Back to Journals » Cancer Management and Research » Volume 10
miRNA-299-5p regulates estrogen receptor alpha and inhibits migration and invasion of papillary thyroid cancer cell
Authors Wang Z, He L, Sun W, Qin Y, Dong W, Zhang T, Zhang P, Zhang H
Received 4 August 2018
Accepted for publication 10 October 2018
Published 22 November 2018 Volume 2018:10 Pages 6181—6193
DOI https://doi.org/10.2147/CMAR.S182625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Zhihong Wang, Liang He, Wei Sun, Yuan Qin, Wenwu Dong, Ting Zhang, Ping Zhang, Hao Zhang
Department of Thyroid Surgery, The First Hospital of China Medical University, Shenyang 110001, China
Background: The incidence of papillary thyroid cancer (PTC) is increasing faster than any other solid tumors worldwide. Invasion and metastasis are the main reasons for the poor prognosis of patients with PTC. Previously, we observed significantly low expression of miRNA-299-5p in invasive PTC tissue samples.
Aim: The present study aimed to determine whether miR-299-5p plays a key role in PTC migration and invasion.
Materials and methods: The miR-299-5p expression level was measured using quantitative real-time PCR in 109 human PTC samples and paired adjacent normal tissues and in the human BCPAP PTC cell line. The effects of miR-299-5p on PTC cell migration and invasion were assessed using wound healing and transwell assays. In addition, we searched for the miR-299-5p target, and the potential mechanism was demonstrated using a reporter assay and rescue experiment.
Results: The expression of miR-299-5p was associated with gender and extrathyroidal extension, and an elevated level of miR-299-5p suppressed BCPAP cell migration and invasion. Estrogen receptor α (ERα) is a direct target of miR-299-5p. The expression level of ERα was significantly higher in PTC tissues and was associated with migration and invasion in PTC cells. Overexpression of ERα could impair miR-299-5p-induced inhibition of migration and invasion. As a key factor of the pathway related to PTC invasion, Gli1 can be combined with ERα and can be regulated by miR-299-5p.
Conclusion: Our data suggested that miR-299-5p could participate in PTC migration and invasion and could be a potential therapeutic target for patients with aggressive PTC tumors.
Keywords: miR-299-5p, papillary thyroid cancer, PTC, estrogen receptor α, ERα, migration, invasion
Introduction
Papillary thyroid cancer (PTC), comprising more than 80% of all thyroid carcinomas, is the prevalent histotype of thyroid cancer.1 It is usually a slow-growing cancer, with a good prognosis and therapeutic response. Although the overall survival from PTC is 97%, patients with advanced PTC have a 5-year survival rate of only 59%.2 Lymph node metastasis (LNM), extrathyroidal extension (ETE), and distant metastasis are associated with advanced tumor node metastasis (TNM) stages and poor prognosis.3 Therefore, efforts should be made to improve the prognosis of these patients with aggressive tumors.
Accumulating evidence suggests that miRNAs are involved in many important physiological and pathological processes, such as cell differentiation, development, and proliferation. miRNAs are widely dysregulated in different cancers including thyroid cancer.4,5 Our previous microarray analysis study showed that miR-299-5p was significantly downregulated in ETE PTC tissues compared with that in non-ETE PTC tissues.6 miR-299-5p is also significantly downregulated and can act as a tumor suppressor in various tumors, including prostate cancer, breast cancer, hepatocellular cancer, and colorectal cancer.7–10 However, the expression, clinicopathological significance, and function of miR-299-5p in PTC require further investigation.
