological Clinic, Tokyo, Japan; 4Department of Global Clinical Research, Chiba University Graduate School of Medicine, Chiba, Japan
*These authors contributed equally to this work
Abstract: Historically, whole brain radiotherapy was administered to most patients with brain metastases. However, over the past three decades, stereotactic radiosurgery (SRS), targeted at individual cranial lesions, has been accepted widely. In this study, based on the authors’ experiences along with published data, recent trends in SRS for brain metastases are discussed. This article focuses on the following issues: 1) How many tumors can or should be treated with SRS? 2) Two-/three-staged SRS for relatively large tumors, 3) post- or preoperative SRS, and 4) repeat SRS.
Keywords: brain metastases, radiotherapy, radiosurgery, gamma knife
Introduction
The late Professor Lars Leksell launched the use of gamma knife radiosurgery (GKRS) for patients with functional neurosurgical disorders, for example, Parkinson’s disease and intractable pain, in 1968.1 Within a few years, he and his colleagues had begun using GKRS to treat patients with cerebral arteriovenous malformations as well as certain benign primary brain tumors, namely, craniopharyngiomas, meningiomas, vestibular schwannomas, and pituitary adenomas.2 After two decades, GKRS was successfully applied to treat brain metastasis (BM) from a recurrent hypernephroma, as first reported by Lindquist.3 Stereotactic radiosurgery (SRS) has since been applied as a primary or boost, with whole brain radiotherapy (WBRT), treatment for growing numbers of BM patients. Many tumors, regardless of whether they are radiosensitive or resistant, single or multiple, can be adequately managed with SRS. This technique is particularly suitable for metastatic lesions because most are well-circumscribed. Therefore, the last decade of the 20th century witnessed a remarkable expansion of SRS, now being used worldwide, as well as various innovations in linac-based systems, that is, Cyber Knife, Synergy, Novalis, Tomotherapy, and so on. Very recently, Kann et al4 reported, based on their series of 75,953 BM patients identified in the National Cancer Database during the 2009 through 2014 period, that the overall utilization rate for SRS rose from 9.8% in 2004 to 25.6% in 2014 (p<0.001), with an average increase of 1.6% annually. The annual increase in SRS application was higher from 2009 to 2014 than from 2004 to 2009 (2.6% vs 0.5% per year, respectively).4
The authors’ personal experiences in using only a gamma knife, as summarized in Tables 1 and 2, are reviewed along with relevant recently-published data. Herein, we focus on current trends in SRS for BMs, especially recently-developed applications, ie, larger BM numbers, larger BM volumes, adjuvant treatment involving surgical removal and repeat SRS. These issues have yet to be fully explored and remain controversial.
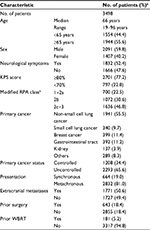  | Table 1 Clinical characteristics before gamma knife radiosurgery Notes: aValues are presented as the number of patients (%) unless otherwise indicated. bRefer to the studies Yamamoto et al.72,73 Abbreviations: KPS, Karnofsky performance score; RPA, recursive partitioning analysis; WBRT, whole brain radiotherapy. |
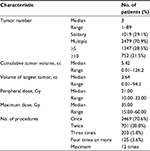  | Table 2 Radiosurgical parameters |
What are the generally accepted concepts?
Numerous publications have focused on radiosurgery for BMs. The authors do not intend to review them comprehensively in this study. Such a review is beyond the scope of this article, but the authors’ personal experiences with 3498 patients (5055 procedures, as of the end of 2017) who have undergone GKRS for BMs since 1998 (Table 1), are summarized in this study along with much of what we have learned from only one prospective observational study (JLGK0901).5,6
- Although the limitation of treatable lesion size is crucial for selecting SRS, tumor control rates of 90%, or even slightly better, can be obtained if one to four lesions which were initially diagnosed and sufficiently small are irradiated with a peripheral dose of at least 20 Gy. In such cases, true recurrence is exceedingly rare.
- The crude SRS-related complication incidence, that is, that of symptomatic radionecrosis of the normal brain, is generally below 3.0% in cohorts including patients with relatively short survival. Not unexpectedly, however, the crude complication incidence exceeds 5%, or even 10%, in a rather special group of patients surviving for 3–5 years, or even longer, after SRS.
- Longer survival cannot be expected because the survival duration depends primarily on the status of non-brain lesions (including the primary tumor). Most patients, 80%–90%, die of causes other than brain tumor progression. Thus, the majority can maintain good brain function until death.
- Factors known to predict longer survival are younger age, female gender, better performance status, absence of neurological symptoms, solitary tumor, controlled primary tumor, and absence of active non-BMs.
