Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Moderate Treadmill Training Induces Limited Effects on Quadriceps Muscle Hypertrophy in Mice Exposed to Cigarette Smoke Involving Metalloproteinase 2
Authors Vieira Ramos G, Sousa Neto IV , Toledo-Arruda AC, Marqueti RC , Vieira RP , Martins MA, Salvini TF, Durigan JLQ
Received 28 June 2021
Accepted for publication 29 September 2021
Published 6 January 2022 Volume 2022:17 Pages 33—42
DOI https://doi.org/10.2147/COPD.S326894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Gracielle Vieira Ramos, 1, 2 Ivo Vieira de Sousa Neto, 3 Alessandra Choqueta Toledo-Arruda, 4, 5 Rita de Cassia Marqueti, 3 Rodolfo P Vieira, 6– 8 Milton A Martins, 5 Tânia F Salvini, 9 João Luiz Quaglioti Durigan 10
1Physical Therapy Division, University of Brasilia, Brasília, DF, Brazil; 2Department of Physical Therapy, University Paulista, Brasília, DF, Brazil; 3Laboratory of Molecular Analysis, Graduate Program of Sciences and Technology of Health, Universidade de Brasília, Brasília, DF, Brazil; 4Faculty of Physiotherapy, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; 5Department of Medicine Clinical (LIM 20), School of Medicine, University of São Paulo, São Paulo, Brazil; 6Universidade Brasil, Post-Graduation Program in Bioengineering, São Paulo, Brazil; 7Laboratory of Pulmonary and Exercise Immunology (LABPEI), Brazilian Institute of Teaching and Research in Pulmonary and Exercise Immunology (IBEPIPE) and Nove de Julho University (UNINOVE), São Paulo, SP, Brazil; 8Federal University of Sao Paulo, Post-Graduation Program in Sciences of Human Movement and Rehabilitation, São Paulo, Brazil; 9Department of Physical Therapy, Federal University of São Carlos, São Carlos, Brazil; 10Rehabilitation Sciences Graduation Program, University of Brasilia, Brasília, DF, Brazil
Correspondence: Gracielle Vieira Ramos
Department of Physical Therapy, University Paulista, Sgas Quadra 913, s/nº - Conjunto B - Asa Sul, Brasília, DF, Brazil
, 70390-130 Email [email protected]
Background: Long-term cigarette smoke (CS) induces substantive extrapulmonary effects, including musculoskeletal system disorders. Exercise training seems to protect long-term smokers against fiber atrophy in the locomotor muscles. Nevertheless, the extracellular matrix (ECM) changes in response to aerobic training remain largely unknown. Thus, we investigated the effects of moderate treadmill training on aerobic performance, cross-sectional area (CSA), fiber distribution, and metalloproteinase 2 (MMP-2) activity on quadriceps muscle in mice exposed to chronic CS.
Methods: Male mice were randomized into four groups: control or smoke (6 per group) and exercise or exercise+smoke (5 per group). Animals were exposed to 12 commercially filtered cigarettes per day (0.8 mg of nicotine, 10 mg of tar, and 10 mg of CO per cigarette). The CSA, fibers distribution, and MMP-2 activity by zymography were assessed after a period of treadmill training (50% of maximal exercise capacity for 60 min/day, 5 days/week) for 24 weeks.
Results: The CS exposure did not change CSA compared to the control group (p> 0.05), but minor fibers in the frequency distribution (< 1000 μm 2) were observed. Long-term CS exposure attenuated CSA increases in exercise conditions (smoke+exercise vs exercise) while did not impair aerobic performance. Quadriceps CSA increased in mice nonsmoker submitted to aerobic training (p = 0.001). There was higher pro-MMP-2 activity in the smoke+exercise group when compared to the smoke group (p = 0.01). Regarding active MMP-2, the exercise showed higher values when compared to the control group (p = 0.001).
Conclusion: Moderate treadmill training for 24 weeks in mice exposed to CS did not modify CSA, despite inducing higher pro-MMP-2 activity in the quadriceps muscle, suggesting limited effects on ECM remodeling. Our findings may contribute to new insights into molecular mechanisms for CS conditions.
