Back to Journals » International Journal of Women's Health » Volume 12
Miscarriage as a Complication of Hidradenitis Suppurativa of the Vulva
Authors Al Ghamdi DS
Received 16 June 2020
Accepted for publication 12 October 2020
Published 29 October 2020 Volume 2020:12 Pages 939—942
DOI https://doi.org/10.2147/IJWH.S268050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Deama Saeed Al Ghamdi
Department of Obstetrics and Gynecology, ABHA Maternity Hospital, King Khalid University, Abha, Assir, Saudi Arabia
Correspondence: Deama Saeed Al Ghamdi Postal Address: 62527 Tel +966566553598
Fax +96617 2312323
Email [email protected]
Abstract: We present a rare case of severe chronic hidradenitis suppurativa (HS) Hurley (stage III) of the vulva complicated by disfiguring draining sinuses, multiple abscesses, and multiple nodules which resulted in miscarriage as a result of ascending infection.
Keywords: hidradenitis suppurativa, miscarriage, abortion, infections
Introduction
Hidradenitis suppurativa (HS), also known as Verneuil’s disease, was first described in 1854 by the French surgeon Aristide Verneuil.1 In March 2009, at the second international symposium organized by the HS Foundation, the disease was defined as a chronic progressive, inflammatory, recurrent debilitating, skin follicular disease that usually presents after puberty with painful deep-seated inflamed lesions in the apocrine gland-bearing areas of the body, most commonly the axillary, inguinal, and anogenital region.2 Disease severity is generally assessed by the Hurley classification.3 Several studies have reported that HS is common in women, with a global prevalence of 1–4%.4 The prevalence of HS in the genital region in one study is about 0.02%, with the armpit of the vulva and groin most frequently affected.5
Severe long-standing HS with interconnected tracts and abscesses has been associated with Crohn's disease, diabetes, obesity, history of smoking, and vulvar cancer.6–8 A diagnosis of HS is made based on clinical pictures and the criteria adopted by the HS Foundation in 2009.2 The assessment using ultrasonography scoring has been shown to be more sensitive, notably in the fistula.9
Treatment options vary based on the severity and degree of the disease ranging from simple topical measures, systemic antibiotics, intralesional steroids, biological agents, hormonal therapy, laser therapy, and surgical excision.10–15
Case Report
We report the case of a 40-year old G6P5+0 with gestation of 16 weeks who initially presented to our clinic in 2010 with abscess formation and multiple sinus tract HS (Hurley stage I–II), involving mainly the vulvar area. Her disease would flare up from time to time, more commonly during her pregnancy. Her only associated comorbidity was obesity with a BMI of 33 kg/m2. Between 2010 and 2018 she was admitted to our hospital three times due to severe pain, itching, multiple painful nodules, deep abscess formation, and sinus discharge. Topical treatment along with clindamycin (1%) and rifampicin (600 mg/day each) given orally was continued for a period of 3 months. She had previously responded well to the above regimens. In 2019, she tried to conceive and during the second trimester of her pregnancy the disease worsened even though she was on the same regimen mentioned above. She came to hospital with a 2-month history of severe pain, fever, severe itching, and vaginal discharge.
Examination of the vulva showed a huge painful polypoidal lymphedematous mass measuring 20x10 cm in the left vulva and 8x3 cm in the right vulva. Additionally, there were foul odors from discharge from multiple sinuses, deep abscesses, cellulitis of the groin, and pus mixed with blood from vaginal discharge. The patient was admitted to hospital and swab cultures were taken from the abscesses, sinus discharge, and cervicovaginal discharge. Anaerobic (Peptostreptococcus anaerobius), and group B-streptococcal bacteria were isolated from both vaginal and sinus discharge. The same organism was found in amniotic fluid analysis, and both organisms were sensitive to ciprofloxacin antibiotics. Routine blood examination showed leukocytosis with leukocytes at 14.3x109, with 34% neutrophils and 34% lymphocytes. Urea, creatinine, and electrolytes were normal. Serological (PCR) and immunological IgM and IgG did not indicate any viral infection. Abdominal ultrasound was normal, and the patient was diagnosed with HS (Hurley stage III) with cervicovaginal infection.
