Back to Journals » Infection and Drug Resistance » Volume 16
Misdiagnosis of Systemic Lupus Erythematosus Combined with Urinary Tuberculosis Leading to Tuberculous Meningitis: A Case Report and Literature Review
Authors Ma H, Wang Y , Liu J , Du L, Wang X , Wang Y
Received 11 May 2023
Accepted for publication 5 July 2023
Published 18 July 2023 Volume 2023:16 Pages 4677—4686
DOI https://doi.org/10.2147/IDR.S420833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Honglei Ma,1 Yuqun Wang,1 Junhong Liu,1 Linping Du,1 Xiaodong Wang,2 Yingliang Wang2
1Affiliated Hospital of Weifang Medical University, School of Clinical Medicine, Weifang Medical University, Weifang, Shandong Province, People’s Republic of China; 2Rheumatology and Immunology, Affiliated Hospital of Weifang Medical University, Weifang, Shandong Province, People’s Republic of China
Correspondence: Yingliang Wang, Affiliated Hospital of Weifang University, No. 2428, Yuhe Road, Kuiwen District, Weifang, Shandong Province, People’s Republic of China, Tel +86 13869663571, Fax +86 5363081201, Email [email protected]
Purpose: To explore the lessons learned from the misdiagnosis of systemic lupus erythematosus (SLE) combined with urinary tuberculosis leading to tuberculous meningitis (TBM) and the diagnosis and treatment of TBM through case reports and review of the literature.
Methods: We report a case of an SLE patient presenting with urinary tuberculosis infection misdiagnosed as interstitial cystitis and complex urinary tract infection, who developed neurological infection after a cystocentesis biopsy and was eventually diagnosed with TBM. In addition, all cases of SLE combined with TBM from January 1975 to February 2022 were summarised and reviewed to compare current diagnostic and treatment strategies for the disease.
Results: The patient suddenly developed neurological symptoms after cystocentesis biopsy, and we detected Mycobacterium tuberculosis in the macrogenomic next-generation sequence (mNGS) of the cerebrospinal fluid. We therefore excluded interstitial cystitis and neuropsychiatric lupus to confirm the diagnosis of Mycobacterium tuberculosis infection leading to urinary tract tuberculosis and TBM.
Conclusion: SLE is complicated by urological tuberculosis, surgery triggering hematogenous dissemination leading to tuberculous meningitis. At the same time, the lack of specificity in the clinical presentation of patients makes it easy to misdiagnose neuropsychiatric lupus and delay treatment, so timely and accurate diagnosis and effective anti-tuberculosis treatment are essential.
Keywords: tuberculosis, meningeal, urinary tuberculosis, lupus erythematosus, systemic, lupus vasculitis, central nervous system
Introduction
SLE is a multi-organ system autoimmune disease that produces a variety of pathogenic autoantibodies and immune complexes. In patients with SLE, 30–50% of morbidity and mortality is attributed to infections, mainly of the respiratory and urinary tract, skin and soft tissues, and blood, while central nervous system infections account for only 3% of all cases of infection.
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis and is one of the leading causes of global ill health and one of the leading causes of death. It is estimated that around a quarter of the world’s population is already infected with TB, and the World Health Organization estimates that the number of new TB diagnoses will rise again to 6.4 million in 2021.1 Central nervous system tuberculosis occurs in about 1% of all patients with active tuberculosis and immunosuppressed adults are more susceptible to disseminated disease and involvement of the central nervous system.2 TBM is the most common form of central nervous system tuberculosis and the most severe form of TB, with 1 in 5 TBM cases resulting in death.3,4 TBM is difficult to distinguish from other causes of meningoencephalitis and the prognosis is generally poor once the neurological symptoms of advanced disease (such as coma, seizures, increased intracranial pressure, and hemiparesis) are present.
The exact mechanism of SLE combined with TBM is unknown and may be related to immune abnormalities associated with SLE, such as immunoglobulin deficiency, complement deficiency, and reduced complement receptor expression, combined with immunosuppression, and hormone induced cellular immune deficiency, leading to an increased chance of pathogenic infection and death.
Because SLE is susceptible to Mycobacterium tuberculosis infection, it has been suggested that isoniazid (INH) may be used in combination with primary therapy to prevent tuberculosis. However, the effectiveness of INH in preventing the development of TB in this group of patients is not known. The balance between SLE and TB treatment after infection with Mycobacterium tuberculosis is also a major challenge.
