Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 13
Mitochondrial Toxicant-Induced Neuronal Apoptosis in Parkinson’s Disease: What We Know so Far
Authors Sivagurunathan N , Gnanasekaran Priyadharshini, Calivarathan L
Received 10 July 2022
Accepted for publication 19 January 2023
Published 26 January 2023 Volume 2023:13 Pages 1—13
DOI https://doi.org/10.2147/DNND.S361526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Thomas Müller
Narmadhaa Sivagurunathan, Priyadharshini Gnanasekaran, Latchoumycandane Calivarathan
Molecular Pharmacology and Toxicology Laboratory, Department of Biotechnology, School of Life Sciences, Central University of Tamil Nadu, Thiruvarur, India
Correspondence: Latchoumycandane Calivarathan, Molecular Pharmacology and Toxicology Laboratory, Department of Biotechnology (Sponsored by DST-FIST), School of Life Sciences, Central University of Tamil Nadu, Thiruvarur, 610005, India, Tel +91-6381989116, Email [email protected]
Abstract: Parkinson’s disease (PD) is one of the most common progressive neurodegenerative diseases caused by the loss of dopamine-producing neuronal cells in the region of substantia nigra pars compacta of the brain. During biological aging, neuronal cells slowly undergo degeneration, but the rate of cell death increases tremendously under some pathological conditions, leading to irreversible neurodegenerative diseases. By the time symptoms of PD usually appear, more than 50 to 60% of neuronal cells have already been destroyed. PD symptoms often start with tremors, followed by slow movement, stiffness, and postural imbalance. The etiology of PD is still unknown; however, besides genetics, several factors contribute to neurodegenerative disease, including exposure to pesticides, environmental chemicals, solvents, and heavy metals. Postmortem brain tissues of patients with PD show mitochondrial abnormalities, including dysfunction of the electron transport chain. Most chemicals present in our environment have been shown to target the mitochondria; remarkably, patients with PD show a mild deficiency in NADH dehydrogenase activity, signifying a possible link between PD and mitochondrial dysfunction. Inhibition of electron transport complexes generates free radicals that further attack the macromolecules leading to neuropathological conditions. Apart from that, oxidative stress also causes neuroinflammation-mediated neurodegeneration due to the activation of microglial cells. However, the mechanism that causes mitochondrial dysfunction, especially the electron transport chain, in the pathogenesis of PD remains unclear. This review discusses the recent updates and explains the possible mechanisms of mitochondrial toxicant-induced neuroinflammation and neurodegeneration in PD.
Keywords: apoptosis, mitochondrial dysfunction, mitochondrial toxicant, neurodegenerative disorder, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder, and its symptoms appear at the age of 50 and above and worsen as aging progresses. According to the National Institutes of Environmental Health Sciences (USA), at least 500,000 Americans live with PD, although some estimates may be much higher than reported. This movement disorder results from the loss of dopaminergic neurons in the substantia nigra pars compacta of the brain. The major risk factors for PD include familial history, aging, and exposure to toxicants present in our environment. Pathogenic mutations of PARK7 (encoding DJ-1), α-synuclein, parkin, PINK1, or LRRK2 in PD cause alterations in mitochondrial dynamics.1 PD exhibits motor symptoms, including tremors, rigidity, bradykinesia, and stooping posture. Some patients show non-motor symptoms such as constipation, loss of sense of smell, sleep, and genitourinary disorders.2 The cellular hallmarks of PD include protein misfolding, aggregation of α-synuclein, lysosomal and proteasomal dysfunctions, neuroinflammation, neuronal cell death, calcium homeostasis, and mitochondrial dysfunction.3,4 Mitochondrial damage-associated molecular patterns are also known to contribute to the pathogenesis of PD.5 Even though recent research has provided many pharmacological therapies and surgeries, such as deep brain stimulation, the definitive treatment for PD is yet to be discovered.6 Levodopa is the primary therapeutic drug in treating PD, whereas the other pharmacological agents used are monoamine oxidase-B inhibitors and catechol-O-methyltransferase inhibitors, amantadine, and anticholinergic agents.7
Several studies have shown that mitochondrial dysfunction is one of the critical factors for the selective degeneration of dopaminergic neurons in the PD model. Mitochondria are essential in maintaining normal neuronal function and viability, and their dysfunction progresses to programmed cell death8 or necroptosis.9 The mitochondrial electron transport chain (ETC) is the primary intracellular site for producing ATP, which is essential for the survival of neurons. Under physiological conditions, the amount of ROS generated from the ETC is insignificant.10 However, ROS production is markedly elevated under pathological conditions or in the presence of ETC inhibitors, with mitochondrial complex III and I as the main production sites.11,12 Several studies support the impaired function of mitochondria complex I13 in substantia nigra, skeletal muscles, platelets, and leukocytes of PD patients.14 Under pathological conditions, the mitochondrial membrane potential is also altered, resulting in increased mitochondrial membrane permeabilization, swelling, and release of cytochrome c, which further activates the downstream apoptotic signaling cascade. Dysfunctional mitochondria stimulate the mitochondrial damage-associated molecular patterns, which trigger inflammation and apoptosis, promoting the onset of several neurodegenerative diseases. Oxidative phosphorylation also produces highly reactive free radicals, such as superoxide anions, which cause damage to the mitochondrial components by inducing mitochondrial oxidative stress and promoting mitochondrial dysfunction.15,16 Several natural and anthropogenic chemicals contribute to mitochondrial toxicity (Table 1), resulting in mitochondrial dysfunction. Increased production of free radicals production targets the mitochondrial genome, affecting ATP synthesis and ultimately causing cellular damage.17,18 This review discusses the recent updates and novel insight into the cellular and molecular mechanism of mitochondrial toxicants-induced neuroinflammation and neurodegeneration in Parkinson’s disease.
 |
Table 1 Mitochondrial Toxicant-Induced Neurodegeneration in Animal Models and Humans |
Pathophysiological Changes in the Brain of PD Patients
Parkinson’s disease occurs due to the gradual loss of dopamine-producing neuronal cells in the substantia nigra pars compacta (SNpc), which consequently leads to less availability of dopamine. Dopamine is a chemical messenger that transmits signals from SNpc to the corpus striatum to regulate muscular coordination. The presence of Lewy bodies characterizes the fundamental pathophysiology of PD at the cellular level, which is an aggregate of abnormal proteins in the brain, specifically in the region of SNpc.19,20 Lewy bodies are lamellated, eosinophilic cytoplasmic inclusion consisting of a dense core surrounded by a halo of 10 nm wide radiating fibrils. The primary structural protein of the Lewy body is α-synuclein, which localizes specifically to the nerve terminal, with relatively little in the nerve cell body, dendrites, or extrasynaptic sites along the axon in PD.21,22 The primary function of α-synuclein is to regulate the activity of dopamine transporters. However, in pathological conditions, either abnormal levels or a mutated condition of α-synuclein contributes to Lewy bodies, which further causes neuronal cell death, consequently leading to motor dysfunctions. Since the abnormal accumulation of α-synuclein is toxic to cells, it impairs the microtubular transport and subcellular organelles.