- Controversy persists as to whether radiosurgery should be combined with WBRT. A randomized study found the only benefit of combining radiosurgery with WBRT to be that re-treatment, necessitated by new lesions, is significantly reduced. Neither the survival rate nor the local tumor recurrence rate differed significantly between the groups with versus without WBRT.7 However, post-WBRT decline of neurocognitive function was described as being clinically meaningful.8 Very recently, Brown et al stated that, based on their randomized study (Alliance), SRS alone may be a preferred strategy for patients with 1–3 BMs because using SRS alone, as compared with SRS+WBRT, was associated with less cognitive deterioration.9
Can patients with larger numbers of BM be treated?
SRS for 5–10 BMs
“How many tumors can and should be treated with SRS?” has long been the major question for specialists in the field of SRS for BMs. Historically, with a linac system, the upper limit is generally considered to be 3–4 tumors in a single session due mainly to the technical difficulties encountered in dose planning and to the time-consuming procedures required. In contrast, as we reported previously, based on our experiences and those of other groups with GKRS since the 1990s,5,6,10–20 the upper limit for lesion numbers that can be treated in a 1-day session has been 30, or even slightly more, such that the prolonged procedure time again accounts for the limitation in number of tumors. If a patient has more than 40 lesions, the authors recommend that the procedure be divided into two sessions, 1 day apart, with the patient keeping the stereotactic head frame on overnight, or into multiple sessions with intervals of several weeks or months. Also, with a recently developed linac system, more than 10, possibly even more than 20, lesions can be easily irradiated within a day.
Because numerous factors in BM patients impact outcomes, we can no longer rely on a one-size-fits-all treatment paradigm. Still, solid patient selection criteria are necessary for SRS of BMs. Despite a lack of good scientific evidence, WBRT was strongly recommended in most industrialized nations until 2013 while SRS alone for patients with ≥4, or even ≥5, tumors had not as yet become accepted.21 However, a trend for patients with ≥5, or even ≥10, tumors to be considered for SRS alone was already apparent early in this century.22 Since Yamamoto et al reported two BM patients with ≥10 tumors who were successfully managed with SRS,10 retrospective studies of SRS-treated patients with several BMs have been published.10–20 Most notably, the authors conducted a case-matched study to reassess whether SRS alone for tumor numbers ≥5 yielded results different from those of treating 1–4 (548 patients in each group).5 Although the post-SRS overall median survival time (MST) difference, 0.9 months, between the two groups was statistically significant, this difference was not taken to be clinically relevant. The study subjects with tumor numbers ≥5 had non-inferior results, when compared to the other tumor number group, with no major differences being seen in neurological death, local recurrence, repeat SRS required for new tumors, maintenance of good neurological state, and SRS-related complications.
However, the JLGK0901 Study launched a major breakthrough in SRS for BM patients, with the perhaps overly strict criteria of the National Comprehensive Cancer Network Guideline and other guidelines having since been revised.5,6,23 This prospective observational study, including 1194 BM patients, clearly showed the non-inferiority of SRS without WBRT as the initial treatment for those with 5–10 BMs versus patients with 2–4 in terms of overall survival (OS) as well as most secondary end points if the tumor volume did not exceed 10 cc which corresponds to ~2.7 cm in diameter. Considering the present lack of evidence supporting WBRT superiority over SRS alone for patients with 5–10 tumors, their results are considered to constitute the highest level of evidence, to date, which would allow SRS alone to be advocated for such patients.
SRS for ≥10 tumors
Next, a need was recognized to reappraise whether the results of SRS alone for patients with ≥10 BMs are inferior to those of patients with fewer metastatic tumors. In 1998, members of our research group presented the first clinical observations, suggesting the feasibility of SRS for patients with multiple lesions.10 The report described two patients receiving GKRS, one with 37 and the other with 36 intracranial metastatic lesions. Both the patients had lung cancer, and all lesions visualized on magnetic resonance (MR) imaging were irradiated and then confirmed, post-radiosurgically, to have disappeared or undergone marked shrinkage. One of these two patients, who had been symptomatic prior to SRS, showed marked clinical improvement 2 weeks after irradiation. These early experiences, though based only on two patients, who died due to their original tumors 20 and 23 weeks after SRS, respectively, raised the possibility of radiosurgery exerting certain beneficial effects in the end-stage management of carefully selected patients with several intracranial metastases. In other words, maintaining good performance status for a significant portion of a patient’s remaining life might well be possible.