There are still very limited reports of cases of TBM following surgery for SLE combined with urological TB. Here, we provide a case report as a reference and a summary of the available reports on this disease to explore the early diagnosis, treatment strategies, and future directions of exploration in SLE combined with TBM.
Case Report
A 52-year-old man was admitted to our hospital with recurrent episodes of urinary urgency, frequency and painful urination with elevated blood creatinine for 18 months. The patient had a history of systemic lupus erythematosus and lupus nephritis for 14 years. He was routinely treated with “prednisone 10 mg/d, leflunomide 20 mg/d, and hydroxychloroquine 0.4 g/d”. The patient first developed urinary frequency and urgency in 2013, and routine urine tests suggested the presence of white blood cells, which improved with anti-infective treatment. Frequent, urgent and painful urination reappeared in 2019 and anti-infective treatment was given with poor results. The patient’s symptoms persisted and several repeat urine tests showed leukocytes, but urine cultures were not abnormal. The renal function was reviewed on 13 January 2021 for abnormalities and elevated blood creatinine, and herbal treatment was given.
On July 11, 2022, he was admitted to our nephrology department for treatment. A routine urine examination showed 434.1/μL white blood cells, 53.7/μL red blood cells and 64.9/μL bacteria (Figure 1). Blood sedimentation 44 mm/h; C-reactive protein 16.82 mg/L; no abnormalities in routine blood (Table 1); renal function: creatinine 124 μmol/L, urea 8.58 mmol/L; no abnormalities in urine culture. Anti-nuclear antibody +1:320; anti-dsDNA antibody 131.1 IU/mL. Urological ultrasound: right kidney stone, left hydronephrosis and left ureteral dilatation. Computed Tomography (CT) of kidneys and bladder: 1. Stones in both kidneys; 2. Thickening of the left posterior wall of the bladder with hydronephrosis of the left kidney and dilated effusion of the left ureter; 3. Cyst in the right kidney (Figure 2). We considered complicated urinary tract infections, interstitial cystitis, and poor anti-infective effect. On day 18 of admission, urethral stricture dilatation, transurethral suburethral cystoscopy, hydrodistension and cystocentesis biopsy were performed. Pathology showed a microscopic examination of transverse muscle and a little fibrous and fatty tissue. On the 24th day of admission, the urine test was repeated: white blood cells 951.3/μL, red blood cells 1258.8/μL and bacterial count 274.7/μL. The patient requested to be discharged after his condition was more stable than before.
 |
Figure 1 The number of leukocytes, red blood cells and bacteria in the patient’s urine routine during hospitalization. |
 |
Figure 2 CT of the bladder: the blue arrow marks the location of the patient’s bladder wall thickening. |
 |
Table 1 Routine Blood Test |
On 16 August 2022, the patient was readmitted to the hospital with “increased urinary frequency, urgency and hematuria of the naked eye one week after surgery”. The patient had an elevated body temperature of 38.8°C. And We found him to be unresponsive when we talked to him. Blood count: white blood cells 10.22*10^9/L, red blood cells 4.36*10^12/L, platelets 203*10^9/L; Urine routine: leucocytes 2618.50/μL, bacterial count 2937.7/μL and a large number of red blood cells. Renal function: creatinine 121.0 μmol/L, urea 5.64 mmol/L. We gave bladder irrigation and piperacillin sodium tazobactam anti-infective treatment.
Sudden onset of unconsciousness on the 3rd day of admission, and cranial magnetic resonance imaging (MRI) of the brain showed: 1. ventral diffusion-weighted imaging (DWI) punctate high signal in the pontine brain; 2. bilateral frontal lobes with a little lacunar cerebral infarction; 3. mild demyelination changes in the white matter of the brain around the ventricles bilaterally; 4. mild septal sinusitis; 5. right vertebral artery terminal stenosis, right posterior cerebral artery P3 segment, anterior cerebral artery local mild stenosis, cerebrovascular atherosclerotic changes (Figure 3). We considered excluding the possibility of cerebral hemorrhage and cerebral infarction. And no meningeal enhancement changes were seen. The patient had a recurrent elevated temperature and was unconscious. We, therefore, concluded that lupus encephalopathy could not be ruled out and added methylprednisolone 40 mg/d. The symptoms did not improve significantly and there were still recurrent fevers and occasional headaches.