Unfortunately, on the second day of her admission, the patient went into late miscarriage due to ascending infection from her disease. A multidisciplinary team comprising of an infectious disease specialist, dermatologist, plastic surgeon, and gynecologist were involved in the care of this patient. The gross description of the placenta showed a yellow-green discoloration and cloudy amniotic fluid. A microscopic picture showed neutrophil infiltrate of membrane overlying chorionic plate, the amnion and upper chorion are infiltrated with macrophage and neutrophil in addition to vasculitis of chorionic plate with acute deciduitis. She was treated in hospital for 1 week with intravenous ciprofloxacin (1000 mg/day) in addition to the previously mentioned regimen. Her symptoms improved, and she was discharged on the same medications and followed weekly for 3 months thereafter. By her last visit her infection had improved and she was advised to go for surgery. She subsequently underwent surgery by a plastic surgeon to remove the bulk of the offending tissue and wound closed primarily (Figures 1 and 2). Post-operatively, the patient was treated with both doxycycline orally (100 mg twice a day) and topical clindamycin (1% twice a day) and followed until complete resolution, which was achieved 9 months post-operation.
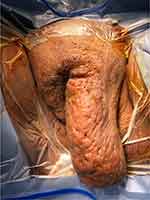 |
Figure 1 Vulva showed a huge polypoidal mass measuring 20x10cm in the left vulva and 8x3cm in the right vulva. |
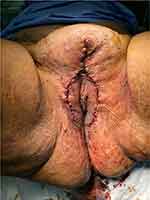 |
Figure 2 Postoperative picture showing primary closure of wound. |
Discussion
Our case highlights the rare consequences of HS, where long standing disease superimposed on chronic inflammation of the vulva led to a miscarriage. To our knowledge, this type of complication has never been reported before in the literature. Infection of the vulva can present as complex differential diagnoses to the treating doctors ranging from superficial skin infection to life-threatening necrotizing fasciitis.16 The subcutaneous anatomy of the vulva can facilitate rapid spread to other tissues with significant morbidity and mortality. Ineffective antibiotics or deferring necessary surgical treatment of HS have proven to be costly to the patient.17 Bacterial infection with Staphylococcus, Escherichia coli, and Streptococcus is considered a secondary event in the pathogenesis of HS.18 These types of bacteria have previously been reported to cause miscarriage in the field of obstetrics and gynecology.18,19 Furthermore, cervicovaginal infection has been significantly associated with preterm and term premature rupture of the membrane, miscarriage, and intrauterine death.20 These pathological processes clearly explain the mechanism that led to our patient’s miscarriage.
Histopathology results showed only acute and chronic inflammatory cells infiltrated the adnexal structures in the dermis and no evidence of malignancy. Any biopsy taken from long standing disease should always be investigated for squamous cell cancer.21 HS is a chronic inflammatory disease that may affect sexual and erectile dysfunction and adversely affect quality-of-life,22 and this case emphasizes the importance of recognizing HS in these areas to facilitate better early treatment. Furthermore, a multidisciplinary approach is recommended, especially in pregnant women, to avoid the risk of ascending infection and possible miscarriage. Hurley stage II and III HS is better managed surgically for complete eradication of disease.
Conclusion
Long standing HS of the vulva profoundly impairs a patient’s quality-of-life, even in mild cases. Early recognition and treatment of vulvar HS disease is essential. Surgical intervention for Hurly stage II and III disease, especially in the vulvar region, is necessary to avoid serious complications such as miscarriage and malignancy.
Ethical Approval
Obtained from local ethical committee in King Khalid University.
Informed Consent
Written and informed consent from the patient was obtained for publication of case details and images.
Acknowledgments
The author would like to acknowledge Dr. Hisham M. O. Kheiralla from the department of plastic surgery and Dr. Waleed Houshimi from the department of obstetrics and gynecology for their critical review and feedback on the manuscript.
Funding
The author received no financial support for the research, authorship, or publication of this article.