Suzuki et al conducted the first retrospective study on SRS for ≥10 BM.11 In 2000, they reported, based on 24 patients, that although the post-SRS MST was only 11 weeks with cumulative survival rates of 70.4%, 49.3%, and 12.3% at the 12th, 24th, and 36th post-SRS month, respectively, none of their patients died due to brain disease progression. In 2008, Kim et al described 26 patients receiving GK SRS.13 According to their retrospectively obtained results, post-SRS MST was 34 weeks, the local control rate 79.5%. Among 18 patients who died, causes of death could not be determined in two, but were confirmed in the other 16 to be non-brain diseases in six and brain diseases in 10. Univariable analyses demonstrated synchronous presentation, higher Karnofsky Performance Score (better than 80%) and controlled primary diseases to be favorable prognostic factors. In 2009, Yamamoto et al, employing a data set of 456 non-lung cancer patients including 82 with ≥10 BMs, described post-SRS results focusing on multiple BMs.14 One of their major conclusions, from this retrospective study, was that despite tumor number having a significant impact on the duration of survival, ~85% of patients died of causes other than brain disease progression, regardless of the number of tumors.
In 2000, Chang et al studied 323 BM patients who underwent GKRS.15 Their patients were divided into four groups according to BM numbers: Group 1, 1–5; Group 2, 6–10; Group 3, 11–15; and Group 4, ≥16. According to their analysis, neither survival times nor local tumor control rates differed significantly among the four groups, although the probability of new lesion development in the brain was noted to be greater in Group 4.
In 2012–2014, three studies evaluated the outcomes in patients with ≥10 BMs treated with SRS.16,18,20 Rava et al reported, based on 53 patients with ≥10 BMs treated with SRS (mean tumor number: 11) whose post-SRS MSTs exceeded 6 months, that aggressive local treatment is still an option, although rapid central nervous system failure is to be anticipated.16 Grandhi et al reported, based on 61 patients with ≥10 BMs receiving SRS (mean, 13 tumors), whose post-SRS MST was 4 months, that SRS can be applied safely and effectively for treating intracranial disease with a high local control rate in patients with ≥10 BMs.18 In those with fewer tumors, a non-melanomatous primary lesion, controlled systemic disease, and a low recursive partitioning analysis (RPA) class, SRS might well be one of the most effective treatments currently available. Their conclusion was that SRS can reasonably be regarded as a first-line treatment. Patients with breast cancer constituted a group of individuals likely to experience major benefit from SRS alone, with both survival and the time until central nervous system recurrence being prolonged. The present authors conducted a case-matched study to reappraise whether treatment outcomes were truly inferior for tumor numbers ≥10 versus 2–9. We compared group A, with 2–9 tumors, to group B harboring ≥10 tumors (467 patients each in groups A and B).20 No significant difference in post-SRS MSTs (months) was detected between the two groups (7.1/group A vs 6.9/group B, hazard ratio [HR]: 1.238 [95% confidence interval {CI}: 0.835–1.834], p=0.29). Other post-SRS treatment results, that is, neurological death-free survival time and cumulative incidences of local recurrence, the need for repeat SRS to manage new lesions, neurological deterioration, and SRS-related complications, were not inferior in the group B as compared to the group A patients. These observations allowed us to conclude that patients with ≥10 tumors are not unfavorable candidates for SRS alone (Table 3).
Nevertheless, we should keep in mind that survival is determined mainly by systemic disease rather than intracranial status. Although debates continue as to whether >5 intracranial lesions at the time of SRS tend to be associated with more new lesions at the first follow-up MR imaging, thereby warranting additional treatment including WBRT or further SRS in a short interval, extensive intracranial and extracranial disease burdens remain a concern.
Is SRS for multiple BMs safe?