Mutation of α-synuclein leads to aggregation, delaying the fusion of phagosomes with lysosomes during mitophagy. Recently, exosomes have been shown to play multiple roles in PD-associated inflammation that transports toxic α-synuclein oligomers into the extracellular environment causing activation of microglia and astrocytes to secrete exosomes,23 proposing a novel mechanism for linking mitochondrial dysfunction to systemic inflammation associated with PD. Postmortem brain tissues of patients with PD support the hypothesis that mitochondrial dysfunctions contribute to the pathogenesis of PD. Further, identifying mutant genes such as PINK1, DJ-1, PRKN, and LRRK-2 involved in oxidative stress and mitochondrial dysfunction also confirms the hypotheses that mitochondrial dysfunction mediates dopaminergic neurodegeneration in PD.24 The dopaminergic neurons of SNpc synthesize the neurotransmitter dopamine from DOPA and transport it to the axon terminals in the striatum. Neurochemical analysis shows a reduction in the striatal dopamine content by over 80% in PD patients, parallel with the loss of dopaminergic neurons from the SNpc. However, positron emission tomography shows that the symptoms of PD appear only when the striatal dopamine falls below 40% of the normal range. Experimentally-induced lesions of the nigrostriatal tract or chemically-induced dopamine depletion exhibit the triads of cardinal motor symptoms in PD,25 such as bradykinesia, rigidity, and tremors, which are due to more complex changes in the neurochemicals, including acetylcholine, noradrenaline, 5-HT, and GABA apart from dopamine. The severity of symptoms correlates with degeneration of the dopaminergic nigrostriatal pathway and dopamine depletion in the striatum.26 It has been shown that up to 13% of dopaminergic neurons die every decade, suggesting that more cases of age-related Parkinson’s will occur as people live longer. Unfortunately, by age 80, a person may have lost at least 80% of his/ her dopaminergic neurons, but not everyone loses the cells at the same rate or develops PD.
Mitochondrial Toxicants and Neurodegeneration
Mitochondria play a crucial role in maintaining the survival of cells by regulating physiological and pathological mechanisms, including ATP generation, metabolism signal transduction, immune response, and apoptosis.27,28 Several chemicals are well known to target mitochondria, including drugs, pesticides, and environmental contaminants (Table 1). Mitochondria are more vulnerable to exposure to toxicants due to high lipid content on membranes and negative charges of the mitochondrial matrix. Apart from beneficial effects, some pharmaceutical drugs act as mitochondrial toxicants targeting the ETC. Mitochondrial toxicants also cause direct inhibition of biochemical processes, including the oxidation of fatty acids, the citric acid cycle, and mitochondrial protein synthesis. An electrochemical gradient is established during oxidative phosphorylation, which is the primary driving force for ATP synthesis.29,30 Under pathological conditions or exposure to mitochondrial toxicants, excessive production of free radicals occurs via oxidative phosphorylation, causing oxidative stress that leads to mitochondrial membrane depolarization and cytochrome c release, resulting in downstream activation of apoptotic signaling. Many mitochondrial toxicants have been shown to induce neuronal cell death, resulting in various neurodegenerative disorders, including PD, AD, HD, and ALS.31–34 MPTP, peroxynitrite, trichloroethane, paraquat, rotenone, lidocaine, carbon monoxide, and heavy metals are some mitochondrial toxicants known to induce Parkinson’s or PD-like symptoms.35
MPTP and Parkinson’s Disease
Accidental exposure to synthetic drugs contaminated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) by intravenous street drug abusers exhibited symptoms remarkably similar to idiopathic PD.36 MPTP is a lipophilic compound that can cross the blood-brain barrier and reach the central nervous system, which is taken up by the glial cells. The 1-methyl-4-phenyl pyridinium (MPP+) is an active metabolite of the neurotoxin MPTP that causes parkinsonian syndrome in humans and animals. MPP+ is well known to inhibit NAD(H)-linked mitochondrial oxidation, acts as a selective dopaminergic neurotoxin, and induces superoxide anion and hydroxyl radicals, which causes oxidative stress-mediated neuronal cell death.37 MPTP metabolism is a complicated multistep process; it is converted into MPP+ in the astrocytes and released into the neuronal microenvironment, where they specifically bind to the dopamine transporter to enter the dopaminergic neurons. Astrocytes contain MAO-B, which is actively involved in converting MPTP to MPP+. Once within the dopaminergic neuronal cells, MPP+ enters the mitochondria and interferes with the complex I of the ETC, which ultimately leads to apoptosis of the dopaminergic neuronal cells,38–40 suggesting that mitochondria are the preferential target for MPP+ in causing neurodegeneration. MPTP is capable of causing clinical symptoms in humans and monkeys indistinguishable from PD.41 MPTP exerts its effect by inducing oxidative stress and the formation of inclusion bodies with abnormal α-synuclein proteins, thereby causing accelerated neurodegeneration in the substantia nigra pars compacta and striatum.42,43 Since MPTP induces selective neuronal cell death of dopaminergic neurons, it is also considered the standard mouse model for PD research. Interestingly, the C57BL/6 strain is more vulnerable to MPTP-induced mitochondrial dysfunctions, perturbations in energy metabolism, and motor defects than the BALB/c strain.44 One of the characteristic features of PD which is not reproduced in animal models is that the SNpc lesion does not coincide with the Lewy body formation in the brain.45
Peroxynitrite and Parkinson’s Disease
Nitric oxide reacts with superoxide anion, forming peroxynitrite (ONOO−), a highly reactive oxidizing and nitrating agent causing adverse biological effects.46 Peroxynitrite reacts with the cellular DNA to cause single-stranded breaks.47 Due to its ability to cause oxidation and nitration of various biomolecules, it is implicated in the pathogenesis of various diseases, including neurodegenerative diseases.47,48 The origin of peroxynitrite is either in extramitochondrial compartments or directly produced within the mitochondrial matrix. The diffusion of peroxynitrite into the mitochondria leads to alterations in calcium homeostasis and mitochondrial energy production, resulting in increased membrane permeability.49 In addition, ONOO− is an effective inhibitor of enzymes in the mitochondrial respiratory chain, resulting in decreased ATP synthesis.50–52 Several studies have shown that peroxynitrite inhibits most components of the electron transport chain, including complex I,53 complex II,54 complex III,55 and complex V,56 by oxidation or nitration of complex proteins.50,51 Peroxynitrite also leads to DNA damage, inactivation of enzymes, ionic pumps, proteins, and disruption of cellular membranes, which contribute to mitochondrial dysfunctions and promote apoptosis.51 In the postmortem brain of PD patients, peroxynitrite-mediated oxidative and nitrative stresses contribute to the pathogenesis.57,58 As this compound is highly reactive with protein molecules, some modified proteins accumulate and are involved in the onset and progression of PD.59,60 Impairment of mitochondrial complex I is also consistently observed in the postmortem PD brain, and peroxynitrite is suspected to be involved with the inhibition via nitrosylation and nitration of mitochondrial complex I.61 Nitration of tyrosine hydroxylase, the initial and rate-limiting enzyme in the biosynthesis of dopamine, is inhibited by peroxynitrite, leading to failure in the synthesis of dopamine.62 Peroxynitrite also targets the intracellular glutathione in substantia nigra by inactivating glutathione reductase, the enzyme responsible for the regeneration of glutathione from its oxidized form.63