The reason for high incidence of PTC in women is unclear. Studies have revealed that the estrogen receptor (ER), especially ERα, plays an important role in the development and progression of PTC.11,12 Upregulation of ERα expression in PTC cells increased the activity and secretion of MMP-2 and MMP-9 and promoted cell invasion and metastasis.13 Our previous study indicated that the expression of ERα was significantly associated with gender and ETE in PTC tissues. Moreover, ERα can promote the invasion of the ERα-positive PTC cell line BCPAP when treated with the ERα-selective agonist 1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT).14,15
The transcription factor Gli1 is both a downstream effector of the Hedgehog (Hh) pathway and a target protein regulated by this pathway, and its expression is a hallmark of activation of the Hh pathway.16 Some studies suggest the relationship between Gli1 expression and tumor invasion and metastasis.17,18 Our previous study showed that the expression of Gli1 in the Hh signaling pathway in PTC tissues was significantly higher than that in normal tissues, and its expression level correlated with tumor size, extrathyroidal invasion, and LNM.19 Studies on breast cancer have shown that overexpression of ERα in ER-positive breast cancer cells can upregulate the transcriptional activity of Gli1 and even promote the effect of Gli1 protein on cell invasion and metastasis.20,21
We propose the following hypothesis: in ERα-positive cell lines, miR-299–5 p may inhibit the invasion of PTC cells by targeting ERα to affect the expression of Gli1.
Materials and methods
A total of 109 human PTC tissues and 109 paired adjacent normal thyroid tissues were obtained from patients undergoing curative-intent surgery in Department of Thyroid Surgery, the First Hospital of China Medical University (Shenyang, China) between 2010 and 2014. All tissues were immediately frozen in liquid nitrogen and stored at −80°C until processing. The inclusion criteria were as follows: all patients received initial surgery and none of the patients in our group had undergone prior oncological surgery or head and neck irradiation. Clinicopathological information, such as tumor size, the presence of ETE, and LNMs, were collected from the relevant medical records. ETE was defined as invasion of an adjacent tissue or an organ or the skeletal muscle outside the isthmus.22 Our study was approved by the ethics committee of the First Hospital of China Medical University, Shenyang, China. Written informed consent was obtained from all study participants.
Cell culture and transfection
The ERα-positive human PTC cell line, BCPAP, was bought from the Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China) and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS (RPMI 1640+10% FBS+1% nonessential amino acids+1% sodium pyruvate+1% Glutamax).23,24 The miR-299-5p mimic, inhibitor, and their respective negative control (NC) were purchased from Gene Pharma (Shanghai, China). siRNAs to silence ERα were designed and synthesized by Gene Pharma. The oligonucleotide sequences were as follows: miR-299-5p mimic (sense): 5′-UGGUUUACCGUCCCACAUACAU-3′, NC (sense): 5′-UUCUUCGAACGUGUCACGUTT-3′. ERα-siRNAs sequences used were 5′-CGAGUAUGAUCCUACCAGATT-3′ (sense); the following nonsense siRNA was used as a NC: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense). Gli1-siRNAs sequences used were 5′-AACUCCACAGGCAUACAGGAU-3′ (sense); NC: 5′-AACGUACGCGGAAUACAACGA-3′ (sense). Lipofectamine 2000 was used for transfection (Thermo Fisher Scientific, Waltham, MA, USA). The PTC cell line treated with the transfection reagent alone was used as a mock control. The normal human thyroid follicular epithelial cell line, Nthy-ori 3-1, was purchased from the European Collection of Authenticated Cell Culture (ECACC, UK).
Lentivirus and its NC were designed and synthesized by Genechem (Obio Technology Corp., Ltd., Shanghai, China). A total of 3×105 cells/mL were seeded in each well of a six-well plates and then infected with pLenti-EF1a-EGFP-P2A-Puro-CMV-ESR1-3Flag (vector-ERα) or plenti-CMV-3FLAG-GLI1-PGK-Puro (vector-Gli1), empty vector (EV; NC group), and no infection (nontransfected control group) by replacing the medium with infection medium containing recombinant vectors at a multiplicity of infection (MOI) of 40 plaque forming units per cell and 10 µL of 1 mg/mL polybrene (Obio Technology Corp., Ltd.). Plates were then incubated for 24 hours before their media were changed to fresh, virus-free media. Cells were treated with puromycin (1 µg/mL; InvivoGen, San Diego, CA, USA) to produce stable transfected cells (vector-ERα, vector-Gli1, and EV) for further experiments.
Total RNA extraction and quantitative real-time reverse transcription PCR (qRT-PCR) detection
Total RNA was isolated using RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China). The 638315 Mir-X™ miRNA First-Strand Synthesis Kit (Takara Biotechnology Co., Ltd.) was used to transcribe total RNA to cDNA. qRT-PCR was performed using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) on the LightCycler 480 system (Roche Diagnostics, Indianapolis, IN, USA). The qRT-PCR cycling profile was as follows: 95°C for 30 seconds, followed by 40 cycles at 95°C for 5 seconds and 60°C for 30 seconds. GAPDH and U6 were used as references for mRNAs and miRNAs, respectively.