In a phantom experiment, the first author and another group of colleagues analyzed cumulative whole brain irradiation doses based on the treatment protocol for a patient with 48 lesions.10 The estimated cumulative irradiation doses were 2.60–6.69 Gy at sites located some distance from the targets, indicating that whole brain irradiation was not delivered at unacceptably high doses. It is noteworthy that these results are highly consistent with those described in this study. Yang et al, based on their dose–volume histogram analysis using a model with placement of 25 targets within the whole brain followed by irradiation with a maximum dose of 40 Gy, reported that the 50% whole brain dose was no more than 5 Gy.24 Furthermore, Boone et al reported recently, based on their experiences managing 10 patients with 6–15 BMs treated using a linear accelerator system, that the largest calculated cumulative dose to the entire brain was ~5.0 Gy.25 In 2002, the first author and another group of colleagues, studying a series of 80 patients with ≥10 BMs (median: 17, maximum: 43) undergoing SRS, estimated that the absorbed doses to the whole brain ranged from 2.16 to 8.51 Gy (median: 4.71 Gy).12 They also reported, based on 167 BM patients surviving more than 3 years after SRS (including 11 with ≥10 BMs), that tumor numbers had no impact on the incidence of SRS-related complications (HR: 1.066, 95% CI: 0.968–1.131, p=0.1567).26
Nevertheless, the safety and efficacy of this approach, using alternative technologies (eg, single isocenter linac techniques) and fractionation schemes (eg, 3–5 fraction schemes), have not yet been confirmed, and further studies are awaited. In fact, Ma et al found that normal brain volumes receiving 4 and 12 Gy were higher with a linac-based SRS platform than with Gamma Knife Perfexion, in patients with 3, 6, 9, and 12 irradiated tumors.27 However, this issue remains controversial.28–30
Two/three-staged treatment and fractionated GKRS for relatively large lesions
In general, it is recommended that a BM with a diameter exceeding 3 cm be surgically excised. However, some patients have contraindications for general anesthesia or refuse highly invasive operative procedures. In such cases, as Higuchi et al noted, three-staged GKRS (3-st-GK-Tx) is useful.31 According to their report describing 43 patients, a 10.0 Gy peripheral dose is delivered in each procedure with a 2-week interval. The overall MST after 3-st-GK-Tx was 8.8 months (95% CI: 6.5–11.1 months), and the actuarial survival rate was 62.5% at the 6th and 26.4% at the 12th post-3-st-GK-Tx month. The treatment results obtained by the present authors with this strategy were, surprisingly, quite similar to those of Higuchi et al’s patient group.32 In our 78 patients who underwent 3-st-GK-Tx, the overall MST after 3-st-GK-Tx was 8.1 (95% CI: 5.6–12.0) months, while the actuarial survival rate was 55.1% at the 6th and 35.2% at the 12th post-3-st-GK-Tx month. The incidences of neurological death, neurological deterioration, salvage SRS for new lesions, local recurrence, and treatment-related complications did not differ significantly between these two groups. Thus, we could reasonably conclude that carefully selected patients with relatively large BMs are favorable candidates for 3-st-GK-Tx.
Because three procedures are regarded as being burdensome for both patients and physicians, two-staged GKRS (2-st-GK-Tx) was proposed by Yomo et al.33,34 Their treatment strategy involved total doses of 20–30 Gy being delivered in two sessions with an interval of 3–4 weeks. Their treatment results and recently reported study results are summarized in Table 4.35–37
  | Table 4 List of studies regarding two-staged and three-stage gamma knife radiosurgery (GKRS) for patients with large brain metastases Abbreviation: NA, not available. |
The next question that specialists in this field needed to tackle was whether 3-st-GK-Tx or 2-st-GK-Tx is a better treatment. Recently, Serizawa et al38 conducted a multi-institutional retrospective study (JLGK1601, UMIN ID; 000022152) to reappraise which of these two protocols, that is, 3-st-GK-Tx or 2-st-GK-Tx, yielded better results. Their analyses revealed that there were no significant differences in several outcomes between the two strategies.38
Oligo-fractionated SRT using a GK
Oligo-fractionated SRT (3–5 fractions) has been widely applied for managing relatively large BMs. However, formerly, GK was rarely employed for oligo-fractionation because the standard GKRS technique requires a pin-based head frame. For the past few years, the Elekta Extend bite-block palatal vacuum immobilization system has been available. This system facilitates performing oligo-fractionated GK SRT. McTyre et al39 reported on 34 patients with meningiomas, pituitary adenomas, vestibular schwannomas, hemangiomas, or BMs. Although follow-up was brief, they concluded that fractionated GK SRT using this system was well tolerated in patients receiving treatments for large tumors. However, the Extend system is not yet in widespread use due to its technical complexity.39
A few years ago, an innovative gamma unit model, the Leksell Gamma Knife Icon (Elekta, A.B., Stockholm, Sweden), became available. This new model is anticipated to make fractionated GK SRS more accessible because it simplifies immobilization by eliminating either the conventional pin-fixation frame or the Extend bite block, with a mask fixation system being used instead.