Trichloroethane and Parkinson’s Disease
Trichloroethylene (TCE) is a chlorinated hydrocarbon used in the industrial degreasing of metals and as a secondary solvent in adhesive paint and polyvinyl chloride production. TCE is detected in air, soil, and food and enters the human body through inhalation, ingestion, or skin. Hence, it breaches the central nervous system due to high lipid solubility and slowly induces anesthesia.64
TCE is transformed to chloral by cytochrome P450, converting it to a trichloroethylene oxide intermediate.65 Chloral is one of the precursors of 1-trichloromethyl-1,2,3,4-tetrahydro-b-carboline, also known as TaClo,66,67 which is readily produced from the biogenic amine tryptamine and chloral under physiological conditions.67 TCE is structurally similar to the MPTP has led to speculation that it could cause PD.68 TaClo, one of the mitochondrial toxicants, is cytotoxic to the primary culture of dopaminergic neurons,69 which further induces apoptosis by blocking the transfer of electrons from complex I to ubiquinone in both rat brains homogenates and liver submitochondrial particles.70,71 Similarly, animal models exposed to TCE have shown nigrostriatal degeneration, which led to the mitochondrial dysfunction and the development of parkinsonian features.64,72
Paraquat and Parkinson’s Disease
Paraquat (PQ) is a bipyridyl compound commonly used as a contact herbicide and is poorly absorbed when inhaled, but when ingested orally, it causes severe sickness and death. PQ exposure leads to cytotoxicity by inducing oxidative stress and mitochondrial dysfunction.73 PQ induces the production of superoxide in the complex I of ETC, thereby causing extensive mitochondrial oxidative damage in mammalian systems.74 PQ exposure decreases the activity of mitochondrial complex I and mitochondrial transmembrane potential and induces the release of cytochrome c from mitochondria.75 Paraquat is a well-known neurotoxicant associated with an increased risk of developing PD and PD-like symptoms.76,77 In experimental animals, PQ induces oxidative stress, resulting in the death of dopaminergic neurons and causing behavioral abnormalities similar to patients with PD.78 The production of free radicals and activation of cholinergic and glutamatergic transmission is also connected to PQ-induced neurotoxicity.79 PQ significantly increases the in vitro rate of α-synuclein fibril formation and aggregation, with dose-dependent accelerating effects.80 Furthermore, the amount of α-synuclein increases considerably in the brain of mice exposed to PQ.80
Rotenone and Parkinson’s Disease
Rotenone (RO) is an isoflavone insecticide obtained from the roots and stems of various plants, including Derris, Tephrosia, Lonchocarpus, and Mundulea. According to WHO, rotenone is a moderately hazardous substance; however, it is mildly toxic to humans and other mammals but highly toxic to insects and fishes. After intravenous injection in mouse or rat, rotenone is metabolized in the liver by NADP-linked hepatic microsome enzymes to rotenolone I and II, hydroxy-, and dihydroxy-rotenone, which is used as a biomarker for rotenone poisoning in blood, urine, feces, and liver.81 Due to its lipophilicity, rotenone enters the cells without transporters and can readily cross the blood-brain barrier. Rotenone then enters the brain and is accumulated in the cellular organelles such as mitochondria.82 The mode of action of rotenone includes the inhibition of oxidation of NADH to NAD as well as the mitochondrial respiratory chain. Rotenone induces mitochondrial dysfunction by inhibiting the complex I of the ETC, which leads to oxidative stress, and apoptosis.83,84 Rotenone induces apoptosis by enhancing the production of mitochondrial reactive oxygen species, cytochrome c release, and activation of caspase-3, followed by DNA fragmentation.85 Chronic exposure to rotenone increases the risk of PD-like features in various animal models. In vivo experimental model shows RO induces loss of dopaminergic neurons in the substantia nigra, one of the characteristic features of PD neuropathology.86 Rotenone also replicates the clinical features of PD, including the aggregation of α-synuclein and the formation of Lewy bodies.87
Pyrethroid and Parkinson’s Disease
A pyrethroid is a class of synthetic insecticides, which targets the nervous system of insects and other species, and is widely used due to its high efficacy and low environmental persistence. There are different types of pyrethroids, such as deltamethrin, cypermethrin, and permethrin, and exposure to such pyrethroids leads to progressive neurodegenerative diseases. Exposure to pyrethroids affects the mitochondrial membrane potential and inhibits the complex I function.88,89 Deltamethrin causes mitochondrial dysfunction with decreased membrane potential, increased permeability, and cytochrome c release.90,91 Rats exposed to cypermethrin exhibit changes in the mitochondrial proteome profile of the substantia nigra and striatum.92 Deltamethrin has been shown to cause apoptosis in the cerebral cortex, hippocampus, and striatal neuronal cells.88,93 Permethrin raises α-synuclein, decreases striatal dopamine levels, causes oxidative stress, and inhibits ETC’s mitochondrial complex I.88
Iron and Parkinson’s Disease
Iron is necessary for almost all living species and is essential in heme-containing proteins and iron-sulfur clusters, which are involved in various physiological processes, including oxygen transport, mitochondrial respiration, and DNA synthesis.94 Free iron enters cells and accumulates in mitochondria, inhibiting oxidative phosphorylation, promoting free radical generation, catalyzing lipid peroxidation, and ultimately causing cell death.95 Iron accumulation within cells and tissues impairs the redox equilibrium, forming lipid peroxide through ferroptosis. Ferroptosis is a non-apoptotic, and iron-dependent form of cell death mainly occurs in the brain due to high lipid content. Due to compromised blood-brain barrier in older adults, poor recycling or redistribution of iron in the brain, and prolonged neuroinflammation,96,97 have been shown to increase iron buildup in the substantia nigra, globus pallidus, putamen, and caudate nucleus.98 The pathogenesis of many neurodegenerative disorders is implicated with the buildup of iron in specific brain locations in the CNS.99 Iron accumulation in the SNpc has been recognized as one of the key pathological features of PD100 and in the experimentally-induced mouse models of PD.101