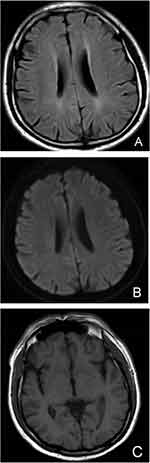 |
Figure 3 (A): FLAIR sequence of head MRI; (B): DWI sequence of head MRI; (C): T1 sequence of head MRI. Head MRI did not show meningeal enhancement. |
A repeat cranial MRI on the 9th day of admission showed no specific abnormalities and an initial pressure of 125 mmH2O was measured by parallel lumbar puncture. Cerebrospinal fluid laboratory results indicated: lactate dehydrogenase 71 U/L, glucose 1.22 mmol/L, chloride 113.2 mmol/L, adenosine dehydrogenase 4.8 U/L, protein 1.660 g/L, cell count 110*10^6/L, immunoglobulin G 216.0 mg/L, immunoglobulin M 4.5 mg/L, immunoglobulin A 94.4 mg/L (Table 2). No antacid bacilli were detected in the cerebrospinal fluid concentrate collection test. The cerebrospinal fluid culture was not abnormal. The cerebrospinal fluid antibody test for Mycobacterium tuberculosis was negative. Cerebrospinal fluid DNA quantification of Mycobacterium tuberculosis was negative. Cerebrospinal fluid fungal microscopy was unremarkable. Multiple blood cultures were unremarkable. We then discussed with the family about sending the cerebrospinal fluid out for mNGS.
 |
Table 2 Cerebrospinal Fluid Studies |
Day 13 mNGS results showed Mycobacterium tuberculosis complex sequence number 3358 in the cerebrospinal fluid. At this point, the patient’s diagnosis of tuberculous meningitis was clarified. Meanwhile, we applied the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) to score the patient’s current disease activity and the result was a score of 2. The SLE disease was largely inactive. He was transferred to the Infectious Diseases Hospital on 28 August 2022 for continued treatment. Mycobacterium tuberculosis was found in the urine concentrated collector antacid test on 4 September and was treated with isoniazid, rifampicin, ethambutol and pyrazinamide against tuberculosis. At the six-month follow-up, the patient is still on continuous anti-tuberculosis treatment and is in stable condition.
Literature Review
Literature Search Strategy and Selection Criteria
A search of PubMed and China Knowledge Network for the keywords “systemic lupus erythematosus” and “tuberculous meningitis” yielded 115 relevant articles. After the abstract screening, 17 papers were selected. After reading the full text, those without complete data and duplicate cases were excluded and nine papers were retained.” One case report was excluded as the patient was infected with both Mycobacterium tuberculosis and Cryptococcus. Finally, we accurately summarised eight papers.5–12 A total of 46 patients were included (Table 3).
 |
Table 3 Basic Information of All Patients |
Results
By analyzing the results of cerebrospinal fluid tests in 46 patients, we found that patients with SLE combined with TBM generally had increased cerebrospinal fluid pressure, increased cerebrospinal fluid leukocytes and protein, and decreased chloride and glucose levels (Table 4). More importantly, the rate of positive cultures or smears for Mycobacterium tuberculosis in the cerebrospinal fluid is very low.
 |
Table 4 Literature Review of Cerebrospinal Fluid Data and Reference Value Statistics for All Patients |
Discussion
The risk of infection in adults with SLE is two to six times higher than in the general population.13 Susceptibility to a wide range of serious infections has become a major cause of morbidity and mortality in people with SLE.14,15 The risk of death from infection is five times higher in people with SLE than in the general population.16 Potential causes of infection susceptibility in SLE patients include immune dysregulation, the use of glucocorticoids and immunosuppressive drugs.17 Also, the risk of TB increased 7.7-fold at a daily dose of 15 mg prednisone equivalent and 5-fold at a cumulative dose of >1000 mg.18
Tuberculosis is a major infectious disease worldwide. A study that analyzed data from 35 studies, including 46,327 SLE patients from 13 countries on five continents, found that the incidence and prevalence of TB among SLE patients were 1.16 per 100 person-years and 3.59%, respectively.19 A study that included 17,751 SLE patients and 209 SLE patients with CNS infection found that the incidence of CNS infection in SLE patients was 0.012.20 CNS infection has also been found to account for 1.4% of SLE patients, but mortality rates higher than 40% have been reported.21,22 Of these, meningitis is the most common clinical syndrome and Cryptococcus neoformans and Mycobacterium tuberculosis are the most common pathogens.20 Clinicians should therefore maintain a high level of suspicion for SLE patients with suspected CNS infection, especially those on high doses of hormones, to clarify the presence of Cryptococcus or Mycobacterium tuberculosis infection.