Keywords: physical exercise, chronic obstructive pulmonary disease, MMP-2, inflammation
Introduction
According to the World Health Organization (WHO), there are 1.3 billion tobacco users around the world.1 The tobacco epidemic is one of the biggest public health, killing more than 8 million people each year.1,2 Smoking is a major risk factor for premature non-communicable diseases, such as cardiovascular disorders, chronic obstructive pulmonary disease, and several cancers.3 Cigarette smoke (CS) can cause a reduction of functional ability and quality of life and thus represents an enormous healthcare and socioeconomic burden.2,3
Long-term CS can induce substantive extrapulmonary effects, including musculoskeletal system disorders. Kok et al4 have shown that smoking 100g tobacco per week resulted in a reduction of 2.9% in knee muscle strength in young men, independent of daily physical activity and cardiopulmonary fitness. Studies in human and animal’s models also revealed that CS promotes a decrease of ~25% in the fiber cross-sectional area, indicating that CS is a potential risk factor for a low lean body mass and COPD-related muscle weakness. Consequently, smokers are more susceptible to acute or chronic muscle deterioration.5,6
Regarding molecular mechanisms, in vivo and in vitro studies have already reported that CS appears to stimulate protein breakdown through muscle-specific ubiquitin proteolytic pathways, including muscle atrophy F-box (MAFBx),7 muscle ring finger-1 (MuRF1),8 p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK),9 besides up-regulation of myostatin, which is responsible by inhibition muscle growth via protein kinase B inactivation. These regulatory mechanisms promote several detrimental responses in skeletal muscle,10 such as reduction of contractile function and protein synthesis process, which can lead to muscle loss, mainly in the lower limbs.5 CS has several toxins with immunomodulatory effects that might impair fatigue resistance, exercise tolerance, and functional disability, culminating in fiber atrophy and skeletal muscle abnormalities.6,11 Such dysfunction is believed to be associated with impairment of muscle structure (ie, cross-sectional area, fiber-type distribution, capillary density),6 metabolic capacity, and cellular damage.12 However, the changes in muscle extracellular matrix (ECM) remain largely unknown in CS conditions.
The ECM is a 3-dimensional network of macromolecules connecting myofibers architecture and integrating them to ensure optimal force transmission within the skeletal muscle.13 The muscle ECM is essential for structural support, mechanical stability, and transmission signals from cells.14 Strategies to overcome possible harmful effects of ECM function might protect smokers from dysfunctional remodeling and musculoskeletal disorders.15 The modulation of ECM functions is regulated by matrix metalloproteinases (MMPs), a family of zinc-dependent endoproteases that degrade or remodel the ECM proteins, including collagen, fibronectin, and laminin and glycoproteins.16–18 The MMP-2 promotes positive effects in skeletal muscle, such as the release of local growth factors and the stimulation of angiogenesis, proliferation,17 differentiation, and migration of satellite cells to injury sites, allowing tissue repair.19,20 These adaptations are relevant to maintain the physiological functions and functional integrity of skeletal muscle in the setting of skeletal muscle hypertrophy.20
Exercise training modulates muscle ECM remodeling through MMP-2 activity.21 Previous studies showed that MMP-2 exerts essential regulatory roles in muscle fiber repair and connective tissue, controlling muscle plasticity after damage caused by exercise training.14,19,21 It has been shown that MMP-2 activation induces neoangiogenesis, muscle fiber growth via ECM remodeling, which facilitates physiological adaptations to exercise, such as aerobic performance capacity and muscle regeneration.13 Presumably, up-regulated MMP-2 activity might represent a potential mechanism induced by exercise, to protect the peripheral muscle from abnormalities inherent to CS exposure.22 It is known that MMP-2 is considered a critical biomarker for cell signaling, muscle morphogenesis, hypertrophy processes and tissue homeostasis maintenance. Thus, whether aerobic training modulates MMP-2 activity remains a valid question for muscle health and outlines treatment guidance for smokers.
Although mechanistic studies on muscular responses to exercise have increased in the past decade, the interaction of long-term smoking with aerobic exercise training in the perspective of muscle ECM remodeling processes is poorly understood. A better knowledge of the role played by the MMP-2 can help maximize the benefits of exercise in prevention and therapy. This study aimed to investigate the effects of moderate treadmill training on aerobic performance, cross-sectional area (CSA), fiber distribution, and metalloproteinase 2 (MMP-2) activity on the quadriceps muscle in mice exposed to chronic cigarette smoke. We hypothesize that CS would impair muscular performance accompanied by a decrease in the quadriceps muscle’s CSA and fiber distribution, while aerobic exercise training counterbalances such deleterious responses associated with muscle ECM remodeling.
Materials and Methods
Animals and Experimental Design
The current study was part of prior investigation.23 Therefore, most of the methodology (animals, cigarette smoke exposure protocol and treadmill aerobic training) used on this secondary analysis was the same. The details of the experimental design have been reported previously.23 Male C57BL/6 mice (weighing 22 ± 2g; 6–8 weeks old) were randomly divided into four groups: 1) control (n=6); 2) smoke (exposed to cigarette smoke; n=6); 3) exercise (submitted to treadmill training; n=5) and 4) smoke+exercise (submitted to both treadmill training and cigarette smoke exposure; n=5).