Cell migration and invasion assays
Cell migration was evaluated using a wound-healing assay. After 24 hours of transfection, 4×105 cells were cultured in six-well plates until the cell confluence reached 90%, and scratches were created by scraping the cell layer across each well using a 200 µL pipette tip. Wound healing was observed under a light microscope, and images in three observation fields were captured at 0 hour and 24 hours. Transwell invasion assays were performed to determine cell invasion. After 24 hours of transfection, the cells were trypsinized, the cells were suspended into single cells in a serum-free medium, and the concentration of each group was adjusted to 4×104 cells/mL using a serum-free medium. Cell invasion assays in vitro were performed using a Transwell chamber (Costar, Corning, NY, USA) with a polycarbonic membrane (6.5 mm in diameter and 8 µm pore size). Cells were incubated for 36 hours for the invasion assay. Afterward cells that had invaded to the lower surface of the membrane were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet and washed twice with PBS. Stained cells were observed under an inverted microscope to count the cell number within five randomly chosen fields at 100× magnification, and the average number was calculated.
Luciferase reporter assay
The 3′ UTR from ESR1 (encoding ERα) (ESR1-3′UTR [Wt]) and mutant ESR1-3′UTR (Mut) containing the putative binding site of miR-299-5p were generated by PCR amplification, established, and cloned into the firefly luciferase expressing vector pmirGLO (Promega Corporation, Fitchburg, WI, USA) for a dual luciferase reporter assays. The 293 T cell line was purchased from the Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China) and used for luciferase reporter assays. 293 T cells were plated into 24-well plates and cotransfected with 500 ng of the ESR1-Wt or ESR1-Mut reporter vector and 100 nM miR-299-5p mimics or 200 nM of inhibitor using Lipofectamine 2000. After 48 hours, the cells were harvested, and luciferase activity levels were measured using a Dual-Luciferase Reporter Assay System (Promega Corporation) according to the manufacturer’s protocol.
Immunohistochemical analysis
Twenty formalin-stabilized PTC tissues and adjacent normal thyroid tissues were embedded in paraffin and cut into 4 µm sections for use in immunohistochemistry (IHC). Slides were deparaffinized, rehydrated, and subjected to microwave heat antigen retrieval in 0.01 mol/L sodium citrate buffer (pH 6.0) for 20–25 minutes. After blocking endogenous peroxidase activity after 30 minutes at 37°C, the sections were incubated with primary antibodies against ERα (1:200; Abcam, Cambridge, UK) overnight at 4°C. Immunoreactivity was visualized using the chromogen 3, 3′-diamino-benzidine (DAB; Maixin Bio, Fuzhou, China). The slides were then counterstained with hematoxylin, washed, dehydrated with alcohol and xylene, and mounted onto cover slips.
Western blotting analysis
Proteins were extracted from BCPAP cells and PTC and adjacent normal tissue samples from eight patients (four male and four female patients, and four patients were <45 years old and four were ≥45 years old). The protein extracts (20–30 µg) were separated on 10% SDS-PAGE gels and then electrophoretically transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked in 5% skim milk, which was dissolved in Tris-buffered saline with 0.1% Tween-20 (TBS-T) for 2 hours. The membranes were then incubated with primary antibodies for ERα (1:300; Santa Cruz Biotechnology Inc., Dallas, TX, USA), Gli1 (1:100; Santa Cruz Biotechnology Inc.), and GAPDH (1:3,000; Santa Cruz Biotechnology Inc.) overnight at 4°C. The membranes were then washed three times and incubated with a 1:10,000 dilution of horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody and visualized using enhanced chemiluminescence (ECL; Thermo Fisher Scientific).