Interestingly, iron chelation ameliorates neuronal cell death and alleviates motor deficits in animal models,102 and decreases the progression of PD in patients.103 Further, rotenone exposure to rats or monkeys has been shown to cause iron accumulation in the nigral dopaminergic neurons and microglial cells,104 suggesting that the increased iron content of microglia in the PD brain comes from phagocytosis of iron-laden dopaminergic neurons.104 In animal models and patients with PD, transferrin accumulates in dopaminergic neurons, and much of the iron transport protein primarily localizes to mitochondria, and a large proportion of the nigral transferrin is oxidized at cysteine residues, forming an intermolecular disulfide bond in close proximity to the iron-binding site.104 Iron also appears to contribute to the formation of the pathological hallmarks of PD, such as Lewy bodies, intraneuronal proteinaceous inclusions that contain the aggregation-prone protein, the α-synuclein. Neurodegeneration with brain iron accumulation has been reported due to mutations in pantothenate kinase type 2 that also exhibit Lewy pathology labeling for α-synuclein and neuroaxonal spheroids labeling for β- and γ-synuclein.105,106 Specifically, iron associates with α-synuclein, increasing its aggregating and thereby potentiating neurotoxicity.107 Conditions such as neuroferritinopathy and Friedreich ataxia are associated with mutations in genes that encode proteins involved in iron metabolism, and as the brain ages, iron accumulates in regions affected by AD and PD.108 Under the pathological condition, the non-heme iron is in higher concentrations in globus pallidus, substantia nigra, red nucleus, and dentate nucleus,109 and upregulation of iron-related protein, Divalent Metal Transporter Iron Responsive Element (DMT1+IRE), has been observed in dopaminergic neurons and microglia in patients with PD and mouse model of PD.101 Iron metabolism occurs in the mitochondrial matrix, where the Fe-S cluster assembles and incorporates into the electron transport complexes. Under pathological conditions, disproportionate cellular iron is observed due to disruption of Fe-S clusters, resulting in the mitochondrial dysfunction observed in the early pathogenesis of PD.
Aluminum and Parkinson’s Disease
Aluminum (Al) is one of the most prevalent elements since it has only one oxidation state, and it can combine with other metals present in the environment to form a complex. In the experimental animal model, Al has been shown to accumulate in all regions of the brain, with the highest concentration in the hippocampus, the memory and learning center of the brain.110 Al is known to induce ROS, which impairs mitochondrial functions, leading to cellular damage and apoptosis. Al induces cell death by various other mechanisms such as the generation of ROS, the release of intracellular Ca2+ deposits, and perturbation of mitochondrial function.111–113 Al exposure due to high amounts of this element in the environment leads to the formation and accumulation of neurofibrillary tangles, β-amyloid plaques, tau aggregation, and neuronal dysfunction in the central nervous system.114 Al concentrations are also higher in the substantia nigra pars compacta of the PD patients’ brain, indicating a link between PD and Al exposure. Al also promotes the ability of 6 OHDA to cause oxidative damage and neurodegeneration in the mouse model of PD.115
Manganese and Parkinson’s Disease
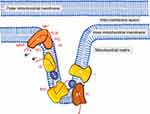
Occupational exposure to manganese causes Manganism, a cognitive disorder with muscle weakness, bent posture, whispering speech, limb tremor, and salivation, symptoms very similar to PD that occur due to the accumulation of Mn in basal ganglia, targeting dopaminergic neurons. Occupational exposures occur mainly in welding and mining as miners are surrounded by dust of Mn and airborne Mn particles, alloy production and processing, and ferromanganese operations, especially in which Mn ore or compounds are turned into steel, and work with agrochemicals. Overexposure to Mn in the ionic forms mainly influences the central nervous system, causes symptoms that increase the possibility of irreversible hippocampal damage, and shows involuntary extrapyramidal symptoms associated with PD. Apart from divalent metal transporter 1 (DMT1), Mn is transported across the cell membrane by voltage-gated Ca2+ channel, Ca2+ coupled ionotropic glutamate receptor, solute carrier 39 (SLC39), ZIP8, and ZIP14. Elevated levels of Mn in the brain also impair iron homeostasis and cause a synergistic effect. Histopathological analysis shows Mn2+ at a low dose induces apoptotic neuronal cell death through a mitochondrial-dependent pathway.8,116 Mn overexposure in rodent and non-human primates results in Mn accumulation in brain regions, including the basal ganglia, further disrupting the GABAergic and dopaminergic neurotransmission and activating glial cells and eventually causing neuronal loss.117 One of the impending mechanisms underlying Mn neurotoxicity is the induction of ROS generation and subsequent oxidative damage to the dopaminergic neurons,8 especially in the globus pallidus, striatum, and substantia nigra,118–120 and antioxidants have been shown to ameliorate the deleterious neurotoxic effects of Mn.121 Mn2+ interferes with oxidative phosphorylation by inhibiting ETC complex activities, especially mitochondrial complex I and III (Figure 1), or decreasing the production of ATP.121–123 In an in vitro study, dopaminergic neuronal cells, not the glial cells exposed to Mn, show inhibition of mitochondrial ETC,122 whereas in vivo exposure to MnCl2 inhibits cytochrome c oxidase.124 Mn exposure further mediates nonenzymatic autoxidation of catecholamines, dopamine to 6-hydroxydopamine that further potentiate neurotoxicity and also explain the reason for the depletion of dopamine from the specific regions of the brain following Mn exposure.125,126 Accumulation of Mn in the striatal regions inhibits tyrosine hydroxylase activity, affecting dopamine synthesis.127 Mitochondria sequester intracellular Mn through the Ca2+ uniporter,128 and the specificity for Mn accumulation in GP and striatum is likely correlated with Mn transporter distribution.
Conclusion
Besides providing beneficial effects to society, pesticides, heavy metals, drugs, and natural and anthropogenic chemicals present in our environment pose a significant threat to our health. Although some serve a vital purpose, exposure to these chemicals causes cellular toxicity, contributing to the pathogenesis of many diseases, including neurodegenerative diseases. Several of these chemicals have different mechanisms in causing pathogenesis; however, most chemicals directly or indirectly target the mitochondrial electron transport system. At the organelle level, mitochondria are involved in fundamental processes, including energy production, reactive oxygen species generation, calcium signaling, mitochondrial dynamics, and apoptosis. This review elaborates on the molecular mechanism of mitochondrial toxicant-induced neurodegeneration and its role in the pathogenesis of PD (Figure 1), signifying the need to develop innovative strategies for studying this organelle at the cellular and molecular levels. Remarkably, mitochondria are unintended drug targets of many pharmaceutical and therapeutic agents that can cause mitochondrial dysfunction by altering the membrane potential and electron transport chain. The brain heavily depends on oxidative phosphorylation for survival and appears to be the main target for many mitochondrial toxicants. Interestingly, each chemical does not induce the same effect on the population in developing neurodegenerative diseases, which varies on the time of exposure, duration of exposure, and the metabolic conditions of an individual. Furthermore, all the individuals exposed to pesticides or mitochondrial toxicants do not have, or develop the symptoms or neurodegeneration similar to Parkinson’s disease at the same rate due to genetic diversity. Due to predisposition, some individuals are more prone to react when they are exposed to mitochondrial toxicants even at a lower concentration. However, it is inconclusive about the specific dose of exposure that correlate with neurodegeneration. Understanding the mechanism of action of chemical agents contributing to neurodegeneration is essential to developing a neuroprotective strategy.