The animals were kept in plastic cages under controlled environmental conditions (12-hour light/dark cycles) with water and standard chow ad libitum (Socil, São Paulo). The present study was approved by the Animal Research Ethics Committee of the University of São Paulo (protocol number: CAPpesq/025/10). The research has been carried out in accordance with the Guide for care and use of laboratory animals24 and international principles for research involving animals (ARRIVE 2.0).25
Cigarette Smoke Exposure Protocol
Animals were exposed to 12 commercially filtered cigarettes per day (0.8 mg of nicotine, 10 mg of tar, and 10 mg of CO per cigarette). The exposure was performed, placing the animals into a box (inhalation chamber), maintaining controlled CO levels (250 to 350 ppm) for 30 min/day,−1 5 days/week for 24 weeks. Control animals were exposed to the same protocol but using room air.22,23 The experimental model increases the density of inflammatory cells (macrophages), MCP-1 expression, and ROS production in bronchoalveolar lavage while reducing IL-10 levels.23 These effects were accompanied by reduced tissue damping and pulmonary elastance, hallmarks of emphysema.23
Treadmill Aerobic Training and Test
Before treadmill aerobic training, the animals were submitted to an exercise adaptation period (3 days, 15 min/day, 25% inclination, and 0.2 km/h). Subsequently, the mice were submitted to a physical test to evaluate their maximal exercise capacity (100%) in physical training. The animals were trained at 50% of maximal exercise capacity for 60 min/day, 5 days/week, for 24 weeks. Both treadmill aerobic training and CS exposure started on the same day and continued for 24 weeks. CS exposure was accomplished after 1 hour of physical training.23
Euthanasia
The animals were euthanized using an intraperitoneal injection of xylazine solution (12 mg/kg of body weight) and ketamine (95 mg/kg of body weight) 48h after the end of the experimental period.
Histology
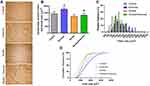
For histology analysis, we fixed the samples with 4% paraformaldehyde in PBS at room temperature for 24 h. Samples were embedded in OCT (and histological muscle cross-sections were obtained (one section of 10μm) in a cryostat microtome (Microm HE 505, Jena, Germany). Samples were kept in 70% ethanol. Sections were incubated with a dual endogenous enzyme–blocking reagent (Dako, Carpinteria, CA, USA) to quench any endogenous peroxidases. Slides were incubated with a solution containing the primary antibodies in 1% bovine serum albumin in 0.1M PBS overnight in the dark at 4°C. We performed immunohistochemical to evaluated the primary antibody (Mouse Anti-Collagen IL-10 Monoclonal Antibody, 1:100, Santa Cruz, Dallas, USA), but the primary antibody was not detected. A Universal LSAB + Kit/HRP, Rabbit/Mouse/Goat (Dako) was used to detect immunoactivity according to the manufacturer’s instructions. To develop the signal, we used DAB (Dako) followed by a hematoxylin counterstain. The slides were rinsed, dehydrated, and coverslipped with Permanent Mounting Medium (Thermo Fisher Scientific). Images from four different muscle regions of each animal (4 per group) were obtained by a digital camera Axioplan (Carl Zeiss, Oberkochen, Germany) at 10X amplification, coupled to a binocular microscope (Olympus® BX51). The CSA of 400 fibers was randomly chosen from each image. Boundaries of individual muscle fibers were delineated, and fiber CSA was determined from the number of pixels within the outlined fiber using the ImageJ software (National Institutes of Health, Bethesda, MD). All images were analyzed by the same researcher in a blinded design, in which the analyzer was not aware of the experimental group. Fiber area-frequency histograms and cumulative frequency distribution were calculated as previously described.26,27
Zymography
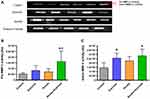
Tissue extraction and zymography analysis have been performed according to the protocol used in other studies published by our laboratory.22,28 Quadriceps extracts (25 mg) was incubated in 2 mL of extraction buffer (10 mmol.L–1 cacodylic acid (pH 5.0), 0.15mol.L–1 NaCl, 1mol.L–1 ZnCl2, 20mmol. L–1 CaCl2, 1.5 mmol. L–1 NaN3, 0.01% Triton X-100 (v/v)) at 4 °C overnight with continuous mixing. After this period, the solution was centrifuged for 20 min (13,000 g at 4 °C). Protein concentrations were measured with a BCA protein assay kit (Thermo Scientific, USA).