Surgical removal and SRS
Postoperative SRS
Historically, combining surgical removal and subsequent WBRT was the gold standard for managing patients with a single, relatively large BM, whether symptomatic or not. This approach was based on the Patchell et al report showing that adjuvant WBRT following total removal of a single brain BM significantly reduced local recurrence and remote tumor development rates as compared with those in patients undergoing surgical resection alone.40 However, they employed a total WBRT dose of 50.4 Gy/28 fr which is very high and would never be applied today. Thus, Kocher et al conducted a randomized controlled trial designed to compare treatment results between two patient groups, one receiving surgery/SRS plus WBRT with a total dose of 30 Gy/10 fr and the other surgery/SRS alone.41 However, local recurrence and neurological death incidences (27% and 27.8%) were far higher than those obtained by Patchell et al (10.2% and 14.4%). In addition, as noted above, WBRT carries the risk of deterioration of neurocognitive function in relatively long surviving patients.8,42 As the present authors described in another report,43 diffuse white matter change, which is suspected to possibly increase the risk of future dementia, was detected by MR imaging in 8%, 50%, 63%, and 84% of patients, respectively, 6, 12, 18, and 24 months after WBRT. Therefore, the current trend favors withholding WBRT until it is necessary, that is, until the development of meningeal or miliary dissemination for which there are no other therapeutic options. Retrospective studies on SRS for postoperative irradiation, reported in the past decade, are summarized in Table 5.44–54
In the authors’ own series of 209 patients who underwent surgical tumor removal plus GK SRS without WBRT, local control failure at the resection site was documented in 10.0%, while 30.6% of these patients developed remote lesions. As presented in Table 5, the MST was 9.8 months, the local recurrence rate 10.0%, and the neurological death rate 19.0%, results very similar to those obtained by Patchell et al.40 However, the remote recurrence rate (30.6%) in our present study was lower than that in Patchell et al’s observation group (36.9%).40 It is widely accepted that remote recurrence is more frequent in patients with multiple BMs than in those harboring only a single lesion. Although the results reported by Patchell et al were based entirely on patients with a solitary lesion, 79% of patients in our study had multiple tumors. Furthermore, we consider these differences to reflect the quality of neuroimaging techniques, especially MR imaging, which has advanced remarkably over the past 20 years. The patients in Patchell et al’s study were treated over a 10-year period before 1998, while our patients were all treated in the decade after 1998. With an MR imaging unit capable of higher performance, smaller lesions can be detected much earlier and promptly treated by GK SRS.55 In fact, the majority of recently published studies have demonstrated higher rates of remote recurrence (maximum of 72.3%) than the 45.6% in Patchell et al’s observation group (Table 5).40
Preoperative SRS
As already mentioned, the relatively high incidence of remote recurrence remains the most serious weakness of postoperative SRS. In particular, cerebrospinal fluid-seeding occurred during follow-up in several patients who had received postoperative SRS. Therefore, we previously reported that the incidence of subdural seeding was significantly lower in patients given preoperative GKRS (14.3%/12 months after treatment) than in those receiving postoperative GKRS (61.5%/12 months after treatment, adjusted HR: 9.095, 95% CI: 1.107–74.704, p=0.0339), although neither MST (8.9 months/post-op vs 10.5/pre-op, HR: 1.067, 95% CI: 0.510–2.227, p=0.8638) nor 12th-posttreatment month incidences of new lesions being detected in the brain parenchyma (32.8%/post-op vs 42.1/pre-op, adjusted HR: 1.359, 95% CI: 0.331–5,581, p=0.6703) differed significantly between these two groups.51
Asher et al reported, based on 47 BM patients given SRS followed by surgical removal, respective cumulative survival rates of 77.8% and 60.0% at 6 and 12 months and cumulative local control rates of 97.8%, 85.6%, and 71.8% at 6, 12, and 24 months.56 According to the results of their analyses, only 14.8% of the patients received WBRT. Local failure was more likely with lesions >10 cc (p=0.01) and >3.4 cm (p=0.014), with trends being noted for surface lesions (p=0.066) and eloquent areas (p=0.052). They concluded that their results demonstrated overall safety and local control equal to, or even better than, those of other published approaches, suggesting the feasibility of this approach as a novel treatment for BM.
Multiple GK SRS procedures
Although WBRT is generally considered to not be repeatable, SRS has overcome this limitation. Therefore, a growing number of BM patients have recently been treated with SRS twice, three times, or even more. Recent retrospective or prospective studies based on more than 1000 BM patients given SRS alone have demonstrated that re-SRS for new tumors was required in 22%–34% of all cases.5,57–60 While a number of retrospective studies have documented re-SRS to be safe and effective, all were based on relatively small patient numbers (Table 6).61–66
  | Table 6 List of studies regarding repeat radiotherapy Abbreviations: GKRS, gannma knife radiosurgery; SRS, seteretactic radiosurgery; SRT, stereotactiv radiotherapy; NA, not available. |
Koiso et al carried out a retrospective study that was based on a rather large sample size (859 patients) and employed robust statistical methods, that is, competing risk analyses for secondary end points.67 They found that post-2nd SRS MST was 7.4 (95% CI: 7.0–8.2) months. The respective actuarial survival rates were 58.2% and 34.7% at the 6th and 12th post-2nd SRS month. Actuarial neurological death-free survival rates were 94.4% at the 6th and 86.6% at the 12th post-2nd SRS month. The cumulative incidences of local recurrence were 11.2% and 14.9% at the 12th and 24th post-2nd SRS month, respectively. The respective cumulative incidences of neurological deterioration were 4.5%, 5.8%, 6.7%, 7.2%, and 7.5% at 12, 24, 36, 48, and 60 months after the 2nd SRS. SRS-related complications were documented in 25 patients (2.9%). The cumulative incidences of complications were 1.4%, 2.0%, 2.4%, 3.0%, and 3.0% at 12, 24, 36, 48, and 60 months after the 2nd SRS, respectively. Koiso et al67 concluded that post-2nd SRS results, not only OS but other secondary end points as well, were not inferior to those after the 1st SRS. Most notably, maintenance of good neurological condition can be anticipated even at the 5th post-2nd SRS year in more than 90% of patients.