The medical history of this patient has the following characteristics. First, the patient had a previous history of SLE and lupus nephritis and had been treated with hormones and immunosuppressants for a long time. In the past three years, he had recurrent urinary frequency, urgency, and painful urination. White blood cells were seen in the patient’s urine routine on several retests, while no bacterial growth in urine culture. Concomitant application of antibiotic treatment is less effective. Secondly, the patient was diagnosed with a “complicated urinary tract infection and Interstitial cystitis” during his hospitalization, but no pathogens were found in several pathogenic tests, and the anti-infective effect was poor. Third, cerebrospinal fluid testing was refined and no pathogens were detected, but glucose and chloride levels were significantly low. The neurologist thought that tuberculous meningitis was ruled out, so the cerebrospinal fluid was sent out for mNGS testing and Mycobacterium tuberculosis was found. After a definite diagnosis, the patient was transferred to an infectious disease hospital for anti-tuberculosis treatment and his symptoms gradually improved.
Because the definitive diagnosis of renal tuberculosis is often delayed by clinical and radiological manifestations, many of its symptoms resemble those of conventional bacterial cystitis, and suspicion is aroused only when antibiotics are ineffective.23 Therefore, we should consider the possibility of renal tuberculosis in patients with SLE who present with refractory urinary tract infections, where leukocytes are repeatedly detected in the urine and urine cultures are negative, and where antibiotic therapy is ineffective. To determine the presence of Mycobacterium tuberculosis infection, in addition to urine culture, we can also do a urine staining test for concentrated concentrations of Mycobacterium antacid. Secondly, patients with urinary tract infections, especially if the pathogen is not known, should preferably not undergo invasive urinary tract surgery to prevent dissemination of the pathogen into the bloodstream, with serious consequences. Thirdly, in this case, there were no signs of meningitis or lupus encephalopathy on multiple cranial CT and MRI and no Mycobacterium tuberculosis was seen on cerebrospinal fluid examination. It is difficult to distinguish between brain infection and lupus vasculitis in patients with SLE, both clinically and radiologically, and the low rate of positive cerebrospinal fluid smears often delays diagnosis, leading to the progression of infection and neurological complications.24 Tuberculous meningitis, therefore, requires repeated investigations, with at least three consecutive cerebrospinal fluid tests and bacterial pathogenic tests. Cerebrospinal fluid (CSF) examination is key to the diagnosis of most patients with suspected intracranial infection. Increased protein content (>0.45 g/L), decreased glucose content (<2.50 mmol/L), increased white blood cell count (>8 × 106/L) and a decreased chloride content (chloride content) in the CSF are associated with TBM.25 In addition, the greater the likelihood of glucose and protein separation, the greater the likelihood of a diagnosis of TBM. However, typical tuberculous cerebrospinal fluid changes, such as elevated cerebrospinal fluid proteins and reduced sugar and chloride ions, are currently considered to be of limited value for the early diagnosis of TBM, with a high rate of false positives.26 In this case, although the cerebrospinal fluid protein was elevated and the sugar and chloride ions were reduced early on, no pathogenic microorganisms were identified. Finally, we applied mNGS and found the presence of Mycobacterium tuberculosis in the cerebrospinal fluid, which confirmed the diagnosis. mNGS is a new clinical technology, the integrated analysis of microbial and hosts genetic material in patient samples. Whole-genome sequencing of cultured microbial isolates using NGS for biotyping, epidemiology, susceptibility prediction and virulence factor determination promises to improve our ability to diagnose, investigate and follow-up infectious diseases.27 Since it was first reported in 2014, NGS has been used to detect pathogens in cerebrospinal fluid for rapid and effective diagnosis of CNS infectious diseases.28 Although conventional tests offer a fairly cost-effective and rapid method, they are limited to the diagnosis of the most common known infections, and mNGS is clearly beyond the reach of conventional tests for the detection of unknown pathogenic microorganisms and mixed infections. Limitations in clinical testing methods often lead to incorrect dosing and delayed treatment, so early refinement of mNGS to clarify the presence or absence of pathogenic infection is essential.