Disclosure
The author has no conflicts of interest to declare.
References
1. Verneuil A. Etudes sur les tumeurs de la peau; de quelques maladies des glandes sudoripares. Arch Gen Med. 1854;4(447):693.
2. Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61(1):51–57. doi:10.1016/j.jaad.2009.02.013
3. Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. Dermatol Surg. 1989;729–739.
4. Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi:10.1016/j.jaad.2008.06.020
5. Zimman S, Comparatore M, Vulcano A, Absi M, Mazzuoccolo L. Hidradenitis suppurativa: estimated prevalence, clinical features, concomitant conditions, and diagnostic delay in a university teaching hospital in Buenos Aires, Argentina. Actas Dermosifiliogr. 2019;110(4):297–302. doi:10.1016/j.ad.2019.01.004
6. Vilarrasa ER, González YL. Clinical features of hidradenitis suppurativa and Crohn disease: what do these two entities have in common? Actas Dermosifiliogr. 2016;10(7):21–26.
7. Chan MP, Zimarowski MJ. Vulvar dermatoses: a histopathologic review and classification of 183 cases. J Cutan Pathol. 2015;42(8):510–518. doi:10.1111/cup.12541
8. Rekawek P, Mehta S, Andikyan V, Harmaty M, Zakashansky K. Squamous cell carcinoma of the vulva arising in the setting of chronic hidradenitis suppurativa: a case report. Gynecol Oncol Rep. 2016;16:28. doi:10.1016/j.gore.2016.03.002
9. Nazzaro G, Passoni E, Muratori S. Comparison of clinical and sonographic scores in Hidradenitis suppurativa and proposal of a novel ultrasound scoring system. Eur J Dermatol. 2018;28(6):845–847.
10. Church J, Fazio V, Lavery I, Oakley J, Milsom J. The differential diagnosis and comorbidity of hidradenitis suppurativa and perianal Crohn’s disease. Int J Colorectal Dis. 1993;8(3):117–119. doi:10.1007/BF00341181
11. Sartorius K, Boer J, Jemec GB. Topical Treatment. Hidradenitis Suppurativa. Springer; 2006.
12. O’Malley GF, Dominici P, Giraldo P, et al. Routine packing of simple cutaneous abscesses is painful and probably unnecessary. Acad Emerg Med. 2009;16(5):470–473. doi:10.1111/j.1553-2712.2009.00409.x
13. Gener G, Canoui-Poitrine F, Revuz J, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219(2):148–154. doi:10.1159/000228334
14. Van Der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology. 2009;219(2):143–147. doi:10.1159/000228337
15. Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62(2):205–217. doi:10.1016/j.jaad.2009.06.050
16. Sartorius K, Lapins J, Jalal S, Emtestam L, Hedberg M. Bacteraemia in patients with hidradenitis suppurativa undergoing carbon dioxide laser surgery: detection and quantification of bacteria by lysis-filtration. Dermatology. 2006;213(4):305–312. doi:10.1159/000096194
17. Jemec GB. Hidradenitis suppurativa. NEJM. 2012;366(2):158–164. doi:10.1056/NEJMcp1014163
18. Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116–133. doi:10.1093/humupd/dmv041
19. Benedetto C, Tibaldi C, Marozio L, et al. Cervicovaginal infections during pregnancy: epidemiological and microbiological aspects. J Matern Fetal Neonatal Med. 2004;16(2):9–12. doi:10.1080/jmf.16.2.9.12
20. Manolitsas T, Biankin S, Jaworski R, Wain G. Vulval squamous cell carcinoma arising in chronic hidradenitis suppurativa. Gynecol Oncol. 1999;75(2):285–288.
21. Kerdel F, Menter A, Micheletti R, editors. Hidradenitis suppurativa: update on diagnosis and treatment. Semin Cutan Med Surg. 2014.
22. Cuenca-barrales C, Montero-vilchez T, Szepietowski J, Matusiak L, Molina‐Leyva A. Sexual impairment in a patient with hidradenitis suppurativa. A system reviews. Eur Acad Dermatol Venerol. 2020. doi:10.1111/jdv16726
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