Samples of six animals (control and smoke) or five (exercise and smoke+exercise) were evaluated, and each of them was normalized for the total amount of protein (30μg). The samples were analyzed by electrophoresis in a polyacrylamide gel containing 10% SDS and gelatin at a final concentration of 1 mg/mL. The gelatinolytic activity was manifested as horizontal white bands on a blue background. MMP-2 activity was determined by densitometric scanning of the bands (ImageScanner III, Lab- Scan 6.0). The band intensity averages were measured using Image Master 2D Platinum 7.0 software and were conducted by a blinded researcher, attenuating possible bias related to this process. MMP2 activity bands were identified according to their molecular weight (pro-MMP-2; 64 kDa: 62 kDa: active-MMP-2) according to previous studies.14,21,29
The assurance of the analysis accuracy, the gels for zymography were prepared simultaneously using the same solutions. Gel electrophoresis was carried out simultaneously using fresh buffers at the same condition and temperature (inside the fridge) to minimize the variation between the gels. Furthermore, protein normalization, voltage, and time during electrophoresis, and gel staining background have been carefully standardized. A same experienced researcher performed all gels to minimize the groups’ variation and generate a banding pattern. The band intensities were measured by a blinded researcher, attenuating possible bias related to this process.
Statistical Analysis
Data are presented as mean and standard deviation (SD). Shapiro–Wilk test was used to verify data normality. A two-way mixed ANOVA was used to compare body weight, time running, and speed evaluated in the baseline, 8, 16, and 24 weeks. Compound sphericity was verified by the Mauchly’s test. When the assumption of sphericity was not met, the significance of F-ratios was adjusted according to the Greenhouse–Geisser procedure. A two-way independent ANOVA (smoke and exercise training as factors) was used to compare groups. The Tukey post hoc test was used to identify differences. An alpha level of p ≤ 0.05 was considered significant. The sample size power for all variables was verified post hoc using G*Power version 3.1.3 (Kiel University, Kiel, Germany), with alpha level = 0.05 and power (1 - β) = 0.8. The software GraphPad Prism 7.0 for Mac (San Diego, CA, USA) was used for statistical analysis and graphics design. The Servier Medical Art and Mind the Graph web-based software was used to create the last figure.
Results
Bodyweight Gain and Aerobic Performance Analysis
There were no differences between experimental groups at baseline (p > 0.05). Exercise aerobic training induced body weight gain and improved the time running and speed in both exercise groups (exercise and smoke+exercise) in the 8, 16, and 24 weeks compared to animals not submitted to moderate treadmill training (p =0.01; p =0.01 and p =0.01; Figure 1A–C). Regarding the time effect, we observed similar performance after 16 and 24 weeks of training (p > 0.05). There were no significant differences between control and smoke groups or between exercise and smoke+exercise groups (p > 0.05).
Cross-Sectional Area and Fibers Size Distribution
Figure 2A shows the CSA quadriceps muscle qualitative analysis of histological sections in all experimental groups. Cigarette smoke exposure for 24 weeks in a condition of physical inactivity did not promote change in quadriceps CSA compared to the control group (p<0.05, Figure 2B), but smaller fibers in the relative frequency (250 to 750 µm2) was observed in the smoke group compared to other groups (Figure 2C). Furthermore, there is a significant shift to the left in the cumulative frequency distribution for the Smoke group (Figure 2D). However, higher fibers in the relative frequency (1250 to 1750 µm2) were observed in the smoke+exercise compared to the smoke group (Figure 2C). Moderate treadmill training induced an increase of CSA in the exercise group (p=0.001, Figure 2B), but this effect is not observed in mice exposed to CS (smoke+exercise vs smoke; p<0.05).
MMP-2 Activity
Figure 3A shows optical densitometry of zymographic bands in all experimental groups. There was higher pro-MMP-2 activity in the smoke+exercise group when compared to the control and smoke groups (p = 0.01; Figure 3B). Regarding active MMP-2 activity, exercise and smoke+exercise groups showed the highest values compared to the control group (p = 0.001; Figure 3C).
Discussion
This study demonstrates that long-term CS exposure did not change CSA compared to the control group, but minor fibers in the frequency distribution (250 to >1000µm2) were observed in the smoke group. Moreover, CS exposure attenuated quadriceps muscle CSA increases in the exercise condition (smoke+exercise vs exercise) while it did not impair aerobic performance indicators (speed and time running). Contrary to our hypothesis, moderate treadmill training did not appear to influence CSA and active MMP-2 activity in the CS condition (smoke+exercise vs Smoke), whereas it increased in nonsmoker mice. These data indicate limited effects of moderate aerobic exercise on ECM remodeling of skeletal muscle during CS exposure. Our findings may contribute to new insights into molecular mechanisms and training adaptations for CS conditions and open new avenues for therapeutic interventions. A schematic representation of experimental groups, analysis, and main findings was created to clarify the results of the present study (Figure 4).