Several prognostic grading indexes have been proposed for patients with newly diagnosed BM. However, little is known about prognostic grading indexes for patients receiving salvage treatments, that is, surgery, WBRT, or SRS/SRT, and so on. We tested, in a data set of 804 re-GKRS patients, the validity of applying five prognostic indices, RPA, Score Index for Radiosurgery, Basic Score for Brain Metastases, Graded Prognostic Assessment, and Modified RPA.68–74 Among these five systems, based on patient number proportions, MST separation among three/four groups, and/or detailed reflection of status changes, the Modified RPA system was concluded to be the most applicable to re-SRS patients.74 Very recently, a unique grading system, BM velocity (a cumulative number of new brain tumors divided by an interval [years] between the day of the first SRS and the day when follow-up MR imaging showed new BMs), which was specific for post-SRS salvage SRS, was proposed. However, no validity tests using different data sets have been published.75
Interpretations
Finally, all specialists working in this field would be well advised to keep in mind the words of Lindquist and Steiner,76 “Although effective, it must be realized that radiosurgery at best only kills intracranial tumor cells. Suffering should not be prolonged by treatment of terminal patients. Which tumors should be treated? How many tumors can and should be treated?” The gradual diminution of consciousness with the progression of BM, which inevitably occurs, might be Nature’s way of relieving the suffering of terminally ill cancer patients. Recent advances in multidisciplinary management strategies do, however, allow physicians to relieve much of the suffering associated with these end-stage diseases. Furthermore, as physicians, we should always accept the challenge of managing patients with very complex disorders, so long as the patients themselves want to continue active treatment efforts. We should also keep in mind that maintenance of good neurological function and, ultimately, a reduced neurological death incidence, are now recognized as being crucial for managing BM patients.
Disclosure
The authors report no conflicts of interest for this work
References
Leksell L. Cerebral radiosurgery. I. Gammathalamotomy in two cases of intractable pain. Acta Chir Scand. 1968;134:585–595. | ||
Backlund EO. Gamma knife, the early story: memories of a privileged man. Prog Neurol Surg. 2007;20:21–32. | ||
Lindquist C. Gamma knife surgery for recurrent solitary metastasis of a cerebral hypernephroma: case report. Neurosurgery. 1989;25:802–804. | ||
Kann BH, Park HS, Johnson SB, Chiang VL, Yu JB. Changing practice patterns and disparities in the United States. J Natl Compr Canc Netw. 2017;15:1494–1502. | ||
Yamamoto M, Serizawa T, Shuto T, et al. Results of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective study. Lancet Oncol. 2014;15:387–395. | ||
Yamamoto M, Serizawa T, Higuchi Y, et al. A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 Study Update): irradiation-related complications and long-term maintenance of Mini-Mental State Examination scores. Int J Radiat Oncol Biol Phys. 2017;99:31–40. | ||
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491 | ||
Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trail. Lancet Oncol. 2009;10:1037–1044. | ||
Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–409. | ||
Yamamoto M, Ide M, Jimbo M, et al. Gamma knife radiosurgery with numerous target points for intracranially disseminated metastases: early experience in three patients and experimental analysis of whole brain irradiation doses. In: Kondziolka D, editor. Radiosurgery 1997. Vol 2. Basel: Karger; 1998:94–109. | ||
Suzuki S, Omagari J, Nishio S, Nishiye E, Fukui M. Gamma knife radiosurgery for simultaneous multiple metastatic tumors. J Neurosurg. 2000;93(Suppl 3):30–31. | ||
Yamamoto M, Ide M, Nishio S, Urakawa Y. Gamma knife radiosurgery for numerous brain metastases: Is this a safe treatment? Int J Radiat Oncol Biol Phys. 2002;53:1279–1283. | ||
Kim CH, Im YS, Nam DH, Park K, Kim JH, Lee JI. Gamma knife radiosurgery for ten or more brain metastases. J Korean Neurosurg Soc. 2008;44:358–363. | ||
Yamamoto M, Barfod BE, Urakawa Y. Gamma knife radiosurgery for brain metastases of non-lung cancer origin: focusing on multiple brain lesions. Prog Neurol Surg. 2009;22:154–169. | ||
Chang WS, Kim HY, Chang JW, Park YG, Chang JH. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113(Suppl):73–78. | ||
Rava P, Leonard K, Sioshansi S, et al. Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2011;119:457–462. | ||
Yamamoto M, Kawabe T, Barfod BE. How many metastases can be treated with radiosurgery? Prog Neurol Surg. 2012;25:261–272. | ||