Coimmunoprecipitation
For coimmunoprecipitation of Gli1 with ERα, the following lysis buffer was used: 20 mM Tris (pH 7.9), 150 mM NaCl, 1 mM EDTA, 0.3% Triton-X-100, proteinase inhibitor cocktail (Roche Applied Science, Penzberg, Germany), and halt phosphatase inhibitor cocktail (Thermo Fisher Scientific). The cell lysates were subjected to immunoprecipitation with anti-Gli1 antibodies (Santa Cruz Biotechnology Inc.) or anti-ERα antibodies (Santa Cruz Biotechnology Inc.). The eluates were separated by SDS-PAGE, and Western blotting analysis was performed using the anti-ERα antibodies (1:1,000) and anti-Gli1 antibodies (1:1,000).
Statistical analyses
All statistical analyses were performed using SPSS Statistics (version 20.0; IBM Corporation, Armonk, NY, USA) and GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA). The results are presented as mean±SD and median (IQR). The relative expression levels of mRNAs or miRNAs were calculated using the 2−ΔCt method (cycle threshold [Ct]). The Mann–Whitney U test, Student’s t-test, and ANOVA tests were used to compare data from independent groups. Bonferroni correction was used to counteract the problem of multiple comparisons. The chi-squared test was applied to examine the relationship between miR-299-5p expression and the clinicopathological characteristics. P-value <0.05 was considered statistically significant.
Results
miR-299-5p was downregulated in PTC tissues and cell lines and was associated with gender and ETE
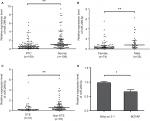
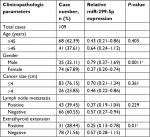
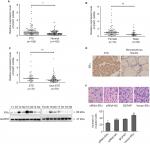
The expression of miR-299-5p was significantly lower in human PTC tissues (n=109) compared with that in paired adjacent normal thyroid tissues (Figure 1A; P<0.001). To further investigate the clinical significance of miR-299-5p, we examined the correlations between miR-299-5p expression and the clinicopathological features in PTC tissues. As shown in Table 1, there were significant differences in the expression levels of miR-299-5p (relative quantification value) with regard to gender (74 females vs 35 males; Figure 1B; P=0.0011). We compared the miR-299-5p expression between the same number of male and female samples (n=35; Figure S1). The estrogen levels get lower with decreased ovulation in women older than 45 years. Thus, the analysis of ERα expressions in PTC was performed in those patients of reproductive age (≤45 years old) and advanced reproductive age (>45 years old). We provided the differences in miR-299-5p expression levels in males and females in different age subgroups. In female group, miR-299-5p expression was significantly lower in patients aged 45 years or younger than that in patients older than 45 years. The opposite result was found in the male group (Figure S2A and B). In addition, miR-299-5p was strongly associated with ETE in PTC (31 ETE vs 78 non-ETE; Figure 1C; P=0.01), which was consistent with the previous microarray data. We detected miR-299-5p expression in ETE vs non-ETE patients in gender-specific and age-specific manners (Figure S3A–D). The patients with ETE included eight with perithyroid muscle invasion, 11 with recurrent laryngeal nerve, three with trachea, two with esophagus, five with carotid artery sheath, and two with jugular vein. miR-299-5p was downregulated in PTC cell lines (BCPAP) compared with that in normal human thyroid follicular epithelial cell line (Nthy-ori 3-1; Figure 1D; P<0.05). miR-299-5p inhibited the migration and invasion of PTC cells
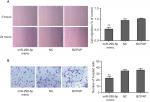
A wound healing assay and a transwell assay were used to assess the effect of miR-299-5p on migration and invasion, respectively, of PC cells in vitro. The results showed that miR-299-5p overexpression reduced the migration ability of BCPAP cells compared with that of the NC (Figure 2A; P<0.001). Moreover, as shown in Figure 2B, the Transwell assays indicated that overexpression of miR-299-5p decreased the invasion abilities of BCPAP cells (P=0.002). Taken together, these data indicated that miR-299-5p could inhibit migration and invasion in BCPAP cells.