Acknowledgments
CL greatly acknowledges the Science and Engineering Board (DST-SERB), University Grants Commission (UGC) and the Indian Council of Medical Research (ICMR), Government of India for the financial support in the form of Core Research Grant, Startup Research Grant, and Extramural Adhoc Research Grant, respectively.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–273. doi:10.1016/j.neuron.2014.12.007
2. Aubignat M, Tir M, Krystkowiak P. Les symptômes non-moteurs de la maladie de Parkinson de la physiopathologie au diagnostic précoce [Non-motor symptoms of Parkinson’s disease from pathophysiology to early diagnosis]. Rev Med Interne. 2021;42(4):251–257. French. doi:10.1016/j.revmed.2020.06.019
3. Antony PM, Diederich NJ, Kruger R, Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013;280(23):5981–5993. doi:10.1111/febs.12335
4. Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9(7):a028035. doi:10.1101/cshperspect.a028035
5. Zambrano K, Barba D, Castillo K, et al. Fighting Parkinson’s disease: the return of the mitochondria. Mitochondrion. 2022;64:34–44. doi:10.1016/j.mito.2022.02.003
6. Beitz JM. Parkinson’s disease: a review. Front Biosci. 2014;6(1):65–74. doi:10.2741/S415
7. Bene R, Antic S, Budisic M, et al. Parkinson’s disease. Acta Clin Croat. 2009;48(3):377–380.
8. Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313(1):46–55. doi:10.1124/jpet.104.078469
9. Iannielli A, Bido S, Folladori L, et al. Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in Parkinson’s disease models. Cell Rep. 2018;22(8):2066–2079. doi:10.1016/j.celrep.2018.01.089
10. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784–44790. doi:10.1074/jbc.M207217200
11. Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279(47):49064–49073. doi:10.1074/jbc.M407715200
12. Larsen SB, Hanss Z, Kruger R. The genetic architecture of mitochondrial dysfunction in Parkinson’s disease. Cell Tissue Res. 2018;373(1):21–37. doi:10.1007/s00441-017-2768-8
13. Gonzalez-Rodriguez P, Zampese E, Stout KA, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599(7886):650–656. doi:10.1038/s41586-021-04059-0
14. Monzio Compagnoni G, Di Fonzo A, Corti S, Comi GP, Bresolin N, Masliah E. The role of mitochondria in neurodegenerative diseases: the lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol Neurobiol. 2020;57(7):2959–2980. doi:10.1007/s12035-020-01926-1
15. Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–230. doi:10.1016/S0891-5849(00)00317-8
16. Meyer JN, Hartman JH, Mello DF. Mitochondrial toxicity. Toxicol Sci. 2018;162(1):15–23. doi:10.1093/toxsci/kfy008
17. Meyer JN, Chan SSL. Sources, mechanisms, and consequences of chemical-induced mitochondrial toxicity. Toxicology. 2017;391:2–4. doi:10.1016/j.tox.2017.06.002
18. Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8(21):2003–2014. doi:10.3969/j.issn.1673-5374.2013.21.009
19. Sherer TB, Chowdhury S, Peabody K, Brooks DW. Overcoming obstacles in Parkinson’s disease. Mov Disord. 2012;27(13):1606–1611. doi:10.1002/mds.25260
20. Gazewood JD, Richards DR, Clebak K. Parkinson disease: an update. Am Fam Physician. 2013;87(4):267–273.
21. George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15(2):361–372. doi:10.1016/0896-6273(95)90040-3
22. Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi:10.1016/0896-6273(95)90302-X
23. Li KL, Huang HY, Ren H, Yang XL. Role of exosomes in the pathogenesis of inflammation in Parkinson’s disease. Neural Regen Res. 2022;17(9):1898–1906. doi:10.4103/1673-5374.335143
24. Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi:10.1146/annurev.neuro.28.061604.135718
25. Latchoumycandane C, Anantharam V, Jin H, Kanthasamy A, Kanthasamy A. Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCdelta in cell culture and animal models of Parkinson’s disease. Toxicol Appl Pharmacol. 2011;256(3):314–323. doi:10.1016/j.taap.2011.07.021
26. Wichmann T, DeLong MR. Pathophysiology of parkinsonian motor abnormalities. Adv Neurol. 1993;60:53–61.
27. Mottis A, Herzig S, Auwerx J. Mitocellular communication: shaping health and disease. Science. 2019;366(6467):827–832. doi:10.1126/science.aax3768
28. Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26(6):711–723. doi:10.1016/j.beem.2012.05.003
29. Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191(4784):144–148. doi:10.1038/191144a0
30. Jayasundara N. Ecological significance of mitochondrial toxicants. Toxicology. 2017;391:64–74. doi:10.1016/j.tox.2017.07.015
31. Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83(1):84–92. doi:10.1016/j.yexmp.2006.09.008
32. Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348(14):1365–1375. doi:10.1056/NEJMra022366