Grandhi R, Kondziolka D, Panczykowski, D, et al. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg. 2012;117:237–245. | ||
Yamamoto M, Kawabe T, Sato Y, et al. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1-4 vs ≥ 5 tumors: clinical article. J Neurosurg. 2013;118:1258–1268. | ||
Yamamoto M, Kawabe T, Sato Y, et al. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for 2-9 vs ≥10 tumors. J Neurosurg. 2014;121(Suppl 2):16–25. | ||
Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. | ||
Knisely JPS, Yamamoto M, Gross CP. Radiosurgery alone for five or more brain metastases: expert opinion survey. J Neuorosurg. 2010;113(Suppl):84–89. | ||
The National Comprehensive Cancer Network Guideline. 2013. Available from: www.ncbi.nlm.nih.gov. Accessed December 31, 2013. | ||
Yang CC, Ting J, Wu X, Markoe A. Dose volume histogram analysis of the gamma knife radiosurgery treating twenty-five metastatic intracranial tumors. Stereotact Funct Neurosurg. 1998;70(Suppl 1):41–49. | ||
Boone RA, Solberg TD, Selch MT, et al. Cumulative dose to targets, critical structures, and the whole brain from the treatment of multiple intracranial metastases. In: Alexander E III, Kondziolka D, Lindquist C, et al, editors. Radiosurgery 1999. Basel: Karger; 2000:247–256. | ||
Yamamoto M, Kawabe T, Higuchi Y, et al. Delayed complications in patients surviving at least 3 years after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85:53–60. | ||
Ma L, Nichol A, Hossain S, et al. Variable dose interplay effects across radiosurgical apparatus in treating multiple brain metastases. Int J Comput Assist Radiol Surg. 2014;9:1079–1086. | ||
Thomas EM, Popple RA, Wu X, et al. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and gamma knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75:409–417. | ||
Ma L, Sahgal A, Larson DA. Letter: volumetric arc therapy (RapidArc) vs gamma knife radiosurgery for multiple brain metastases. Neurosurgery. 2015;76:E353. | ||
Thomas EM, Popple RA, Markert JM, Fiveash JB. In reply: volumetric arc therapy (RapidArc) vs gamma knife radiosurgery for multiple brain metastases: not only a dosimetric issue. Neurosurgery. 2015;77:E311. | ||
Higuchi Y, Serizawa T, Nagano O, et al. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009;74:1543–1540. | ||
Yamamoto M, Higuchi Y, Serizawa T, et al. Three-Stage Gamma Knife Treatment for Metastatic Brain Tumors Larger than 10 cc: A Two Institute Study including Re-Analyses of Results Published by Higuchi and Colleagues Using Competing Risk Analysis. Paper present at: the 19th International Leksell Gamma Knife Society Meeting, Dubai; March 6, 2018; United Arab Emirates. | ||
Yomo S, Hayashi M, Nicholson C. A prospective pilot study of two-session Gamma Knife surgery for large metastatic brain tumors. J Neurooncol. 2012;109:159–165. | ||
Yomo S, Hayashi M. A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma Knife stereotactic radiosurgery. Radiat Oncol. 2014;9:132. | ||
Dohm Q, McTyre ER, Okoukoni C, et al. Staged stereotactic radiosurgery for large brain metastases: Local control and clinical outcomes of a one-two punch technique. Neurosurgery. 2017 Epub Jul 7. | ||
Hasegawa T, Kato T, Yamamoto T, et al. Multisession gamma knife surgery for large brain metastases. J Neurooncol. 2017;131:517–524. | ||
Angelov L, Mohammadi AM, Bennett EE, et al. Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg. Epub 2017 Sep 22. | ||
Serizawa T, Higuchi Y, Yamamoto M, et al. Comparison of treatment results between 3- and 2-staged gamma knife radiosurgery for large brain metastases: a multi-institutional retrospective study (JLGK1601). Paper present at: the 5th Asian Leksell Gamma Knife Society Meeting, Cheju; November 3, 2017; Taiwan. | ||
McTyre E, Helis CA, Farris M, et al. Emerging indications for fractionated gamma knife radiosurgery. Neurosurgery. 2017;80:210–216. | ||
Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. | ||
Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. | ||
DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. | ||
Yamamoto M. Radiosurgery for metastatic brain tumors. Prog Neurol Surg. 2007;20:106–128. | ||
Bahl G, White G, Alksne J, Vemuri L, Spear MA. Focal radiation therapy of brain metastases after complete surgical resection. Med Oncol. 2006;23:317–324. | ||
Kim PK, Ellis TL, Stieber VW, et al. Gamma knife surgery targeting the resection cavity of brain metastasis that has progressed after whole-brain radiotherapy. J Neurosurg. 2006;105(Suppl):75–78. | ||
Yamamoto M, Kawabe T, Barfod BE, et al. Can pre-operative GKRS prevent meningeal dissemination in brain MET patients? A case-matched study. Paper presented at: 10th Congress of International Stereotactic radiosurgery Society; 2011; Paris. | ||
Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187–193. | ||