ERα is a direct target of miR-299-5p
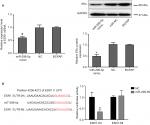
To investigate the mechanism of miR-299-5p in PTC, we screened for target genes of miR-299-5p using the target prediction tools such as TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org). Both prediction tools indicated that ESR1 (encoding ERα) could be a direct target of miR-299-5p. As predicted, we observed that overexpression of miR-299-5p decreased the expression of ERα at both mRNA and protein levels in BCPAP cells (Figure 3A). To assess whether miR-299-5p could directly bind to the 3′-UTR of ESR1 mRNA, the wild-type ESR1-3′UTR (Wt) and mutant ESR1-3′UTR (Mt) containing the putative binding site of miR-299-5p were established and cloned into a luciferase expressing vector. The results demonstrated that overexpression of miR-299-5p significantly suppressed the luciferase activity of the wild-type ESR1-3′UTR but failed to affect the mutant ESR1-3′UTR (Figure 3B). These data indicated that miR-299-5p could directly bind to the 3′-UTR of ESR1.
ERα was overexpressed and associated with migration and invasion in PTC
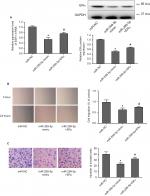
To further evaluate the effect of ERα on PTC development, we detected ERα expression in PTC using qRT-PCR, Western blotting, and IHC. PCR analysis revealed that ESR1 levels were significantly upregulated in PTC tissues (n=109) compared with those in paired adjacent normal thyroid tissues (Figure 4A; P=0.003). The expression of ESR1 was significantly higher in the female group than in the male group (Figure 4B; P=0.009) and in the ETE group compared with that in the non-ETE group (Figure 4C; P=0.01), which was consistent with our previous work. We compared the ESR1 expression between same number of male and female samples (n=35). The results still showed that ESR1 expression in the female group was significantly higher than that in the male group (Figure S4). The IHC results showed strong positive staining for ERα in PTC tissues, whereas staining was absent or sporadic in adjacent normal thyroid tissues (Figure 4D). Moreover, Western blotting analysis of samples from eight patients also revealed that the level of ERα in PTC tissues was higher than that in adjacent normal thyroid tissues (Figure 4E). We demonstrated that overexpression of ERα increased the invasion abilities of BCPAP cells (P=0.002), while inhibiting ERα expression decreased the invasion abilities, as assessed using transwell assays (P=0.0011; Figure 4F). These results demonstrated that ERα was overexpressed and associated with migration and invasion in PTC.
Overexpression of ERα could impair the miR-299-5p-induced inhibition of migration and invasion
Next, we adapted a “rescue” strategy to further confirm whether the inhibition of migration and invasion effects of miR-299-5p was mediated via ERα. The vector-ERα was first transfected into BCPAP cells to construct a stable ERα overexpression BCPAP cell line. The PCR and Western blotting results indicated that overexpression of ERα significantly rescued the inhibition of ERα expression in the presence of miR-299-5p mimic (Figure 5A). Wound healing assays showed that overexpression of ERα significantly rescued the migration inhibition role of miR-299-5p (Figure 5B). Transwell assays showed that overexpression of ERα significantly attenuated inhibition of the invasion induced by miR-299-5p (Figure 5C). These results demonstrated that miR-299-5p inhibited migration and invasion by targeting ERα in BCPAP cells.
miR-299-5p regulated migration and invasion through the ERα/Gli1 complex
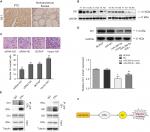
To better understand the underlying molecular mechanism of miR-299-5p-mediated inhibited migration and invasion of PTC, we detected the Gli1 protein that is associated with the ERα pathway. IHC revealed that the protein levels of Gli1 were strongly and positively stained in PTC tissues (Figure 6A). Western blotting results showed that the protein level of Gli1 in PTC tissues was higher than that in adjacent normal thyroid tissues (Figure 6B). Overexpression of Gli1 increased the invasion abilities of BCPAP cells (P=0.002), while inhibiting Gli1 expression decreased their invasion abilities, as assessed using Transwell assays (P=0.03; Figure 6C). Therefore, we hypothesized that miR-299-5p might inhibit the migration and invasion of PTC cells by targeting ERα to affect the expression of Gli1. To confirm our hypothesis, we first detected whether miR-299-5p could also regulate the protein level of Gli1. The results indicated that overexpression of miR-299-5p reduced the protein level of Gli1. Next, Western blotting results indicated that overexpression of ERα significantly rescued the inhibition of Gli1 expression in the presence of miR-299-5p mimic (Figure 6D). In addition, BCPAP cell lysates were coimmunoprecipitated with anti-ERα antibodies, followed by Western blotting assay with anti-Gli1 antibodies. A 120 kDa band corresponding to Gli1 was found in the input group, and the group coimmunoprecipitated with anti-ERα antibodies (Figure 6E). Our results revealed that Gli1 is a direct client protein of ERα and that miR-299-5p might inhibit migration and invasion by regulating the ERα/Gli1 complex.