33. Badley AD, Dockrell D, Paya CV. Apoptosis in AIDS. Adv Pharmacol. 1997;41:271–294.
34. Tatton WG, Olanow CW. Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta. 1999;1410(2):195–213. doi:10.1016/S0005-2728(98)00167-4
35. Meyer JN, Leung MC, Rooney JP, et al. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134(1):1–17. doi:10.1093/toxsci/kft102
36. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi:10.1126/science.6823561
37. Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65(2):135–172. doi:10.1016/s0301-0082(01)00003-x
38. Hisahara S, Shimohama S. Toxin-induced and genetic animal models of Parkinson’s disease. Parkinsons Dis. 2010;2011:951709. doi:10.4061/2011/951709
39. Nicotra A, Parvez SH. Cell death induced by MPTP, a substrate for monoamine oxidase B. Toxicology. 2000;153(1–3):157–166. doi:10.1016/S0300-483X(00)00311-5
40. Perry TL, Yong VW, Jones K, et al. Effects of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and its metabolite, N-methyl-4-phenylpyridinium ion, on dopaminergic nigrostriatal neurons in the mouse. Neurosci Lett. 1985;58(3):321–326. doi:10.1016/0304-3940(85)90074-6
41. Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2(1):141–151. doi:10.1038/nprot.2006.342
42. Purisai MG, McCormack AL, Langston WJ, Johnston LC, Di Monte DA. Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis. 2005;20(3):898–906. doi:10.1016/j.nbd.2005.05.028
43. Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. doi:10.1007/s00702-017-1686-y
44. Pathania A, Garg P, Sandhir R. Impaired mitochondrial functions and energy metabolism in MPTP-induced Parkinson’s disease: comparison of mice strains and dose regimens. Metab Brain Dis. 2021;36(8):2343–2357. doi:10.1007/s11011-021-00840-2
45. Blandini F, Armentero MT. Animal models of Parkinson’s disease. FEBS J. 2012;279(7):1156–1166. doi:10.1111/j.1742-4658.2012.08491.x
46. Ascenzi P, Di Masi A, Sciorati C, Clementi E. Peroxynitrite-An ugly biofactor? Biofactors. 2010;36(4):264–273. doi:10.1002/biof.103
47. Ahmad R, Hussain A, Ahsan H. Peroxynitrite: cellular pathology and implications in autoimmunity. J Immunoassay Immunochem. 2019;40(2):123–138. doi:10.1080/15321819.2019.1583109
48. Dawson TM, Dawson VL. Nitric oxide signaling in neurodegeneration and cell death. Adv Pharmacol. 2018;82:57–83.
49. Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–680. doi:10.1038/nrd2222
50. Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383(3–4):401–409. doi:10.1515/BC.2002.044
51. Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33(11):1451–1464. doi:10.1016/S0891-5849(02)01111-5
52. Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308(1):89–95. doi:10.1006/abbi.1994.1013
53. Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658(1–2):44–49. doi:10.1016/j.bbabio.2004.03.016
54. Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64(5):1965–1972. doi:10.1046/j.1471-4159.1995.64051965.x
55. Guidarelli A, Fiorani M, Cantoni O. Enhancing effects of intracellular ascorbic acid on peroxynitrite-induced U937 cell death are mediated by mitochondrial events resulting in enhanced sensitivity to peroxynitrite-dependent inhibition of complex III and formation of hydrogen peroxide. Biochem J. 2004;378(Pt 3):959–966. doi:10.1042/bj20031167
56. Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328(2):309–316. doi:10.1006/abbi.1996.0178
57. Ebadi M, Sharma SK. Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid Redox Signal. 2003;5(3):319–335. doi:10.1089/152308603322110896
58. Ebadi M, Sharma SK, Ghafourifar P, Brown-Borg H, El Refaey H. Peroxynitrite in the pathogenesis of Parkinson’s disease and the neuroprotective role of metallothioneins. Methods Enzymol. 2005;396:276–298.
59. Picon-Pages P, Garcia-Buendia J, Munoz FJ. Functions and dysfunctions of nitric oxide in brain. Biochim Biophys Acta Mol Basis Dis. 2019;1865(8):1949–1967. doi:10.1016/j.bbadis.2018.11.007
60. Torreilles F, Salman-Tabcheh S, Guerin M, Torreilles J. Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev. 1999;30(2):153–163. doi:10.1016/S0165-0173(99)00014-4
61. Chinta SJ, Andersen JK. Nitrosylation and nitration of mitochondrial complex I in Parkinson’s disease. Free Radic Res. 2011;45(1):53–58. doi:10.3109/10715762.2010.509398
62. Ara J, Przedborski S, Naini AB, et al. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad Sci U S A. 1998;95(13):7659–7663. doi:10.1073/pnas.95.13.7659
63. Bharath S, Andersen JK. Glutathione depletion in a midbrain-derived immortalized dopaminergic cell line results in limited tyrosine nitration of mitochondrial complex I subunits: implications for Parkinson’s disease. Antioxid Redox Signal. 2005;7(7–8):900–910. doi:10.1089/ars.2005.7.900
64. Zaheer F, Slevin JT. Trichloroethylene and Parkinson disease. Neurol Clin. 2011;29(3):657–665.
65. Bringmann G, God R, Feineis D, Janetzky B, Reichmann H. TaClo as a neurotoxic lead: improved synthesis, stereochemical analysis, and inhibition of the mitochondrial respiratory chain. J Neural Transm Suppl. 1995;46:245–254.
66. Bringmann G, God R, Feineis D, et al. The TaClo concept: 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline (TaClo), a new toxin for dopaminergic neurons. J Neural Transm Suppl. 1995;46:235–244.
67. Bringmann G, Hille A. Endogenous alkaloids in man, VII: 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline--a potential chloral-derived indol alkaloid in man. Arch Pharm. 1990;323(9):567–569. doi:10.1002/ardp.19903230903
68. Kochen W, Kohlmuller D, De Biasi P, Ramsay R. The endogeneous formation of highly chlorinated tetrahydro-beta-carbolines as a possible causative mechanism in idiopathic Parkinson’s disease. Adv Exp Med Biol. 2003;527:253–263.
69. Yang Y, Pang B, Liu Z, et al. 1-Trichloromethyl-1,2,3,4-tetrahydro-beta-carboline (TaClo) induces the apoptosis of dopaminergic neurons via oxidative stress and neuroinflammation. Oxid Med Cell Longev. 2019;2019:1292891. doi:10.1155/2019/1292891
70. Rausch WD, Abdel-mohsen M, Koutsilieri E, Chan WW, Bringmann G. Studies of the potentially endogenous toxin TaClo (1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline) in neuronal and glial cell cultures. J Neural Transm Suppl. 1995;46:255–263.
71. Keane PC, Kurzawa M, Blain PG, Morris CM. Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;2011:716871. doi:10.4061/2011/716871
72. Keane PC, Hanson PS, Patterson L, et al. Trichloroethylene and its metabolite TaClo lead to degeneration of substantia nigra dopaminergic neurones: effects in wild type and human A30P mutant alpha-synuclein mice. Neurosci Lett. 2019;711:134437. doi:10.1016/j.neulet.2019.134437
73. Shokrzadeh M, Shaki F, Mohammadi E, Rezagholizadeh N, Ebrahimi F. Edaravone decreases paraquat toxicity in a549 cells and lung isolated mitochondria. Iran J Pharm Res. 2014;13(2):675–681.
74. Cocheme HM, Murphy MP. Chapter 22 the uptake and interactions of the redox cycler paraquat with mitochondria. Methods Enzymol. 2009;456:395–417.