The patient in this case was treated for tuberculosis immediately after the diagnosis was made and fortunately improved after aggressive anti-tuberculosis treatment. For patients with rheumatic diseases treated with hormones and those living in countries with a high incidence of tuberculosis, there is a high risk of developing tuberculosis. It has been suggested that INH can be used in combination with primary treatment to prevent TB.29 However, the effectiveness of INH in preventing the development of tuberculosis in this group of patients is unclear. There are also concerns about the potential for the development of multidrug-resistant TB and the cost-effectiveness and safety of INH, which limit its use in general practice for patients at high risk of non-TB disease.30,31 As a result, most national guidelines for TB prevention, especially those relevant to people with rheumatic diseases, do not thoroughly address this issue. Secondly, TBM is a medical emergency and delays in treatment are strongly associated with death. All patients with a suspected diagnosis of TBM should start empirical anti-TB treatment immediately and not wait for microbiological or molecular diagnostic confirmation.2
Conclusion
In summary, early diagnosis of renal tuberculosis is difficult on clinical and imaging grounds alone, so it is best to avoid invasive urinary tract surgery in the presence of urinary tract infection in immunosuppressed patients, especially if the pathogen is not known, to prevent dissemination of the pathogen into the bloodstream. Secondly, when SLE patients present with neurological symptoms, not only should neuropsychiatric lupus be considered, but cerebrospinal fluid tests should also be actively refined to rule out infection. The application of mNGS as a novel diagnostic technique can provide a rapid and accurate test for clinical diagnosis and can play a decisive role in the diagnosis of cases of unexplained infection.
Abbreviations
SLE, systemic lupus erythematosus; TBM, tuberculous meningitis; mNGS, metagenomics next-generation sequencing; TB, tuberculosis; CT, computed tomography; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; CSF, cerebrospinal fluid; INH, isoniazid.
Data Sharing Statement
All the data in this study are included in the published articles.
Ethics Approval and Informed Consent
The patient provided informed consent to publish their case details and any accompanying images. Our institutions do not require ethical approval to report individual cases or series of cases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Fund of Scientific research project of Weifang Health Commission, China (WFWSJK-2022-015); the Fund of Doctoral Fund, China (2022BSQD03); and the Fund of Graduate Student Research Grant from Weifang Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2022; 2022. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
2. Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–187. doi:10.1016/j.jinf.2009.06.011
3. Török ME. Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull. 2015;113(1):117–131. doi:10.1093/bmb/ldv003
4. Seid G, Alemu A, Dagne B, Gamtesa DF. Microbiological diagnosis and mortality of tuberculosis meningitis: systematic review and meta-analysis. PLoS One. 2023;18(2):e0279203. doi:10.1371/journal.pone.0279203
5. Zhou M, Wang H. Clinical analysis of 5 cases of systemic lupus erythematosus combined with tuberculous meningitis. Anhui Med J. 2008;2008(1):71–72.
6. Kong W, Yan J. Presentation and diagnosis of systemic lupus erythematosus with central lesions (with 12 case reports). J Nanhua Univ. 2004;2004(4):545–546.
7. Zou Y, Chen Z, Yimin F. Clinical analysis of 15 case systemic lupus erythematosus with tuberculous meningitis patients. Med Recap. 2009;15(15):2376–2378.
8. Hua B, Wang H, Feng X, Ding C, Liu B, Sun L. A case of systemic lupus erythematosus combined with tuberculous meningitis. Chin J Gen Pract. 2007;6(07):424–426.
9. Wang Y, Tang J, Yueying G, Wang J. Manifestation and diagnosis of 14 patients with systemic lupus erythematosus and centric nervous system infection. Chin J Rheumatol. 2002;2002(1):35–36.
10. Wang J, Zhao H, Dawei H. Clinical analysis of 13 cases of systemic lupus erythematosus combined with tuberculous meningitis. Chin Gen Pract. 2007;2007(4):315–316.
11. Li Y, Zhao H, Zuo X. Systemic lupus erythematosus with lung, brain, liver, and spleen tuberculosis. J Clin Rheumatol. 2012;18(7):385. doi:10.1097/RHU.0b013e31826d67ac
12. Takahashi T, Ogawa K, Sawada S, Nakayama T, Mizutani T. イソニアジド (INH) の髄腔内投与により著明に改善した難治性結核性髄膜炎の1例 [A case of refractory tuberculous meningitis markedly improved by intrathecal administration of isoniazid (INH)]. Rinsho Shinkeigaku. 2003;43(1–2):20–25. Japanese.