 |
Figure 4 Overview of quadriceps muscle remodeling exposed to smoke and aerobic exercise training. (A–C) represents experimental groups, analysis, and main results. Moderate treadmill training for 24 weeks in mice exposed to cigarette smoke did not promote CSA gain, despite inducing higher pro-MMP-2 activity in quadriceps muscle mass. The figure was created in the Servier Medical Art (www.smart.servier.com) licensed under a Creative Commons Attribution 3.0 Unported (free) and Mind the Graph platform (www.mindthegraph.com) licensed under the Attribution-Share Alike 4.0 International license. |
Interestingly, mice exposed to CS for 24 weeks did not present loss of quadriceps muscle mass. Moreover, no increase in CSA was observed after aerobic training in the mice exposed to CS, which might be explained by muscle fiber-type predominance of the quadriceps muscle. For instance, Montes de Oca et al30 reported in vastus lateralis that the harmful effects of CS on capillary numbers and CSA were more pronounced in type I fibers when compared to type II fibers. Nakatani et al31 also noted damages in the soleus muscle (predominance of slow-twitch-type 1) of spontaneously hypertensive rats exposed to CS. It has been also demonstrated that smokers exhibit a lower type I fibers proportion when compared to the healthy aging population.32 Collectively, these studies suggest that the harmful effects of CS on CSA with a predominance of type II fibers, such as quadriceps, would have a later onset compared to slow-twitch fibers that seem to be more sensitive to cigarette toxicity.
It is possible to assume that fibers are not all the same size and might respond to stress or mechanical stimuli differently.11 So, the use of frequency histograms according to diameter provides a more detailed and accurate quantitative analysis of fibers size distribution than reliance on mean size, which is influenced by the size and the relative number of cells with any given diameter. The heterogeneity of fiber size in the present study is consistent with previous reports on human33 and rodent skeletal muscle after CS exposure.11 Such heterogeneous fiber size distribution is believed to be associated with the redistribution of muscle fiber types observed in CS exposure, shifting from type I oxidative muscle fibers to type II glycolytic muscle fibers.11 A non-uniform atrophy response can be a considerable adaptation to the CS condition, generally related to physiological character.
Although CS exposure did not appear to induce substantial muscle atrophy, we showed smaller fibers in the relative frequency (250 to >1000µm2) for mice exposed to CS when compared to all groups (Figure 2). Furthermore, there is a significant shift to the left in the cumulative frequency distribution for the smoke group. Thus, one possibility that CS might trigger intracellular catabolic pathways responsible for muscle degradation signaling should not be ruled out. Other studies reinforce this suggestion, which showed that modulation of cell metabolic pathways could precede the muscle atrophy process.34,35 For instance, muscle-specific E3 ligases (Atrogin-1/MuRF1) were up-regulated in patients with COPD, but it has not been possible to see changes in CSA. This fact could clarify the absence of CSA modifications in the current study.
The extra-pulmonary changes inherent to CS could also reduce muscle endurance and strength, ultimately leading to diminished exercise capacity and regenerative response.11 Curiously, the speed and time running did not differ significantly between mice nonsmokers and smokers, which could be partially explained by similar body weight between groups and substantial metabolic adjustments. Optimal body weight maintenance may compensate the skeletal muscle energy metabolism during exercise to maintain exercise capacity and tolerance.36 Another possible explanation is that long-term aerobic training (24 weeks) may enhance tolerance of metabolic acidosis due to increased muscle buffer capacity and improved ionic regulation (26), thereby enabling power output maintenance during exercise and aerobic performance even in adverse conditions like CS exposure. Additionally, Chen et al37 explain that although skeletal muscle capacity is exhausted on the treadmill, reducing performance exercise induced by smoking occurs only when cardiopulmonary systems reach their maximal capabilities.
Previous studies have noticed that aerobic training might induce muscle hypertrophy, like those observed in resistance training, but in minor magnitude.38,39 This plausible mechanism reduces satellite cell pool activation, which promotes muscle mass increase post-exercise.40,41 Nogueira et al6 reported that mice exposed to tobacco for 8 weeks displayed a reduced number of quiescent satellite cells, transcription factor PAX7 (paired box 7), and capillaries in isolated slow-twitch type I fibers (soleus muscle) when compared with the non-smoking group. These adaptations may explain cellular saturation in which skeletal muscles become less mechanically sensitive with aerobic training. Future studies may wish to compare the effects of different exercise modalities (resistance, aerobic, and combined) on molecular mechanisms after CS exposure.