75. Yang W, Tiffany-Castiglioni E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: involvement of p53 and mitochondria. J Toxicol Environ Health A. 2008;71(4):289–299. doi:10.1080/15287390701738467
76. Chinta SJ, Woods G, Demaria M, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 2018;22(4):930–940. doi:10.1016/j.celrep.2017.12.092
77. Zuo Y, Xie J, Li X, et al. Ferritinophagy-mediated ferroptosis involved in paraquat-induced neurotoxicity of dopaminergic neurons: implication for neurotoxicity in PD. Oxid Med Cell Longev. 2021;2021:9961628. doi:10.1155/2021/9961628
78. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600–609. doi:10.1038/ncpneuro0924
79. Corasaniti MT, Strongoli MC, Rotiroti D, Bagetta G, Nistico G. Paraquat: a useful tool for the in vivo study of mechanisms of neuronal cell death. Pharmacol Toxicol. 1998;83(1):1–7. doi:10.1111/j.1600-0773.1998.tb01434.x
80. Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277(3):1641–1644. doi:10.1074/jbc.C100560200
81. Radad K, Al-Shraim M, Al-Emam A, et al. Rotenone: from modelling to implication in Parkinson’s disease. Folia Neuropathol. 2019;57(4):317–326. doi:10.5114/fn.2019.89857
82. Talpade DJ, Greene JG, Higgins DS
83. Schuler F, Casida JE. Functional coupling of PSST and ND1 subunits in NADH: ubiquinoneoxidoreductase established by photoaffinity labeling. Biochim Biophys Acta. 2001;1506(1):79–87. doi:10.1016/S0005-2728(01)00183-9
84. Yarmohammadi F, Wallace Hayes A, Najafi N, Karimi G. The protective effect of natural compounds against rotenone-induced neurotoxicity. J Biochem Mol Toxicol. 2020;34(12):e22605. doi:10.1002/jbt.22605
85. Li N, Ragheb K, Lawler G, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278(10):8516–8525. doi:10.1074/jbc.M210432200
86. Landau R, Halperin R, Sullivan P, et al. The rat rotenone model reproduces the abnormal pattern of central catecholamine metabolism found in Parkinson’s disease. Dis Model Mech. 2022;15(1). doi:10.1242/dmm.049082
87. Sharma M, Kaur J, Rakshe S, Sharma N, Khunt D, Khairnar A. Intranasal exposure to low-dose rotenone induced alpha-synuclein accumulation and Parkinson’s like symptoms without loss of dopaminergic neurons. Neurotox Res. 2022;40(1):215–229. doi:10.1007/s12640-021-00436-9
88. Mohammadi H, Ghassemi-Barghi N, Malakshah O, Ashari S. Pyrethroid exposure and neurotoxicity: a mechanistic approach. Arh Hig Rada Toksikol. 2019;70(2):74–89. doi:10.2478/aiht-2019-70-3263
89. Gassner B, Wuthrich A, Scholtysik G, Solioz M. The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J Pharmacol Exp Ther. 1997;281(2):855–860.
90. Hirano T, Suzuki N, Ikenaka Y, Hoshi N, Tabuchi Y. Neurotoxicity of a pyrethroid pesticide deltamethrin is associated with the imbalance in proteolytic systems caused by mitophagy activation and proteasome inhibition. Toxicol Appl Pharmacol. 2021;430:115723. doi:10.1016/j.taap.2021.115723
91. Chen D, Huang X, Liu L, Shi N. Deltamethrin induces mitochondrial membrane permeability and altered expression of cytochrome C in rat brain. J Appl Toxicol. 2007;27(4):368–372. doi:10.1002/jat.1215
92. Agrawal S, Singh A, Tripathi P, Mishra M, Singh PK, Singh MP. Cypermethrin-induced nigrostriatal dopaminergic neurodegeneration alters the mitochondrial function: a proteomics study. Mol Neurobiol. 2015;51(2):448–465. doi:10.1007/s12035-014-8696-7
93. Park YS, Park JH, Ko J, Shin IC, Koh HC. mTOR inhibition by rapamycin protects against deltamethrin-induced apoptosis in PC12 Cells. Environ Toxicol. 2017;32(1):109–121. doi:10.1002/tox.22216
94. Gozzelino R, Arosio P. Iron homeostasis in health and disease. Int J Mol Sci. 2016;17(1):130. doi:10.3390/ijms17010130
95. Baranwal AK, Singhi SC. Acute iron poisoning: management guidelines. Indian Pediatr. 2003;40(6):534–540.
96. Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–352. doi:10.1016/j.neurobiolaging.2007.07.015
97. Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65(3):199–203. doi:10.1097/01.jnen.0000202887.22082.63
98. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13(10):1045–1060. doi:10.1016/S1474-4422(14)70117-6
99. Carocci A, Catalano A, Sinicropi MS, Genchi G. Oxidative stress and neurodegeneration: the involvement of iron. Biometals. 2018;31(5):715–735. doi:10.1007/s10534-018-0126-2
100. Lhermitte J, Kraus WM, McAlpine D. Original papers: on the occurrence of abnormal deposits of iron in the brain in parkinsonism with special reference to its localisation. J Neurol Psychopathol. 1924;5(19):195–208. doi:10.1136/jnnp.s1-5.19.195
101. Salazar J, Mena N, Hunot S, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105(47):18578–18583. doi:10.1073/pnas.0804373105
102. Nunez MT, Chana-Cuevas P. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals. 2018;11(4):109. doi:10.3390/ph11040109
103. Devos D, Moreau C, Devedjian JC, et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal. 2014;21(2):195–210. doi:10.1089/ars.2013.5593
104. Mastroberardino PG, Hoffman EK, Horowitz MP, et al. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol Dis. 2009;34(3):417–431. doi:10.1016/j.nbd.2009.02.009
105. Galvin JE, Giasson B, Hurtig HI, Lee VM, Trojanowski JQ. Neurodegeneration with brain iron accumulation, type 1 is characterized by alpha-, beta-, and gamma-synuclein neuropathology. Am J Pathol. 2000;157(2):361–368. doi:10.1016/S0002-9440(10)64548-8
106. Wakabayashi K, Fukushima T, Koide R, et al. Juvenile-onset generalized neuroaxonal dystrophy (Hallervorden-Spatz disease) with diffuse neurofibrillary and lewy body pathology. Acta Neuropathol. 2000;99(3):331–336. doi:10.1007/s004010050049
107. Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103(1):17–37. doi:10.1111/j.1471-4159.2007.04764.x
108. Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–873. doi:10.1038/nrn1537
109. Koeppen AH. The history of iron in the brain. J Neurol Sci. 1995;134:1–9. doi:10.1016/0022-510X(95)00202-D
110. Sanchez-Iglesias S, Soto-Otero R, Iglesias-Gonzalez J, Barciela-Alonso MC, Bermejo-Barrera P, Mendez-Alvarez E. Analysis of brain regional distribution of aluminium in rats via oral and intraperitoneal administration. J Trace Elem Med Biol. 2007;Suppl 21:31–34. doi:10.1016/j.jtemb.2007.09.010
111. Johnson VJ, Kim SH, Sharma RP. Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol Sci. 2005;83(2):329–339. doi:10.1093/toxsci/kfi028