13. Pego-Reigosa JM, Nicholson L, Pooley N, et al. The risk of infections in adult patients with systemic lupus erythematosus: systematic review and meta-analysis. Rheumatology. 2021;60(1):60–72. doi:10.1093/rheumatology/keaa478
14. Iliopoulos AG, Tsokos GC. Immunopathogenesis and spectrum of infections in systemic lupus erythematosus. Semin Arthritis Rheum. 1996;25(5):318–336. doi:10.1016/s0049-0172(96)80018-7
15. Goldblatt F, Chambers S, Rahman A, Isenberg DA. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus. 2009;18(8):682–689. doi:10.1177/0961203308101019
16. Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25(7):727–734. doi:10.1177/0961203315627202
17. Juárez M, Misischia R, Alarcón GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Rheum Dis Clin North Am. 2003;29(1):163–184. doi:10.1016/s0889-857x(02)00100-x
18. Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55(1):19–26. doi:10.1002/art.21705
19. Wu Q, Liu Y, Wang W, et al. Incidence and prevalence of tuberculosis in systemic lupus erythematosus patients: a systematic review and meta-analysis. Front Immunol. 2022;13:938406. doi:10.3389/fimmu.2022.938406
20. Molooghi K, Sheybani F, Naderi H, Mirfeizi Z, Morovatdar N, Baradaran A. Central nervous system infections in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus Sci Med. 2022;9(1):e000560. doi:10.1136/lupus-2021-000560
21. Kim JM, Kim KJ, Yoon HS, et al. Meningitis in Korean patients with systemic lupus erythematosus: analysis of demographics, clinical features and outcomes; experience from affiliated hospitals of the Catholic University of Korea. Lupus. 2011;20(5):531–536. doi:10.1177/0961203310384495
22. Zandman-Goddard G, Shoenfeld Y, Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38(7):473–485. doi:10.1080/08916930500285352
23. Arenas Miras Mdel M, Hidalgo Tenorio C, Jimenez Alonso J. Tuberculosis in patients with systemic lupus erythematosus: spain’s situation. Reumatol Clin. 2013;9(6):369–372. doi:10.1016/j.reuma.2012.06.011
24. Sanna G, Bertolaccini ML, Khamashta MA. Neuropsychiatric involvement in systemic lupus erythematosus: current therapeutic approach. Curr Pharm Des. 2008;14(13):1261–1269. doi:10.2174/138161208799316401
25. Yang Y, Qu XH, Zhang KN, et al. A diagnostic formula for discrimination of tuberculous and bacterial meningitis using clinical and laboratory features. Front Cell Infect Microbiol. 2019;9:448. doi:10.3389/fcimb.2019.00448
26. Cao D, Wang T, Wang Y, Han J. Analysis of cases with cerebrospinal fluid characteristics similar to tuberculous meningitis. Biomed Res Int. 2022;2022:9692804. doi:10.1155/2022/9692804
27. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi:10.1146/annurev-pathmechdis-012418-012751
28. Zanella MC, Lenggenhager L, Schrenzel J, Cordey S, Kaiser L. High-throughput sequencing for the aetiologic identification of viral encephalitis, meningoencephalitis, and meningitis. A narrative review and clinical appraisal. Clin Microbiol Infect. 2019;25(4):422–430. doi:10.1016/j.cmi.2018.12.022
29. Gaitonde S, Pathan E, Sule A, Mittal G, Joshi VR. Efficacy of isoniazid prophylaxis in patients with systemic lupus erythematosus receiving long term steroid treatment. Ann Rheum Dis. 2002;61(3):251–253. doi:10.1136/ard.61.3.251
30. Falagas ME, Voidonikola PT, Angelousi AG. Tuberculosis in patients with systemic rheumatic or pulmonary diseases treated with glucocorticosteroids and the preventive role of isoniazid: a review of the available evidence. Int J Antimicrob Agents. 2007;30(6):477–486. doi:10.1016/j.ijantimicag.2007.07.010
31. Park JW, Curtis JR, Lee H, Lee JK, Song YW, Lee EB. Risk-benefit analysis of isoniazid monotherapy to prevent tuberculosis in patients with rheumatic diseases exposed to prolonged, high-dose glucocorticoids. PLoS One. 2020;15(12):e0244239. doi:10.1371/journal.pone.0244239
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