Accumulating evidence suggests that ECM remodeling enables the fiber to expand in girth to afford satellite cell incorporation.42–44 Regulation of MMP-2 activity is a significant factor for successful muscle regeneration. It seems plausible to speculate that compounds and toxins in the CS may hinder MMP-2 secretion or attenuate optimal ECM remodeling, promoting a delay in the MMP-2 activation cascade, which potentially restricts the proliferation, differentiation, and migration of satellite cells. Collectively, these mechanisms might explain why there were no changes in the MMP-2 activity in response to CS exposure. We also observed that moderate treadmill training for 24 weeks was an inefficient stimulus to induce active MMP-2 upregulation in mice exposed to CS. The responsiveness to exercise includes characteristics of the exercise regimen or dose and the response or effect characteristics. These hypotheses are supported by previous studies that showed higher MMP-2 activities in type II fibers following high-intensity aerobic and resistance exercise.14,19,21 Furthermore, Handler-Olsen et al45 showed a significant decrease in intracellular gelatinolytic activity in skeletal muscles of mice exposed to high-intensity interval training, suggesting that exercise intensity is a critical factor capable of reprogramming intracellular MMP-2. Thus, a mechanical-molecular set point may exist for MMP-2 induction in CS conditions.
Recent observations indicate that MMPs are regulated at transcriptional and post-transcriptional levels through their inhibitors (TIMPs).46,47 In the present study, moderate treadmill training for 24 weeks in mice exposed to cigarette smoke-induced higher pro-MMP-2 activity in the quadriceps muscle, but this effect was not observed in the active isoform. It has been demonstrated that higher TIMP-2 activity blocks the pro-MMP-2 activation in skeletal muscle.16 Thus, it may suggest that TIMPs molecular pathways could be involved in the smoke+exercise group. These mechanisms might clarify the distinct MMP-2 isoforms presented here and open the possibility that ECM remodeling may differ between smokers and nonsmokers. Additionally, it is essential to emphasize that the pleiotropic effects of aerobic exercise and the complexity of responses at the molecular level suggest no singular pathway mediating exercise adaptation. Thus, cellular homeostasis is achieved by a delicate balance between multiple pathways.
In a recent review, Marillier et al12 explained a potential regulatory mechanism for limited muscle hypertrophy in smokers. It is well established that hypoxemia and cachexia are debilitating comorbidities associated with CS that indicate the progression towards a more degenerative status and predict lower responses to exercise training once dampen the improvement in oxidative metabolism and muscle endurance.12 These harmful adaptations may clarify lower myoblast regenerative capacity in response to moderate aerobic exercise during CS exposure. The development of effective exercise should include creative protocols based on muscle mechanosensitivity to counterbalance harmful effects inherent to CS condition.
It is essential to point out some limitations of the present study. It was not possible to evaluate the type of muscle fibers and immunohistological analysis of ECM markers and atrophy/hypertrophy signaling pathways. These factors are relevant for muscle hypertrophy modulation in response to exercise training. The evaluation in a single time point also is a considerable limitation since molecular regulations involving MMP-2 activity at different time points might be present since the beginning of CS exposure. Therefore, future studies are necessary to clarify time-course effects on substantial muscle adaptations. The investigation of other proteins and regulatory molecules (eg, intracellular signal transducer and transcription factors) that participate directly in muscle hypertrophy needs to be included in future studies to clarify adjacent mechanisms. Additionally, studies involving different training protocols could help comprehend the potential mechanism involved in muscle plasticity to protect muscle mass from cigarette toxicity.
Conclusion
Moderate treadmill training for 24 weeks effectively increased active MMP-2 activity and CSA in quadriceps muscle in the nonsmoker mice; however, it did not promote CSA increases in CS condition despite inducing higher pro-MMP-2 activity, which suggests limited effects on ECM remodeling. Our findings may contribute to new insights into the molecular mechanism for smokers and open new avenues for therapeutic interventions to treat locomotor muscle dysfunctions.
Data Sharing Statement
The de-identified data sets generated during the study are available from the corresponding author.
Ethics Approval
The present study was approved by the Animal Research Ethics Committee of the University of Sao Paulo (protocol number: CAPpesq/025/10). The research has been carried out in accordance with the Guide for care and use of laboratory animals and international principles for research involving animals (ARRIVE 2.0).