112. Kumar V, Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41:154–166. doi:10.1016/j.neuro.2014.02.004
113. Savory J, Herman MM, Ghribi O. Intracellular mechanisms underlying aluminum-induced apoptosis in rabbit brain. J Inorg Biochem. 2003;97(1):151–154. doi:10.1016/S0162-0134(03)00258-7
114. Oshima E, Ishihara T, Yokota O, et al. Accelerated tau aggregation, apoptosis and neurological dysfunction caused by chronic oral administration of aluminum in a mouse model of tauopathies. Brain Pathol. 2013;23(6):633–644. doi:10.1111/bpa.12059
115. Sanchez-Iglesias S, Mendez-Alvarez E, Iglesias-Gonzalez J, et al. Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J Neurochem. 2009;109(3):879–888. doi:10.1111/j.1471-4159.2009.06019.x
116. Sadeghi L, Tanwir F, Yousefi Babadi V. Physiological and biochemical effects of echium amoenum extract on Mn(2+)-imposed Parkinson like disorder in rats. Adv Pharm Bull. 2018;8(4):705–713. doi:10.15171/apb.2018.079
117. Burton NC, Schneider JS, Syversen T, Guilarte TR. Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol Sci. 2009;111(1):131–139. doi:10.1093/toxsci/kfp124
118. Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W
119. Dobson AW, Weber S, Dorman DC, Lash LK, Erikson KM, Aschner M. Oxidative stress is induced in the rat brain following repeated inhalation exposure to manganese sulfate. Biol Trace Elem Res. 2003;93(1–3):113–126. doi:10.1385/BTER:93:1-3:113
120. Erikson KM, Dorman DC, Lash LH, Dobson AW, Aschner M. Airborne manganese exposure differentially affects end points of oxidative stress in an age- and sex-dependent manner. Biol Trace Elem Res. 2004;100(1):49–62. doi:10.1385/BTER:100:1:049
121. Milatovic D, Yin Z, Gupta RC, et al. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98(1):198–205. doi:10.1093/toxsci/kfm095
122. Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur J Pharmacol. 1995;293(4):377–383. doi:10.1016/0926-6917(95)90058-6
123. Zwingmann C, Leibfritz D, Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab. 2003;23(6):756–771. doi:10.1097/01.WCB.0000056062.25434.4D
124. Singh J, Husain R, Tandon SK, Seth PK, Chandra SV. Biochemical and histopathological alterations in early manganese toxicity in rats. Environ Physiol Biochem. 1974;4(1):16–23.
125. Sloot WN, Gramsbergen JB. Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res. 1994;657(1–2):124–132. doi:10.1016/0006-8993(94)90959-8
126. Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JB. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J Neurochem. 2008;62(1):205–216. doi:10.1046/j.1471-4159.1994.62010205.x
127. Parenti M, Rusconi L, Cappabianca V, Parati EA, Groppetti A. Role of dopamine in manganese neurotoxicity. Brain Res. 1988;473(2):236–240. doi:10.1016/0006-8993(88)90852-9
128. Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20(2–3):445–453.
129. Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3(4):461–491. doi:10.3233/JPD-130230
130. Erekat NS. Apoptosis and its role in Parkinson’s Disease. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Brisbane (AU): Exon Publications; 2018.
131. Mizuno Y, Saitoh T, Sone N. Inhibition of mitochondrial NADH-ubiquinone oxidoreductase activity by 1-methyl-4-phenylpyridinium ion. Biochem Biophys Res Commun. 1987;143(1):294–299. doi:10.1016/0006-291X(87)90664-4
132. Wang YM, Pu P, Le WD. ATP depletion is the major cause of MPP+ induced dopamine neuronal death and worm lethality in alpha-synuclein transgenic C. elegans. Neurosci Bull. 2007;23(6):329–335. doi:10.1007/s12264-007-0049-3
133. Gash DM, Rutland K, Hudson NL, et al. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63(2):184–192. doi:10.1002/ana.21288
134. Liu M, Choi DY, Hunter RL, et al. Trichloroethylene induces dopaminergic neurodegeneration in fisher 344 rats. J Neurochem. 2010;112(3):773–783. doi:10.1111/j.1471-4159.2009.06497.x
135. Liu M, Shin EJ, Dang DK, et al. Trichloroethylene and Parkinson’s Disease: risk assessment. Mol Neurobiol. 2018;55(7):6201–6214. doi:10.1007/s12035-017-0830-x
136. Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med. 2013;62:65–75. doi:10.1016/j.freeradbiomed.2013.01.032
137. Gubellini P, Picconi B, Di Filippo M, Calabresi P. Downstream mechanisms triggered by mitochondrial dysfunction in the basal ganglia: from experimental models to neurodegenerative diseases. Biochim Biophys Acta. 2010;1802(1):151–161. doi:10.1016/j.bbadis.2009.08.001
138. Wolozin B, Golts N. Iron and Parkinson’s disease. Neuroscientist. 2002;8(1):22–32. doi:10.1177/107385840200800107
139. Shi L, Huang C, Luo Q, et al. The association of iron and the pathologies of Parkinson’s Diseases in MPTP/MPP(+)-induced neuronal degeneration in non-human primates and in cell culture. Front Aging Neurosci. 2019;11:215. doi:10.3389/fnagi.2019.00215
140. Sherer TB, Betarbet R, Testa CM, et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23(34):10756–10764. doi:10.1523/JNEUROSCI.23-34-10756.2003
141. Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism Relat Disord. 2003;9(Suppl 2):S59–S64. doi:10.1016/S1353-8020(03)00023-3
142. Perier C, Bove J, Vila M, Przedborski S. The rotenone model of Parkinson’s disease. Trends Neurosci. 2003;26(7):345–346. doi:10.1016/S0166-2236(03)00144-9
143. Tawara T, Fukushima T, Hojo N, et al. Effects of paraquat on mitochondrial electron transport system and catecholamine contents in rat brain. Arch Toxicol. 1996;70(9):585–589. doi:10.1007/s002040050316
144. Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283(4):1786–1798. doi:10.1074/jbc.M708597200
145. Yamada K, Fukushima T. Mechanism of cytotoxicity of paraquat. II. Organ specificity of paraquat-stimulated lipid peroxidation in the inner membrane of mitochondria. Exp Toxicol Pathol. 1993;45(5–6):375–380. doi:10.1016/S0940-2993(11)80433-1
146. Elwan MA, Richardson JR, Guillot TS, Caudle WM, Miller GW. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol Appl Pharmacol. 2006;211(3):188–197. doi:10.1016/j.taap.2005.06.003
147. Hansen MRH, Jors E, Lander F, et al. Neurological deficits after long-term pyrethroid exposure. Environ Health Insights. 2017;11:1178630217700628. doi:10.1177/1178630217700628
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.