Discussion
In a previous study, a microarray identified that miR-299-5p was significantly downregulated in extrathyroid invasion PTC tissues, indicating that miR-299-5p could play important regulatory roles in PTC invasion. In this study, we found that the expression of miR-299-5p was significantly lower in 109 PTC tissues compared with paired adjacent normal thyroid tissues. The low expression of miR-299-5p correlated with gender and ETE. In addition, overexpression of miR-299-5p inhibited ERα expression, thereby suppressing migration and invasion of BCPAP cells. Collectively, our findings suggest that miR-299-5p acts as a PTC suppressor.
The anticancer role of miR-299-5p has been reported in other cancer types, such as breast cancer, hepatocellular cancer, and colorectal cancer.7,8,10 Shevde et al7 reported that miR-299-5p regulates osteopontin (OPN) and that increasing the expression of OPN via decreased levels of miR-299-5p plays an important role in enhancing the proliferation of breast cancer cells. Jin et al8 demonstrated that miR-299-5p could act as a tumor suppressor to mediate the antitumor mechanism of OSW-1 (a saponin with marked antitumor activities) in hepatocellular cancer. In addition, Fateh et al10 showed that the expression level of miR-299-5p was significantly downregulated in a group of colorectal cancer tissues and could be used as a predictive biomarker in the diagnosis of colorectal cancer. In our study, the different expression of miR-299-5p in men and women, and the invasion–suppressive role of miR-299-5p by targeting ERα, indicated the importance of this essential pathway in PTC.
Estrogens are involved in the growth and differentiation of the normal mammary gland, and the biological effect of estrogen is mediated by several receptors, including ERα and ERβ. ERα contributes to the development of gynecological tumors, especially breast cancer. In PTC, overexpression of ERα could modulate MMP-2 and MMP-9 secretion and activity in thyroid cancer cells. Loss of the ER by siRNA transfection resulted in loss of estradiol-mediated migration and invasion of thyroid cancer cells.13 In the present study, we demonstrated the invasion suppression role of miR-299-5 p by functional experiments and identified the miR-299-5p-binding site of ESR1 using a luciferase reporter gene assay.
To further determine the potential molecular mechanism of miR-299-5p in regulating ERα, we focused on the downstream proteins that interact with ERα. The level of Gli1, the key regulator in the Shh pathway, has been reported to correlate with the invasiveness in many different cancers.16 Our previous study reported that Gli1 was significantly overexpressed and correlated with larger tumor size, the presence of ETE, lymph nodes metastasis, and higher TNM stage in PTC.19 In breast cancer, Gli1 may be responsible for the estrogen-induced epithelial–esenchymal transition (EMT) in ER-positive breast cancer cells.20,21 E2 induces transcriptional activation of Gli1 probably by enriching ER occupancy at the Gli1 promoter. Sun et al20 reported that ER expression positively correlated with Gli1 levels. Estrogen may promote ER-positive breast cancer cell development and EMT via Gli1. Our results indicated that overexpression of miR-299-5p reduced the protein level of Gli1 in BCPAP cells. Moreover, a coimmunoprecipitation assay revealed the interaction between ERα and Gli1, which increased our understanding of the molecular interactions between these two pathways.
Our study highlighted the oncotarget potential of miR-299-5p expression in ERα-positive PTC BCPAP cells. Moreover, this is the first study to describe the relationships among miRNAs and the ER and Shh pathways, which might encourage further exploration of the anticancer function of miR-299-5p, although our results need to be validated in a larger sample cohort.
Conclusion
This study revealed that miR-299-5p is significantly downregulated and has clinical significance in PTC migration and invasion. ERα and the ERα/Gli1 complexes were demonstrated to be the direct binding target and functional target of miR-299-5p, respectively. Our data suggested that miR-299-5p could participate in PTC migration and invasion and could be a potential therapeutic target for patients with aggressive PTC tumors (Figure 6F).