Acknowledgments
This project was supported by Sao Paulo Research Foundation (FAPESP) Grant: 2012/15165-2, by Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) and by DPI/DPG n. 01/2021. We also thank Carolina Pinho for the grammar English review and Capes by fellowship research of Ph.D. at Gracielle Vieira Ramos. JLQD has a Research Scholarship in Physical Therapy (Tier 2) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (Process numbers: 312136/2018-8).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Mehrotra R, Grover S, Chandra A. Role of World Health Organization Framework Convention on Tobacco Control Global Knowledge Hub on Smokeless Tobacco. Indian J Med Res. 2018;148(1):7–13. doi:10.4103/ijmr.IJMR_2036_17
2. Peruga A, Lopez MJ, Martinez C, Fernandez E. Tobacco control policies in the 21st century: achievements and open challenges. Mol Oncol. 2021;15(3):744–752. doi:10.1002/1878-0261.12918
3. Sheffer CE, Al-Zalabani A, Aubrey A, et al. The Emerging Global Tobacco Treatment Workforce: characteristics of Tobacco Treatment Specialists Trained in Council-Accredited Training Programs from 2017 to 2019. Int J Environ Res Public Health. 2021;18(5):665. doi:10.3390/ijerph18052416
4. Kok MO, Hoekstra T, Twisk JW. The longitudinal relation between smoking and muscle strength in healthy adults. Eur Addict Res. 2012;18(2):70–75. doi:10.1159/000333600
5. Degens H, Gayan-Ramirez G, van Hees HW. Smoking-induced skeletal muscle dysfunction: from evidence to mechanisms. Am J Respir Crit Care Med. 2015;191(6):620–625. doi:10.1164/rccm.201410-1830PP
6. Nogueira L, Trisko BM, Lima-Rosa FL, et al. Cigarette smoke directly impairs skeletal muscle function through capillary regression and altered myofibre calcium kinetics in mice. J Physiol. 2018;596(14):2901–2916. doi:10.1113/JP275888
7. Petersen AM, Magkos F, Atherton P, et al. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab. 2007;293(3):E843–8. doi:10.1152/ajpendo.00301.2007
8. Tang K, Wagner PD, Breen EC. TNF-alpha-mediated reduction in PGC-1alpha may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol. 2010;222(2):320–327. doi:10.1002/jcp.21955
9. Caron MA, Morissette MC, Theriault ME, Nikota JK, Stampfli MR, Debigare R. Alterations in skeletal muscle cell homeostasis in a mouse model of cigarette smoke exposure. PLoS One. 2013;8(6):e66433. doi:10.1371/journal.pone.0066433
10. Saha Sp, Bhalla DK, Whayne TF
11. Cheung KK, Fung TK, Mak JCW, et al. The acute effects of cigarette smoke exposure on muscle fiber type dynamics in rats. PLoS One. 2020;15(5):e0233523. doi:10.1371/journal.pone.0233523
12. Marillier M, Bernard AC, Verges S, Neder JA. Locomotor Muscles in COPD: the Rationale for Rehabilitative Exercise Training. Front Physiol. 2019;10:1590. doi:10.3389/fphys.2019.01590
13. Zhang Q, Joshi SK, Lovett DH, et al. Matrix metalloproteinase-2 plays a critical role in overload induced skeletal muscle hypertrophy. Muscles Ligaments Tendons J. 2014;4(3):362–370. doi:10.32098/mltj.03.2014.16
14. de Sousa Neto IV, Durigan JLQ, Guzzoni V, et al. Effects of resistance training on matrix metalloproteinase activity in skeletal muscles and blood circulation during aging. Front Physiol. 2018;9:190. doi:10.3389/fphys.2018.00190
15. Martinez-Huenchullan S, McLennan SV, Verhoeven A, Twigg SM, Tam CS. The emerging role of skeletal muscle extracellular matrix remodelling in obesity and exercise. Obes Rev. 2017;18(7):776–790. doi:10.1111/obr.12548
16. Alameddine HS, Morgan JE. Matrix metalloproteinases and tissue inhibitor of metalloproteinases in inflammation and fibrosis of skeletal muscles. J Neuromuscul Dis. 2016;3(4):455–473. doi:10.3233/JND-160183
17. Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr. 2009;3(4):337–341. doi:10.4161/cam.3.4.9338
18. de Sousa Neto IV, Durigan JLQ, Carreiro de Farias Junior G, et al. Resistance training modulates the matrix metalloproteinase-2 activity in different trabecular bones in aged rats. Clin Interv Aging. 2021;16:71–81. doi:10.2147/CIA.S276518
19. Carmeli E, Moas M, Lennon S, Powers SK. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp Physiol. 2005;90(4):613–619. doi:10.1113/expphysiol.2004.029462
20. Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29(2):191–197. doi:10.1002/mus.10529
21. de Sousa Neto IV, Tibana RA, da Cunha Nascimento D, et al. Effects of resistance training volume on MMPs in circulation, muscle and adipose tissue. Int J Sports Med. 2017;38(4):307–313. doi:10.1055/s-0042-123192
22. Vieira Ramos G, Choqueta de Toledo-arruda A, Maria Pinheiro-Dardis C, et al. Exercise prevents diaphragm wasting induced by cigarette smoke through modulation of antioxidant genes and metalloproteinases. Biomed Res Int. 2018;2018:5909053. doi:10.1155/2018/5909053
23. Toledo AC, Magalhaes RM, Hizume DC, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39(2):254–264. doi:10.1183/09031936.00003411