Iwai Y, Yamanaka K, Yasui T. Boost radiosurgery for treatment of brain metastases after surgical resection. Surg Neurol. 2008;69:181–186. | ||
Mathieu D, Kondziolka D, Flickinger JC, et al. Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 2008;62:817–823. | ||
Limbrick DD Jr, Lusis EA, Chicoine MR, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71:280–288. | ||
Jagannathan J, Yen CP, Ray DK, et al. Gamma knife radiosurgery to surgical cavity following resection of brain metastases. J Neurosurg. 2009;111:431–438. | ||
Robbins JR, Ryu S, Kalkanis S, et al. Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. 2012;71:937–943. | ||
Johnson MD, Avkshtol V, Baschnagel AM, et al. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94:537–543. | ||
Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. | ||
Hayashi M, Yamamoto M, Nishimura C, Satoh H. Do recent advances in MR technologies contribute to better gamma knife radiosurgery treatment results for brain metastases? Neuroradiol J. 2007;20:481–490. | ||
Asher AL, Burri SH, Wiggins WA, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. | ||
Karlsson B, Hanssens P, Wolff R, Söderman M, Lindquist C, Beute G. Thirty years’ experience with gamma knife surgery for metastases to the brain. J Neurosurg. 2009;111:449–457. | ||
Serizawa T, Hirai T, Nagano O, et al. Gamma knife surgery for 1-10 brain metastases without prophylactic whole-brain radiation therapy: analysis of cases meeting the Japanese prospective multi-institute study (JLGK0901) inclusion criteria. J Neurooncol. 2010;98:163–167. | ||
Serizawa T, Saeki N, Higuchi Y, et al. Diagnostic value of thallium-201 chloride single-photon emission computerized tomography in differentiating tumor recurrence from radiation injury after gamma knife surgery for metastatic brain tumors. J Neurosurg. 2005;102(Suppl):266–271. | ||
Watanabe S, Yamamoto M, Sato Y, et al. Stereotactic radiosurgery for brain metastases: a case-matched study comparing treatment results for patients 80 years of age versus patients 65-79 years of age. J Neurosurg. 2014;121:1148–1157. | ||
Yamanaka K, Iwai Y, Yasui T, et al. Gamma knife radiosurgery for metastatic brain tumor: the usefulness of repeated gamma knife radiosurgery for recurrent cases. Stereotact Funct Neurosurg. 1999;72(Suppl 1):73–80. | ||
Chen JC, Petrovich Z, Giannotta SL, Yu C, Apuzzo ML. Radiosurgical salvage therapy for patients presenting with recurrence of metastatic disease to the brain. Neurosurgery. 2000;46:860–866. | ||
Shuto T, Fujino H, Inomori S, Nagano H. Repeated gamma knife radiosurgery for multiple metastatic brain tumours. Acta Neurochir (Wien). 2004;146:989–993. | ||
Kwon KY, Kong DS, Lee JI, Nam DH, Park K, Kim JH. Outcome of repeated radiosurgery for recurrent metastatic brain tumors. Clin Neurol Neurosurg. 2007;109:132–137. | ||
Kim DH, Schultheiss TE, Radany EH, Badie B, Pezner RD. Clinical outcomes of patients treated with a second course of stereotactic radiosurgery for locally or regionally recurrent brain metastases after prior stereotactic radiosurgery. J Neurooncol. 2013;115:37–43. | ||
McKay WH, McTyre ER, Okoukoni C, et al. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J Neurosurg. 2017;127:148–156. | ||
Koiso T, Yamamoto M, Kawabe T, et al. Follow-up results of brain metastasis patients undergoing repeat gamma knife radiosurgery. J Neurosurg. 2016;125(Suppl 1):2–10. | ||
Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. | ||
Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–1161. | ||
Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–224. | ||
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. | ||
Yamamoto M, Sato Y, Serizawa T, et al. Sub-classification of recursive partitioning analysis class ii patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys. 2012;83:1933–1405. | ||
Yamamoto M, Serizawa T, Sato Y, et al. Validity of two recently-proposed prognostic grading indices for lung, gastro-intestinal, breast and renal cell cancer patients with radiosurgically-treated brain metastases. J Neurooncol. 2013;111:327–335. | ||
Yamamoto M, Kawabe T, Higuchi Y, et al. Validity of prognostic grading indices for brain metastasis patients undergoing repeat radiosurgery. World Neurosurg. 2014;82:1242–1249. | ||
Farris M, McTyre ER, Cramer CK, et al. Brain metastasis velocity: a novel prognostic metric predictive of overall survival and freedom from whole-brain radiation therapy after distant brain failure following upfront radiosurgery alone. Int J Radiat Oncol Biol Phys. 2017;98:131–141. | ||
Lindquist C, Steiner L. Radiosurgery for tumors. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. 2nd ed. New York: McGraw-Hill; 1996:1887–1907. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