Acknowledgments
This research was supported by National Natural Science Foundation of China (grant number: 81502319), the Liaoning Province PhD Start-up Fund (grant number: 201501008), the Liaoning BaiQianWan Talents Program (grant number: 2014921033), the Science and Technology Project of Shenyang City (grant number: F16-205-1-41), and Natural Science Foundation of Liaoning Province (grant number: 2015020536).
Disclosure
The authors report no conflicts of interest in this work.
References
Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer. 2016;23(4):313–322. | ||
Kunavisarut T. Diagnostic biomarkers of differentiated thyroid cancer. Endocrine. 2013;44(3):616–622. | ||
Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106(3):524–531. | ||
Falcone G, Felsani A, D’Agnano I. Signaling by exosomal microRNAs in cancer. J Exp Clin Cancer Res. 2015;34:32. | ||
Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. | ||
Wang Z, Zhang H, He L, et al. Association between the expression of four upregulated miRNAs and extrathyroidal invasion in papillary thyroid carcinoma. Onco Targets Ther. 2013;6:281–287. | ||
Shevde LA, Metge BJ, Mitra A, et al. Spheroid-forming subpopulation of breast cancer cells demonstrates vasculogenic mimicry via hsa-miR-299-5p regulated de novo expression of osteopontin. J Cell Mol Med. 2010;14(6B):1693–1706. | ||
Jin JC, Jin XL, Zhang X, Piao YS, Liu SP. Effect of OSW-1 on microRNA expression profiles of hepatoma cells and functions of novel microRNAs. Mol Med Rep. 2013;7(6):1831–1837. | ||
Formosa A, Markert EK, Lena AM, et al. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2014;33(44):5173–5182. | ||
Fateh A, Feizi MAH, Safaralizadeh R, Azarbarzin S. Importance of miR-299-5p in colorectal cancer. Ann Gastroenterol. 2017;30(3):322–326. | ||
Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6(11):1771–1779. | ||
Fan D, Liu SY, van Hasselt CA, et al. Estrogen receptor α induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J Clin Endocrinol Metab. 2015;100(4):E561–E571. | ||
Rajoria S, Suriano R, George A, et al. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3’-diindolylmethane in thyroid cancer. PLoS One. 2011;6(1):e15879. | ||
Dong W, Zhang H, Li J, et al. Estrogen Induces Metastatic Potential of Papillary Thyroid Cancer Cells through Estrogen Receptor α and β. Int J Endocrinol. 2013;2013:941568. | ||
Huang Y, Dong W, Li J, Zhang H, Shan Z, Teng W. Differential expression patterns and clinical significance of estrogen receptor-α and β in papillary thyroid carcinoma. BMC Cancer. 2014;14:383. | ||
Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24(18):2001–2012. | ||
Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32(1):368. | ||
Thomas ZI, Gibson W, Sexton JZ, et al. Targeting GLI1 expression in human inflammatory breast cancer cells enhances apoptosis and attenuates migration. Br J Cancer. 2011;104(10):1575–1586. | ||
Dong W, Cui J, Tian X, et al. Aberrant sonic hedgehog signaling pathway and STAT3 activation in papillary thyroid cancer. Int J Clin Exp Med. 2014;7(7):1786–1793. | ||
Sun Y, Wang Y, Fan C, et al. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol Cancer. 2014;13:137. | ||
Xu L, Kwon YJ, Frolova N, et al. Gli1 promotes cell survival and is predictive of a poor outcome in ERalpha-negative breast cancer. Breast Cancer Res Treat. 2010;123(1):59–71. | ||
Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol. 2010;17(2):386–391. | ||
Rajoria S, Suriano R, Shanmugam A, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20(1):33–41. | ||
Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010;36(5):1067–1080. |
Supplementary materials
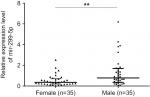  | Figure S1 miR-299-5p expression between male (n=35) and female (n=35) patients. Note: **P<0.01. |
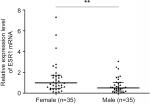  | Figure S4 ESR1 expression between male (n=35) and female (n=35) patients. Note: **P<0.01. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.