24. Council NR. Guide for the Care and Use of Laboratory Animals.
25. Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol. 2020;598(18):3793–3801. doi:10.1113/JP280389
26. de Sousa Neto IV, Carvalho MM, Marqueti RC, et al. Proteomic changes in skeletal muscle of aged rats in response to resistance training. Cell Biochem Funct. 2020;38(4):500–509. doi:10.1002/cbf.3497
27. Oliveira JRS, Mohamed JS, Myers MJ, Brooks MJ, Alway SE. Effects of hindlimb suspension and reloading on gastrocnemius and soleus muscle mass and function in geriatric mice. Exp Gerontol. 2019;115:19–31. doi:10.1016/j.exger.2018.11.011
28. Durigan JL, Peviani SM, Russo TL, et al. Physical training leads to remodeling of diaphragm muscle in asthma model. Int J Sports Med. 2009;30(6):430–434. doi:10.1055/s-0028-1112145
29. Frederiks WM, Mook OR. Metabolic mapping of proteinase activity with emphasis on in situ zymography of gelatinases: review and protocols. J Histochem Cytochem. 2004;52(6):711–722. doi:10.1369/jhc.4R6251.2004
30. Montes de Oca M, Loeb E, Torres SH, De Sanctis J, Hernandez N, Talamo C. Peripheral muscle alterations in non-COPD smokers. Chest. 2008;133(1):13–18. doi:10.1378/chest.07-1592
31. Nakatani T, Nakashima T, Kita T, Ishihara A. Responses of exposure to cigarette smoke at three dosage levels on soleus muscle fibers in Wistar-Kyoto and spontaneously hypertensive rats. Jpn J Pharmacol. 2002;90(2):157–163. doi:10.1254/jjp.90.157
32. Maltais F, LeBlanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154(2 Pt 1):442–447. doi:10.1164/ajrccm.154.2.8756820
33. Chan SMH, Cerni C, Passey S, et al. Cigarette smoking exacerbates skeletal muscle injury without compromising its regenerative capacity. Am J Respir Cell Mol Biol. 2020;62(2):217–230. doi:10.1165/rcmb.2019-0106OC
34. Doucet M, Dube A, Joanisse DR, et al. Atrophy and hypertrophy signalling of the quadriceps and diaphragm in COPD. Thorax. 2010;65(11):963–970. doi:10.1136/thx.2009.133827
35. Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi:10.1016/s0092-8674(04)00400-3
36. Ribisl PM, Lang W, Jaramillo SA, et al. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the Look AHEAD clinical trial. Diabetes Care. 2007;30(10):2679–2684. doi:10.2337/dc06-2487
37. Chen CL, Tang JS, Li PC, Chou PL. Immediate effects of smoking on cardiorespiratory responses during dynamic exercise: arm vs. leg ergometry. Front Physiol. 2015;1:376. doi:10.3389/fphys.2015.00376
38. Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42(2):53–61. doi:10.1249/JES.0000000000000007
39. Grgic J, McLlvenna LC, Fyfe JJ, et al. Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta-analysis. Sports Med. 2019;49(2):233–254. doi:10.1007/s40279-018-1008-z
40. Vieira Ramos G, Pinheiro CM, Messa SP, et al. Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci Rep. 2016;6:18525. doi:10.1038/srep18525
41. Snijders T, Nederveen JP, McKay BR, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. doi:10.3389/fphys.2015.00283
42. Ferre PJ, Liaubet L, Concordet D, et al. Longitudinal analysis of gene expression in porcine skeletal muscle after post-injection local injury. Pharm Res. 2007;24(8):1480–1489. doi:10.1007/s11095-007-9266-8
43. Fukushima K, Nakamura A, Ueda H, et al. Activation and localization of matrix metalloproteinase-2 and −9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ). BMC Musculoskelet Disord. 2007;8:54. doi:10.1186/1471-2474-8-54
44. Kherif S, Lafuma C, Dehaupas M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205(1):158–170. doi:10.1006/dbio.1998.9107
45. Hadler-Olsen E, Solli AI, Hafstad A, Winberg JO, Uhlin-Hansen L. Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J Cell Physiol. 2015;230(1):160–169. doi:10.1002/jcp.24694
46. Kim J, Lee J. Matrix metalloproteinase and tissue inhibitor of metalloproteinase responses to muscle damage after eccentric exercise. J Exerc Rehabil. 2016;12(4):260–265. doi:10.12965/jer.1632640.320
47. Lee YP, Choi DG. MMPs, TIMPs and BMP-4 in medial rectus muscle obtained from intermittent exotropia patients and their clinical correlations. Acta Ophthalmol. 2020;98(1):e107–e112. doi:10.1111/aos.14217
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.